Abstract
Nonhuman primates are widely used models to investigate the neural substrates of human behavior, including the development of higher cognitive and affective function. Due to their neuroanatomical and behavioral homologies with humans, the rhesus macaque monkey (Macaca mulatta) provides an excellent animal model in which to characterize the maturation of brain structures from birth through adulthood and into senescence. To evaluate hippocampal development in rhesus macaques, structural magnetic resonance imaging scans were obtained longitudinally at 9 time points between 1 week and 260 weeks (5 years) of age on 24 rhesus macaque monkeys (12 male, 12 female). In our sample, the hippocampus reaches 50% of its adult volume by 13 weeks of age and reaches an adult volume by 52 weeks in both males and females. The hippocampus appears to be slightly larger at 3 years than at 5 years of age. Male rhesus macaques have larger hippocampi than females from 8 weeks onward by approximately 5%. Interestingly, there was increased variability in hemispheric asymmetry for hippocampus volumes at younger ages than at later ages. These data provide a comprehensive evaluation of the longitudinal development of male and female rhesus macaque hippocampus across development from 1 week to 5 years of age.
Introduction
Recent years have witnessed an increased interest in understanding the role of altered brain developmental trajectories as the basis for the cognitive and functional deficits associated with a number of neurogenetic disorders. Specifically, malformations or abnormal developmental trajectories of the hippocampus have been associated with the cognitive impairments in disorders such as autism, schizophrenia, Down Syndrome, Fragile X-Associated Disorders, 22q11.2 Deletion Syndrome, and Williams Syndrome, among many others (Bauman and Kemper, 1985; DeBoer et al., 2007; Dierssen et al., 1996; Harrison, 1999; Leverenz and Raskind, 1998; Lipska and Weinberger, 2002; Machado and Bachevalier, 2003; Raymond et al., 1996; Saitoh et al., 2001). Unfortunately, it is difficult to evaluate altered developmental trajectories as causal agents for the cognitive deficits in human subjects since imaging studies are generally carried out only after an individual receives a diagnosis (cf., Giedd et al., 2008) which is often several years after birth.
There have been a number of recent developmental neuroimaging studies that provide insights into the maturation of several structures within the human brain (Giedd et al., 2009, 2010; Knickmeyer et al., 2008; Tanaka et al., 2012; Wallace et al., 2006, 2012). Specifically, volumetric changes in the hippocampus have been previously reported in either cross-sectional or somewhat limited longitudinal human neuroimaging studies (Caviness et al., 1999; Casey et al., 2000; Saitoh et al., 2001; Giedd et al., 2006; Lenroot and Giedd, 2006; Thompson et al., 2009). In some studies, when accounting for total cerebral volumes, females demonstrated a disproportionately larger hippocampus than males in 7-to-11-year-old participants (Filipek et al., 1994; Caviness et al., 1996). However, in another study on a different population, Giedd et al. (1996, 1997) reported that between 4 and 18 years of age, males had larger hippocampi than females, but these differences disappeared when total cerebral volume was taken into account. They further demonstrated that females, but not males, exhibited significant changes in hippocampal volume within this age range. Hemispheric differences are supported by repeated observations that the right hippocampus is larger than the left in adults (Jack et al., 2000), children (Giedd et al., 1996; Pfluger et al., 1999; Utsunomiya et al., 1999), and neonates (Thompson et al., 2009). These studies, while providing important contributions to the field, are limited because they are most often cross sectional in nature and encompass relatively wide age ranges. The longitudinal studies in the literature to date have limited data, but this limitation is currently being remedied. Related to the hippocampus, these studies lack data on the very early postnatal time points that are necessary to study its maturation (< 4 years of age; cf., Figure 1 in Giedd et al., 2010).
Nonhuman primates have been widely used animal models to investigate the neural substrates of human behavior, including the development of higher cognitive and affective function as well as to characterize complex social interactions (Amaral et al., 2003; Bauman et al., 2004, 2006; Bechavalier & Málková, 2006). Given the neuroanatomical (Lavenex et al., 2006, 2007) and behavioral (Kalin et al., 1989, 2004) homologies between humans and some nonhuman primate species, the rhesus macaque (Macaca mulatta) provides an excellent candidate animal model in which to characterize the maturation of specific brain structures and major white matter pathways from birth to adulthood. However, there is currently remarkably little information on the longitudinal morphological development of the hippocampus in the macaque monkey.
Another major advantage of the nonhuman primate model is that it can be studied during the very earliest stages of postnatal development (i.e., in utero, as well as son as days to weeks after birth) and can more easily be assessed longitudinally for cognitive, social, and emotional maturation than human children. Additionally, the role for neurogenesis in postnatal development of the hippocampus has been quantified in the rhesus macaque. Specifically, in a cross sectional stereological study, Jabès et al. (2010) report that approximately 30% of the total number of granule cells observed in the dentate gyrus of 5 to 10 year old rhesus macaque monkeys are added to the granule cell layer postnatally; and roughly 25% of these neurons are added within the first three postnatal months. They also reported an overall stable number of granule cells in mature 5 to 10 year old monkeys. These data identify an extended developmental period during which neurogenesis might be modulated to significantly impact the structure and function of the dentate gyrus into adulthood.
Using MRI, Franklin et al. (2000) reported a gender difference in rhesus macaque monkey total brain volume between 1.5 and 7.2 years of age in a cross sectional study. Similar to humans, total cerebral volume was approximately 20% larger in male monkeys relative to females, but no age-related changes were observed in their sample. However, a longitudinal study did reveal a significant increase in total cerebral volume between 1 week and 4 years of age (Málková et al., 2006).
To date, there is only a single MRI study evaluating the early development of the rhesus macaque hippocampus (Payne et al., 2010). Payne et al. (2010) evaluated MRI scans of rhesus macaques from 1 week to 2 years of age for hippocampus, amygdala, and total cerebral volume development. In all, 10 subjects (n = 5 male and n = 5 female) were evaluated longitudinally for hippocampus development.
The present study was designed to extend the findings of Payne et al. (2010) by specifically evaluating the maturation of the hippocampus in 12 male and 12 female rhesus macaques from 1 week of age through 5 years of age. The goal was to compare findings from this larger population of macaque monkeys with findings from human children to determine whether homologies of development exist. We aimed to determine, for example, whether the same degree of variation in overall hippocampal volume in rhesus macaque monkeys mirrored what has been observed in human children. We were also interested in determining whether the sexual dimorphism present in hippocampal development described by Giedd et al. (2012) but not observed by Payne et al. (2010) would be observed in a larger sample of rhesus macaque monkeys.
Materials & Methods
Subjects
All experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and developed through consultation with the veterinary staff at the California National Primate Research Center (CNPRC). The University of California, Davis Institutional Animal Care and Use Committee approved all experimental protocols. Briefly, rhesus macaque monkeys (Macaca mulatta) were studied from birth through five years of age for behavioral and structural brain development. Naturalistic behavioral observations were conducted in their home environments regularly. At periodic intervals (1, 4, 8, 13, 26, 39, 52, 156, and 260 weeks of age), rhesus macaques were brought in from their naturalistic outdoor enclosures for behavioral tests, measurements of physical development, and MRI scans of the brain.
Twenty-eight rhesus macaque monkeys (14 males, 14 females) were selected from the CNPRC in the spring of 2007. Infants were raised in social troops by their biological mothers in outdoor, half-acre enclosures that house 70 to 155 animals. Subject selection was based on characteristics of the mother. Mothers were selected based on the following factors: (1) rank of matriline (high, n = 8; middle, n = 9; low, n = 10); (2) previous reproductive experience (multiparous, n = 25; primaparous, n = 3); (3) absence of previous medical problems such as diabetes, arthritis, etc. These factors were used as selection criteria to allow for an analysis of potential rank in hippocampus volumes as well as to select mothers that were likely to bring the infant primates to term. Three of the subjects were hospitalized during the course of the analysis for symptoms of dehydration caused by bacterial or parasitic gastrointestinal infection. These subjects were successfully treated and remained in the study. Treatment included administration of fluids and antibiotics. Two subjects were removed after 1 year of age due to recurrent illness. One subject was removed from the study at 4 months of age due to non-pathogenic diarrhea that was not responsive to treatment. Therefore, 24 subjects (n = 12 male and n = 12 female) received MRI scans at all ages and only data from these subjects that received all longitudinal measurements will be reported in this manuscript.
Cohort characteristics occasionally changed after the selection of subjects. For example, rank shifted for multiple matrilines. So, the social rank was assessed monthly based on two, 30-minute observations by CNPRC behavioral specialists. All dyadic aggressive and displacement interactions, with and without food as a precipitating stimulus, were recorded and used to determine the hierarchy of the females in each troop. Rank status was determined to have changed when displacements (submission to a lower ranking rhesus macaque in the selection of food) were observed twice for the mother of the infant. Rank was consequently raised for the primate that displayed dominance in the food challenge. Social rank was raised for one primate (low to mid) and shifted downward for two others (mid to low) and (high to mid). For the latter two animals, this shift took place when their mothers were removed from their home enclosures after weaning and the infants remained with their respective matrilines.
Infants were born and reared by mothers that resided in large, 2000 m2 outdoor corrals. All seven of the corrals that housed study animals were chain-link and consisted of grass and gravel ground substrate and included a variety of hanging, climbing, and resting structures. The number of animals that lived in these corrals ranged from 70–155 individuals and all the kin relationships of all the primates were known. Primates were fed twice per day, in the morning and afternoon, with chow (Lab Diet 5047, PMI Nutrition International Inc., Brentwood, MO) and supplemented with fresh fruit and vegetables.
Animal Husbandry
For MRI scans collected at 1, 4, 8, 13, and 26 weeks of age, rhesus macaque infants were relocated with their mothers and were housed together in a standard macaque indoor housing cage (61 cm in width by 66 cm in depth by 81 cm in height) one day prior to behavioral testing. On days when testing was to occur, mothers were lightly sedated with ketamine hydrochloride (7 to 8 mg/kg i.m.) and infants were removed from the cage for testing. Beginning at 39 weeks of age, each rhesus macaque subject was removed from its respective home enclosure without the mother the day prior to behavioral testing and was temporarily housed indoors as described above.
Structural MRI Acquisition
After behavioral testing at the CNPRC, animals were transported to the Imaging Research Center (IRC) for the MRI scan. Each subject was fasted a minimum of two hours prior to sedation for scanning. Subjects were transported from the CNPRC to the IRC by van either in incubators (30.5 cm in width by 30.5 cm in depth and 30.5 cm in height; at 1, 4, 8, and 13 weeks of age) or in a transport box (31.0 cm in width by 51.0 cm in depth by 40.0 cm in height; at 26, 39, 52, 156, 260 weeks of age). Animals were anesthetized and monitored by a veterinarian at 1 and 4 weeks of age, then by an animal health technician from 8–260 weeks of age. Each macaque was sedated with ketamine hydrochloride (1 mg/kg i.m.) during catheter placement and intubation. During the scanning procedures, each rhesus macaque was anesthetized with propofol (2 ml/kg/hr i.v.). The anesthesia rates were managed remotely in the control room of the scanner suite using a Harvard Apparatus 4500 infusion pump (Harvard Apparatus; Holliston, MA). Intravenous saline was administered throughout the scanning procedure to reduce any possibility of dehydration. Heart rate and oxygen saturation were monitored in the control room remotely using a Nonin 8600 pulse oximeter (Nonin; Plymouth, MN). A video camera was also placed at the opening of the scanner bore for visual monitoring of the rhesus macaque on a screen in the control room. Each rhesus macaque was positioned supine on the scanner bed and the head was centered in the RF coil. A heated saline pack and blankets were used to help maintain the body temperature and animal position during the scan. Oxygen was delivered in proximity to the nose at a rate of 0.5 – 1.0 L/hr to maintain oxygen saturation > 90%. A vitamin E capsule was used as a fiduciary to mark the left side of the head during scanning.
MRI data were acquired using a 3T Siemens Trio scanner with a circularly - polarized, 8 - channel dedicated RF head coil with an internal diameter of 18.4 cm (Litzcage, Doty Scientific; Columbia, SC). At each age, a high resolution T1 - weighted magnetization prepared rapid acquisition gradient echo (MP - RAGE) 3D MRI sequence was collected in the sagittal plane (slices = 192; slice thickness = 0.70 mm; number of excitations (NEX) = 1; repetition time (TR) = 2200 ms; echo time (TE) = 4.73 ms; inversion time (TI) = 1100 ms; flip angle = 7°; field of view (FOV) = 180 mm; matrix = 256 × 256). The total scan time for this sequence was 18 min, 37 seconds. Additional sequences were also employed but the resulting data are not reported in this manuscript.
Upon completion of the scans, propofol was discontinued. The total time of sedation ranged from 60 to 90 minutes. During recovery from sedation, the infants were given subcutaneous fluids with 5% dextrose in order to rehydrate and elevate blood glucose levels following fasting. The infants also had access to glucose-enriched water in their incubators. Each macaque was transported back to the CNPRC following the scan and returned to their mothers, and then with their mothers they were returned to their home enclosures at 1, 4, 8, 13, and 26 weeks of age. Each rhesus macaque was returned directly to their home enclosures at 39, 52, 156, and 260 weeks of age.
MRI Processing Pipeline
All MRI processing was carried out using an Apple iMac computer running OSX 10.8.2 with 4 GB RAM (Apple, Inc.; Cupertino, CA). Brains were aligned along the length of the hippocampus and supersampled to .35mm isotropic resolution. Specific codes and protocols used for preprocessing are freely available at http://mrhunsaker.github.io/NeuroImaging_Codes/.
Hippocampus Tracing
For each MRI scan, the hippocampus was manually traced using ANALYZE 11.0 (BIR; Rochester, MN) on an Apple iMac computer running Mac OSX 10.8.2 with 4 GB RAM. All scans at each age were traced a second time using ANALYZE to verify intra-rater reliability (time interval between tracings of the same scan was always > 10 days with multiple scans traced in the interim). A small number of brains at each scan were traced by a second trained technician and inter-rater reliability was maintained > .80. Immediately after tracing, the hippocampus volumes for the right and left hippocampus regions of interest were exported from ANALYZE into a .csv file for later analysis and the object files saved as ANALYZE 7 format (.hdr/.img file pairs) and immediately converted to gzipped NIfTI-1.1 format.
Additionally, all scans were additionally traced one more time using Multi-image Analysis GUI (Mango; University of Texas Health Science Center; San Antonio, TX) and these hippocampal volumes were compared to those obtained using ANALYZE. The volumes for the right and left hippocampus traced in Mango were also exported into a .csv file for later analysis and the region of interest files saved in .hdr /.img format and converted to gzipped NIfTI-1.1 format. Each of the volumes of the individual regions of interest was individually quantified with FSL. For three-dimensional visualization of the reconstructed hippocampus, the bilateral regions of interest were imported into MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/ ) and Mango for 3D rendering.
Tracing Protocol
The tracing protocol used in the present study was published previously by Shamy et al. (2006) with minor modifications for MRI scans obtained from rhesus macaques at ages < 26 weeks of age. Specific landmarks used in the tracing protocol are indicated in the Supplemental Methods. The hippocampus was defined in coronal sections starting at the caudal-most level in which it was clearly visible. The anatomical designations we used were guided by direct observations of age - appropriate histological material, focusing on anatomical landmarks that were clearly identifiable on MRI (cf., Lavenex et al., 2007) as well as descriptions of hippocampus tracing protocols used to delineate the human hippocampus (Schumann and Amaral, 2007; Schumann et al., 2004). The cytoarchitectonic fields included within what we call the hippocampus include the dentate gyrus, the CA fields, the subiculum, and the pre/para-subiculum.
Protocol Modifications Across Ages
For the scans obtained at 26, 39, 52, 156, and 260 weeks of age, the protocol described by Shamy et al. (2006) was easily followed as written (protocol in Supplemental Materials or available online at http://mrhunsaker.github.io/Hippocampus_Protocol/ ). For the scans obtained at 8 and 13 weeks of age, the protocol was slightly modified to include an additional step. These scans were initially viewed in the axial plane and the rostral extent of the hippocampus was traced in the sections in which it could be clearly identified; this clearly established the rostral limit of the hippocampus. Thereafter, the hippocampal tracing protocol was followed as usual using the same neuroanatomical landmarks.
For the scans collected at 1 and 4 weeks of age, the images were first viewed in the axial plane and the rostral border of the hippocampus was traced. The hippocampus was subsequently outlined in the sagittal plane rather than the coronal plane as we found the white matter-grey matter borders easier to discriminate when the images were in this orientation. The 4-week scans were then edited in the coronal view.
For the scans collected at 1 week of age there was a signal inversion (i.e., grey matter appeared as light grey and white matter appeared dark grey). To facilitate identification of the grey mater-white matter border, the grey scale was switched back and forth between standard (black = 0, white = 255) and inverted (black = 255, white = 0) scales as needed for clarity if there were ambiguities. For consistency, the 1-week tracings were edited one final time in the coronal view with the standard greyscale to facilitate comparisons with the other age groups. In all cases, despite the signal inversion, the same neuroanatomical landmarks were used for all scans across all ages.
Statistical Methods
Reliability Analyses
To verify the reliability of hippocampal tracings, each of the scans that were traced at each age were used to quantify intra-rater reliability as one experimenter (MRH) traced all of the scans in this study three times. The intra-rater and inter-rater reliability was evaluated by calling the icc function in the irr library of the R statistical computing language (R 3.0.0; R Development Core Team, 2013). For intra-rater tracings of scans across all ages, the intraclass correlation coefficient for consistency was > .89 (p < .003; .89 for scans collected at 1 week; .93 for scans collected at 4+ weeks, p < .001), and inter-rater reliability was maintained > .80 for scans collected at 1 week of age, and > .85 for scans collected at 4+ weeks.
Statistical Analyses
Due to the longitudinal nature of these data, there was a high correlation among measurements collected close together at early time points and a lower correlation among those collected further apart at the older time points. Linear mixed effects models ANOVA were used to analyze the data, taking into account the uneven spacing between time points of data collection. Although statistical analyses were performed on untransformed data, log10 transformed data are also provided in the figures to visualize the trajectory of growth early in life. Untransformed data are also plotted to facilitate interpretation of nonlinear growth trajectories. No statistical results were considered valid unless they satisfied the following conditions: that α < .05 and β < .20 (and thus statistical power, 1-β, was maintained above .80 for values reported at the p < .05 threshold). Values between p < .05 and p < .10 are reported as statistical trends.
To reduce the possibility of inflated Type I error rates with increasing numbers of statistical tests, permutation tests were performed to confirm any observed effects by calling permutation functions from within the ez, lmPerm, and coin libraries in R. Prior to performing any analyses, the number of bootstraps was standardized at 100,000, a number of repetitions shown to result in reliable confidence intervals for longitudinal data such as those analyzed in the present study. Aside from evaluating hippocampal growth trajectories, regression analyses were performed to evaluate any potential effects of animal weight, sex, age at puberty, or rank across time on hippocampus volumes, as well as any possible hemispheric differences between hippocampi across time.
Results
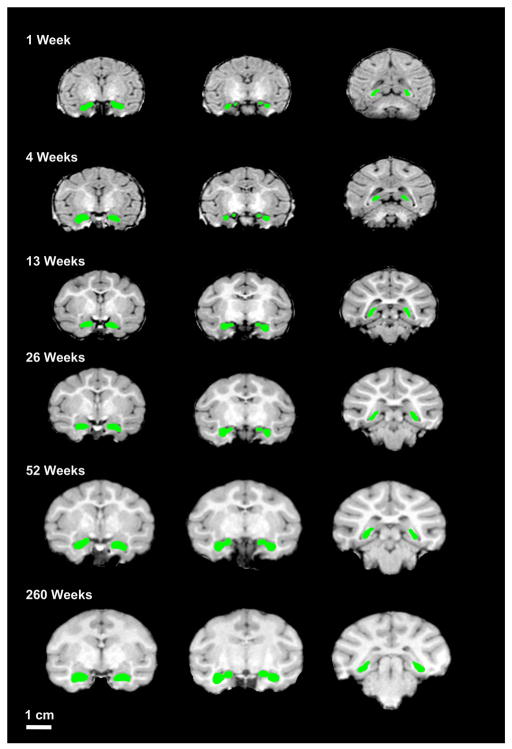
Figure 1 shows representative hippocampal tracings on coronal sections from a single male rhesus macaque monkey across all ages in this study (Lightbox view through the hippocampus at all ages for this same rhesus macaque are available as Supplemental Figures 1–9). Figure 2 shows three-dimensional reconstructions of the hippocampus from the same male subject. What is clear in these figures is that there is a substantial volume increase in the hippocampus of rhesus macaque monkeys over this age range. In both male and female rhesus macaque monkeys, the hippocampus reaches 50% of full adult hippocampus volume at approximately 13 weeks of age. Adult hippocampus volume was reached by 52 weeks of age (Figures 3–4; Tables 1–2). Numerically, both male and female rhesus macaques had greater hippocampus volumes at 156 weeks of age than at either 52 or 260 weeks of age. Both male and female rhesus macaques showed similar developmental trajectories of hippocampus volume increase (Figures 3–4).
Figure 1. Sample hippocampal tracings from a single male rhesus macaque.
Shown are scans at 1 week, 4 week, 26 weeks, 39 weeks, and 260 weeks of age. Note the difference in white matter at the different ages. All scans are shown at the same scale for direct visual comparison. Note the general shape of the traced hippocampus and size relative to the rest of brain at different ages. Scale bar = 1 cm.
Figure 2. Three-Dimensional Renderings of hippocampus for a single male rhesus macaque across all ages.
All renderings are shown at the same orientation and relative scale for direct visual comparison. The renderings for the male rhesus macaque are from the same subject shown in Figure 1. Scale bar = 1 cm.
Figure 3. Longitudinal growth curves for male and female rhesus macaque hippocampus.
All data points are shown. The X axis shows the age of the primate at scanning. Note the nonlinear growth curves and similarity among male and female rhesus macaque primates.
Figure 4. Longitudinal growth curves for male and female rhesus macaque hippocampus.
All data points are shown. The X axis shows the age plotted on a log10 time scale to more clearly show volume differences at different ages.
Table 1. Longitudinal growth of the male rhesus macaque hippocampus.
Volumes are presented as raw values (in mm3). Error given is standard deviation from the mean.
| Age | Left Hippocampus | Right Hippocampus |
|---|---|---|
| 1 week | 162.84 +/− 25.19 | 159.40 +/− 26.44 |
| 4 weeks | 232.42 +/− 23.87 | 225.64 +/− 24.98 |
| 8 weeks | 260.51 +/− 33.43 | 264.42 +/− 32.34 |
| 13 weeks | 310.52 +/− 24.97 | 305.08 +/− 25.06 |
| 26 weeks | 341.42 +/− 40.55 | 342.15 +/− 44.19 |
| 39 weeks | 367.93 +/− 29.21 | 365.17 +/− 42.65 |
| 52 weeks | 398.84 +/− 40.89 | 396.37 +/− 42.60 |
| 156 weeks | 458.06 +/− 32.86 | 458.67 +/− 36.10 |
| 260 weeks | 457.36 +/− 51.32 | 453.31 +/− 52.03 |
Table 2. Longitudinal growth of the female rhesus macaque hippocampus.
Volumes are presented as raw values (in mm3). Error given is standard deviation from the mean.
| Age | Left Hippocampus | Right Hippocampus |
|---|---|---|
| 1 week | 168.04 +/− 28.95 | 159.88 +/− 27.24 |
| 4 weeks | 233.44 +/− 20.57 | 232.10 +/− 22.44 |
| 8 weeks | 256.95 +/− 32.04 | 261.49 +/− 26.39 |
| 13 weeks | 293.00 +/− 31.28 | 286.86 +/− 30.55 |
| 26 weeks | 312.95 +/− 37.48 | 311.10 +/− 44.42 |
| 39 weeks | 350.78 +/− 36.55 | 353.08 +/− 47.31 |
| 52 weeks | 387.15 +/− 43.64 | 385.17 +/− 60.14 |
| 156 weeks | 444.08 +/− 47.59 | 441.91 +/− 43.22 |
| 260 weeks | 445.42 +/− 46.44 | 439.27 +/− 47.65 |
A linear mixed effects model ANOVA was performed on the longitudinal MRI data with sex (male n = 12; female n = 12) as the between groups factor and age (1, 4, 8, 13, 26, 29, 52, 156, 260 weeks of age) and hemisphere (right, left) as repeated within subjects factors, and primate weight at scan, age at puberty, and rank were added as covariates. All statistical results were confirmed with permutation tests to control Type I error. There was no observed main effect for hemisphere, although there was a trend toward the left hemisphere being overall larger in both male and female monkeys (F(1,22) = 3.03, p = .09). There was also no significant age x hemisphere interaction (F(1,207) = 1.92, p = .17), suggesting that the trend of the left hemisphere being larger than the right was maintained across ages.
Within the right hippocampus, there was a main effect of sex (F(1,22) = 5.32, p = .039), a significant main effect of age (F(8,207) = 180.53, p < .0001), but no sex x age interaction (F(8,207) = 1.14, p = .34). The same results were observed for the left hippocampus; there was a main effect of sex (F(1,22) = 4.42, p = .047), a significant main effect of age (F(8,207) = 212.77, p < .0001), but no sex x age interaction (F(8,207) = 1.13, p = .34). In other words, the hippocampus was larger in male rhesus macaques than in the females at all of the analyzed time points. Primate weight, age at puberty, and rank did not significantly affect these effects.
Since the male rhesus macaque hippocampus is significantly larger than the female rhesus macaque hippocampus, further characterizations of the hippocampus growth curves will be reported separately for male and female rhesus macaques.
Male Rhesus Macaque Hippocampus
Figures 3 and 4 and Table 1 demonstrate that the male rhesus macaque hippocampus displays dramatic growth early in life that slows as the animals approach 3 years of age (156 weeks). In fact, the hippocampus volumes are larger at 156 than 260 weeks of age, but this effect failed to reach significance in either hemisphere (lowest p > .23). This observed growth pattern is best modeled as a power function (left hippocampus: 180.46 x0.18292 R2 = .78, right hippocampus: 181.42 x0.18962 R2 = .81) rather than as linear, polynomial, or exponential functions (all R2 for these fits < .53).
To determine if early hippocampus volumes predicted volumes at later ages, an analysis was performed comparing hippocampal volume at 1 week of age with later growth trajectories. This analysis was unable to identify any clear relationships between the hippocampus volume at 1 week of age and hippocampus volumes at 156 or 260 weeks of age (all correlations < .25 and R2 values < .30).
Female Rhesus Macaque Hippocampus
Figures 3 and 4 and Table 2 show that the growth of the female rhesus macaque hippocampus displays a very similar pattern to males; there is substantial growth early in life that slows in rate as the primate approaches 3 years of age (156 weeks). Similar to the males, hippocampal volumes are numerically larger at 156 than at 260 weeks of age, but this effect fails to reach significance in either hemisphere (lowest p = .19). This growth pattern is again best modeled as a power function (left hippocampus: 177.09 x0.17203, R2 = .74, right hippocampus: 181.42 x0.17236, R2 = .71) rather than as linear, polynomial, or exponential functions (all R2 for these fits < .48). Analyses performed on corrected volumes did not change the pattern of effects observed in our raw volume statistical analysis.
To determine if early hippocampus volumes predicted volumes at later ages, an analysis was performed comparing hippocampal volume at 1 week of age with later growth trajectories. This analysis was unable to identify any clear relationships between the hippocampal volume at 1 week of age and hippocampal volumes at 156 or 260 weeks of age (all correlations < .31 and R2 values < .21).
Hemispheric Differences in Hippocampal Volume
As mentioned above, there was a trend toward the left hippocampus volume being greater than the right hippocampus volume at any scan age (lowest p value p < .084 at 1 week of age in males). However, there appears to be a numerically greater disparity between the hippocampal volumes of the left and right hemispheres at earlier, rather than later ages. Figure 5 demonstrates this as a plot of hemispheric asymmetry for hippocampal volume as a function of age computed using the following formula:
Figure 5. Hemispheric asymmetry for all rhesus macaque hippocampus.
The Y axis compares the volume of the right hippocampus to the volume of the let hippocampus in each individual primate. A larger left hippocampus is indicated by a data point above the baseline. The X axis shows the age of the primates.
Negative values correspond to a larger right hippocampus and positive values correspond to larger left hippocampus. When this hemispheric asymmetry was analyzed (i.e., number of primates with left hippocampus larger than right compared to the number of primates with the right hippocampus larger than the left within each age), there was a significant effect for asymmetry with more leftward than rightward asymmetry for both males and females (males p < .031; females p < .028). This suggests that although subtle, the left hippocampus has a significant tendency to be larger in volume than the right hippocampus in this cohort; and this result was present in both male and female rhesus macaques.
For analysis, the coefficient of variance (population standard deviation / population mean) was computed for each group. The 1-week time point shows the greatest coefficient of variance (males: right .16, left .16; females: right .17, left .18), whereas the next highest coefficient of variance is .13 and .12 for males at 26 weeks of age. Comparing these measures of variance across age demonstrated that for the 1 and 8 week time points, there was higher variability than at other ages in both sexes (males p values range: [.011, .023], females p values range [.009, .019]. There is a possibility that the difficulty in tracing the 1-week hippocampus may have contributed somewhat to this variability. But, due to the consistent, high intra-rater reliability, we interpret the increased variance as real biological variability for hippocampal volume across individuals early in life.
Discussion
This report provides a thorough characterization of the developmental trajectory of the rhesus macaque (Macaca mulatta) hippocampus from the early postnatal period (e.g., 1 week) through 5 years of age. Briefly, 24 rhesus macaque primates (12 male and 12 female) were longitudinally scanned in this study 9 times from 1 week of age until 260 weeks of age. We found that there was a reliable nonlinear growth in the young rhesus hippocampus in both male and female monkeys until between 52 and 156 weeks of age when there was a dramatic reduction in the growth rate, as previously observed by Payne et al. (2010). Interestingly, it appeared that between 156 and 260 weeks of age there was a numerical decrease in hippocampus volume.
When hemispheric differences were analyzed, it appears that, although small in magnitude, there is a reliable hemispheric difference in hippocampus volumes; with the left hippocampus being larger than the right. This was particularly striking at 1 week of age, at which point it seemed that hemispheric asymmetry was at it’s highest within our dataset. It appears from Figure 5 that the hemispheric asymmetry was present at all ages, but decreased in magnitude with increasing age. Adjusting the hippocampus volumes for total brain volume did not alter any of these observations.
Over the first five years of life in the rhesus macaque, hippocampi reached near adult size by one year of age, gaining about half of the original volume. Sexual dimorphism at this early age was largely limited to the brain. Males and females had very similar growth trajectories for the brain; though females started out and remained smaller than males.
Comparison of current results with previous MRI studies on the nonhuman primate
Prior to the current report, the only study to previously evaluate the early longitudinal development of the rhesus macaque hippocampus was a report from Payne et al. (2010). They evaluated MRI scans of rhesus macaques from 1 week to 2 years of age for hippocampus, amygdala, and total cerebral volume development. In all, 10 subjects (5 male and 5 female) were scanned at different time points (2–9 scans/subject in males and 6–8 scans/subject in females). They reported that the rhesus macaque hippocampus exhibited age-related changes throughout the first 2 years of life that were not explained by overall brain development. This growth appeared to be a power function with very rapid growth during the first 3–6 months of life, followed by a slowing of the growth trajectory. They found hippocampus volumes increased 110.86% in females and 117.05% in males over the 2-year study. They also reported that the left hippocampus was significantly larger than the right hippocampus. In their study, the most substantial volumetric increases were seen within the first month, with the rate of hippocampus maturation stabilizing around 11 months of age. We replicated all of these findings in our more comprehensive dataset.
Unlike the Payne et al., (2010) study, however, we did see a small, but significant sexual dimorphism in hippocampus development. We suggest that this was possible since in the present report we were able to scan 12 male and 12 female rhesus macaques at the same 9 timepoints through 5 years of life, thus providing a larger dataset that was sufficient to identify these rather small differences. Although in global analyses it was unclear if there were hemispheric differences in hippocampus volume, we were able to identify a tendency in this population of rhesus macaques for the left hippocampus to have a larger volume than the right. This finding replicates similar hippocampal asymmetry previously reported by Payne et al., (2010).
We attribute any differences between the present result and the findings from the Payne et al. (2010) study to two factors. The first factor is the increased resolution and contrast in the current MRI scans that was sufficient to allow a careful tracing of each hippocampus at each time point followed by 3D reconstruction and volumetric analysis. This increased MRI scan resolution made it feasible to trace the extent of the hippocampus from the rostral-most section containing the head of the hippocampus to the posterior tail of the hippocampus adjacent to the retrosplenial cortex. If there were volumetric differences in shape due to either age or hemispheric asymmetry, then having MRI scans at high enough resolution to allow the experimenter the ability to use a more comprehensive tracing protocol would uncover differences that would otherwise remain hidden. Secondly, the fact that we had complete series of 9 MRI scans spanning the first 5 years of life for all 24 rhesus macaque primates in this study may also have contributed to our ability to identify sex differences for hippocampus volume as the Payne et al. (2010) study did not have as comprehensive a dataset as that reported in the present manuscript.
Furthermore, although speculative, some differences between the results of the present study and Payne et al. (2010) may be due to the rearing conditions of the primates. The present experiment was conducted with rhesus macaques that lived in large troops in outdoor corrals that had a relatively normal hierarchal social structure. The primates studied by Payne et al. (2010) were nursery reared and had much more limited access to social stimuli. As Payne et al. (2010) asserted, these early life differences in social interaction and housing conditions may affect neuroanatomical development. The present data support the conclusions from Payne et al. (2010) and suggest there is a need for more naturalistic, social rearing in rhesus macaque neuroimaging experiments.
Comparison with MRI studies of the development of the human hippocampus
It is important to note that for any comparison with humans, it must be understood that the rhesus macaque matures at a must faster rate. In fact, it has been suggested that primates reach puberty at 4–5 years of age and have a life span of approximately 35 years (Rawlins et al., 1984, 1986). As such, longitudinal growth trajectories of the brain in monkeys from birth through adolescence could be completed in four years. In contrast to cross-sectional data, longitudinally acquired data have less inter-subject variability because subjects are compared to their own baseline scans across time points. Therefore, growth curves for a group can be reliably generated using fewer subjects; longitudinal studies also permit the examination of individual variation in growth. More importantly, longitudinal imaging studies of rhesus macaques would begin to fill the gap in knowledge from birth to early childhood, during which the majority of brain growth occurs.
Between humans and rhesus macaque primates the key differences lie in the rate and quantity of growth. The macaque brain progresses through stages of development relatively sooner in the lifespan than humans. Global brain growth also differs. Macaque brains are closer than humans to their adult size at birth (25% of adult volume in humans, 64% in rhesus macaques) and the period of rapid postnatal growth is shorter (4–5 years of rapid postnatal growth in humans, 6–8 months of rapid postnatal growth in rhesus macaques; Huppi et al., 1998, 2006; Courchesne et al., 2000; Malkova et al., 2006; present dataset). However, the regional patterns of development closely parallel what is known for humans (Sowell et al., 2004a,b).
Recent developmental neuroimaging studies have begun to provide insights into the maturation of the human brain (Giedd et al., 2009, 2010; Knickmeyer et al., 2008; Tanaka et al., 2012; Wallace et al., 2006, 2012). Unfortunately, primarily due to the cross sectional nature of the studies, pediatric neuroimaging still has only provided at best an incomplete dataset concerning normative brain development, particularly when researchers try to focus on the development of different brain areas.
Volumetric changes in the hippocampus as a function of age have been previously reported in both longitudinal and cross-sectional human neuroimaging studies (Caviness et al., 1999; Casey et al., 2000; Saitoh et al., 2001; Giedd et al., 2006; Lenroot and Giedd, 2006; Thompson et al., 2009). But, these reports are discrepant across studies. For example, when accounting for total cerebral volumes, females demonstrated a disproportionately larger hippocampus than males in 7-to-11-year-old participants in one set of studies (Filipek et al., 1994; Caviness et al., 1996). However, Giedd et al. (1996, 1997) using a different population of subjects reported that between 4 and 18 years of age, males had larger hippocampi than females, although these differences disappeared when total cerebral volume was taken into account. They then went on to demonstrate that females, but not males, exhibited significant changes in hippocampal volume within this age range. Hemispheric differences in hippocampus volume are somewhat more consistent, with repeated observations that the right hippocampus is larger than the left in adults (Weis et al., 1992; Jack et al., 2000), in children (Giedd et al., 1996; Pfluger et al., 1999; Utsunomiya et al., 1999), and in neonates (Thompson et al., 2009). These studies, while providing important contributions to the field, suffer from the limitation that they are either cross sectional in nature and encompass relatively wide age ranges or else longitudinal and encompass a very limited and narrow age range. As pertaining to the hippocampus, these studies also lack data on the very early postnatal time points that are necessary to study hippocampal maturation (< 4 years of age; cf., Figure 1 in Giedd et al., 2010). Additionally, it is interesting that the hemispheric difference in hippocampus volume as reported by Payne et al. (2010) and in the present report are opposite to those reported in humans (i.e., in humans the right hippocampus is larger than the left whereas in rhesus macaques the left has a greater volume than the right).
The present report provides a first critical step toward modeling the normal development of the human brain. These data provide a foundation against which pediatric and adolescent neuroimaging studies can build. Importantly, the level of experimental control in rhesus macaque MRI research (i.e, ability to anesthetize subjects, relatively long scan times, application of increasingly high field MRI, etc.) allows the field to ask very specific questions that are not yet be answerable in humans due both to technical and ethical limitations.
One notable finding within our dataset was a numerical increase in hippocampus volume at 3 years relative to 5 years of age. This is noteworthy because this finding supports reports in humans that there is a peak in gray matter volume followed by subsequent volume reductions during childhood and adolescence in both males and females (Geidd et al., 2012; Lenroot et al., 2007). This along with the present data concerning sex differences in hippocampus volumes as well as hemispheric differences support the use of the rhesus macaque as a valid model for MRI-based studies of human brain development.
Comparison of MRI findings with histological data on the development of macaque monkey hippocampus
Neuroimaging studies in rhesus macaque monkeys could provide critical information about the relationships between MRI volumetric changes and morphological changes during development. This is possible since the pattern of cell proliferation within the rhesus monkey dentate gyrus (Jabès et al., 2010) is similar to the pattern observed in humans (Seress, 1992, 1998; Seress et al., 2001). Age-related changes in hippocampal volume are attributable to a number of factors including neurogeneisis, dendritic arborization and synaptogenesis (cf., Eckenhoff and Rakic, 1988cf., Eckenhoff and Rakic, 1991; Seress, 1992). For example, stereological studies have demonstrated that greater than 30% of the neurons destined for the granule cell layer of the dentate gyrus are generated postnatally (Jabès et al., 2010, 2011). These quantitative evaluations of neuron number in the different regions of the rhesus monkey hippocampal formation across early postnatal ages into adulthood extended the results of previous studies (Keuker et al., 2003) and thus provides a comprehensive, reliable reference regarding neuron numbers in the macaque monkey hippocampal formation. They found that neurogenesis in the dentate gyrus occurred at a relatively high rate within the monkeys’ first postnatal year (Jabès et al., 2010), impacting granule cell number and therefore dentate gyrus structure for a longer developmental period than previously thought (Eckenhoff and Rakic, 1988).
Jabès et al. (2010, 2011) demonstrated that the number of principal neurons in the other regions of the monkey hippocampal formation is stable between birth and young adulthood (5–9 years of age). These data are consistent with earlier observations that neurogenesis ceases prenatally in these hippocampal subregions regions in monkeys (Rakic and Nowakowski, 1981) and humans (Seress et al., 2001). Aside from neuron number, Jabès et al. (2010, 2011) also demonstrated that there is a significant increase in the volume of the neuropil. Specifically, in the dentate gyrus the polymorphic layer shows an increase in volume, suggesting protracted maturation within this region. Similarly, whereas the mossy fibers (the axons of the granule cell neurons) that are already established at birth have mature-looking synapses, and the thorny excrescences present on the CA3 pyramidal cells are also adult-like, the dendritic arborization and the synaptic organization in the molecular layer of the dentate gyrus demonstrate significant postnatal maturation.
Likewise, cells of the CA1 and CA3 fields do not show an “adult-like” morphology until between 6 months and 1 year of age (Lavenex et al., 2007). Although there appears to be some level of agreement between the maturational changes observed histologically and the current developmental trajectories, more direct comparisons are needed to elucidate candidate neuronal mechanisms driving the gross morphological changes captured by imaging techniques.
Further quantitative data suggest a differential maturation of distinct functional circuits within the monkey hippocampal formation during early postnatal development. The protracted period of neuronal addition and maturation in the dentate gyrus is accompanied by the late maturation of specific layers in distinct hippocampal regions that are located downstream from the dentate gyrus within the hippocampal loop of information processing. This suggests that the developmental regulation of neurogenesis might be a limiting factor in the maturation of defined hippocampal circuits and specific memory functions. In contrast, the early maturation of defined layers in distinct hippocampal regions, which receive direct projections from the entorhinal cortex, suggests that specific circuits and certain “hippocampus-dependent” memory functions might appear earlier than others during postnatal development. The stereological findings mentioned above are supported by findings at the molecular level showing major and consistent changes in gene expression patterns between birth and 6 months of age in CA1 and between 1 year of age and young adulthood in CA3 (Lavenex et al., 2011). In addition, other functions subserved by subcortical-hippocampal circuits might mature even earlier than those subserved by neocortical-hippocampal circuits. Primates, with their relatively slow developmental time scale compared with rodents, might enable researchers to understand better how the structural development of distinct hippocampal circuits might contribute to the functional maturation of distinct “hippocampus-dependent” memory processes.
Implications of developmental trajectories of the hippocampus for relationship to neurodevelopmental disorders
The dramatic volumetric increases observed during the first 5 years of life in male and female rhesus macaques indicate that this period in neurodevelopment may be highly susceptible to perturbation. Disruption of normal developmental processes during this time may have long-lasting or permanent effects on brain structure and function. Previous studies have demonstrated that neonatal damage to the amygdala and hippocampus results in permanent changes in behavioral and neurocognitive function (cf., Bachevalier et al., 1999a, b; Alvarado et al., 2002; Bauman et al., 2004, 2008). In addition, many human neuropsychological disorders, such as autism, schizophrenia, and Down and Williams syndromes, among many others, are characterized by behavioral abnormalities consistent with malfunctions of the hippocampal formation (Uecker et al., 1993; Dierssen et al., 1996; Raymond et al., 1996; Leverenz and Raskind, 1998; Harrison, 1999; Saitoh et al., 2001; Machado and Bachevalier, 2003; Bauman and Kemper, 1985, 2005). However, structural brain imaging of patient populations has not consistently identified specific brain anomalies (Csernansky et al., 1998; Courchesne et al., 2001; Schumann et al., 2004; Hazlett et al., 2005). One factor that may contribute to this inconsistency is the necessary reliance on group differences at an arbitrary age. In fact, it has been shown that age may play a factor in whether amygdala volumes are reduced or increased in volume relative to control populations in an autism sample (cf., Schumann et al., 2004). Longitudinal studies focusing on developmental trajectories are likely to be a crucial step to identifying putative structural aberrations in patient populations.
Supplementary Material
Figure S1. Lightbox through the hippocampus of a 1 week old rhesus macaque primate.
Figure S2. Lightbox through the hippocampus of a 4 week old rhesus macaque primate.
Figure S3. Lightbox through the hippocampus of a 8 week old rhesus macaque primate.
Figure S4. Lightbox through the hippocampus of a 13 week old rhesus macaque primate.
Figure S5. Lightbox through the hippocampus of a 26 week old rhesus macaque primate.
Figure S6. Lightbox through the hippocampus of a 39 week old rhesus macaque primate.
Figure S7. Lightbox through the hippocampus of a 52 week old rhesus macaque primate.
Figure S8. Lightbox through the hippocampus of a 156 week old rhesus macaque primate.
Figure S9. Lightbox through the hippocampus of a 260 week old rhesus macaque primate.
Acknowledgments
The authors would like to thank Aaron Lee for technical assistance. The authors also appreciate the assistance of Jul Lea Shamy, Ph.D. who provided a preliminary protocol for outlining the hippocampal formation in the rhesus macaque monkey.
Funding: This research was supported by NIH Grants R01 NS016980 and R37 MH57502.
References
- Alvarado MC, Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005;15:118–131. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, Gee JC. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49:2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Alvarado MC, Malkova L. Memory and socioemotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychiatry. 1999;46:329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1999;113:1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Malkova L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behav Neurosci. 2006;120:989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Caviness VSJ, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Caviness VSJ, Lange NT, Makris N, Herbert MR, Kennedy DN. MRI-based brain volumetrics: emergence of a developmental brain science. Brain Dev. 1999;21:289–295. doi: 10.1016/s0387-7604(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Wu Z, Lee A, Simon TJ. Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behav Brain Funct. 2007;3:54. doi: 10.1186/1744-9081-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierssen M, Vallina IF, Baamonde C, Lumbreras MA, Martinez-Cue C, Calatayud SG, Florez J. Impaired cyclic AMP production in the hippocampus of a Down syndrome murine model. Brain Res Dev Brain Res. 1996;95:122–124. doi: 10.1016/0165-3806(96)00071-5. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. J Neurosci. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Res Dev Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VSJ. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Kraemer GW, Shelton SE, Baker E, Kalin NH, Uno H. Gender differences in brain volume and size of corpus callosum and amygdala of rhesus monkey measured from MRI images. Brain Res. 2000;852:263–267. doi: 10.1016/s0006-8993(99)02093-4. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–12. doi: 10.1002/9780470751251.ch9. discussion 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3:19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Stockman M, Weddle C, Liverpool M, Alexander-Bloch A, Wallace GL, Lee NR, Lalonde F, Lenroot RK. Anatomic magnetic resonance imaging of the developing child and adolescent brain and effects of genetic variation. Neuropsychol Rev. 2010;20:349–361. doi: 10.1007/s11065-010-9151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Jabes A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal fneurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabes A, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J Comp Neurol. 2011;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CRJ, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kalin NH. Studying non-human primates: a gateway to understanding anxiety disorders. Psychopharmacol Bull. 2004;38(Suppl 1):8–13. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. Postnatal development of the primate hippocampal formation. Dev Neurosci. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Sugden SG, Davis RR, Gregg JP, Banta Lavenex P. 1. Developmental regulation of gene expression and astrocytic processes may explain selective hippocampal vulnerability. Hippocampus. 2001;21(2):142–9. doi: 10.1002/hipo.20730. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp Neurol. 1998;150:296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4:469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006;24:3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, Scheck R, Hahn K. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia. 1999;40:414–423. doi: 10.1111/j.1528-1157.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. Demography of free-ranging Cayo Santiago macaques. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques: History, Behavior, and Biology. Albany: State University of New York Press; 1986. [Google Scholar]
- Rawlins RG, Kessler MJ, Turnquist JE. Reproductive performance, population dynamics and anthropometrics of the free-ranging Cayo Santiago rhesus macaques. J Med Primatol. 1984;13:247–259. [PubMed] [Google Scholar]
- Raymond GV, Bauman ML, Kemper TL. Hippocampus in autism: a Golgi analysis. Acta Neuropathol. 1996;91:117–119. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Karns CM, Courchesne E. Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain. 2001;124:1317–1324. doi: 10.1093/brain/124.7.1317. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Kwon H, Reiss AL, Amaral DG. Hippocampal size positively correlates with verbal IQ in male children. Hippocampus. 2007;17:486–493. doi: 10.1002/hipo.20282. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L. Morphological variability and developmental aspects of monkey and human granule cells: differences between the rodent and primate dentate gyrus. Epilepsy Res Suppl. 1992;7:3–28. [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004a;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004b;24:8223– 8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Matsui M, Uematsu A, Noguchi K, Miyawaki T. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev Neurosci. 2012;34:477–487. doi: 10.1159/000345152. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Egan GF, Inder TE. MR-determined hippocampal asymmetry in full-term and preterm neonates. Hippocampus. 2009;19:118–123. doi: 10.1002/hipo.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uecker A, Obrzut JE. Hemisphere and gender differences in mental rotation. Brain Cogn. 1993;22:42–50. doi: 10.1006/brcg.1993.1023. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol. 1999;20:717–723. [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Lightbox through the hippocampus of a 1 week old rhesus macaque primate.
Figure S2. Lightbox through the hippocampus of a 4 week old rhesus macaque primate.
Figure S3. Lightbox through the hippocampus of a 8 week old rhesus macaque primate.
Figure S4. Lightbox through the hippocampus of a 13 week old rhesus macaque primate.
Figure S5. Lightbox through the hippocampus of a 26 week old rhesus macaque primate.
Figure S6. Lightbox through the hippocampus of a 39 week old rhesus macaque primate.
Figure S7. Lightbox through the hippocampus of a 52 week old rhesus macaque primate.
Figure S8. Lightbox through the hippocampus of a 156 week old rhesus macaque primate.
Figure S9. Lightbox through the hippocampus of a 260 week old rhesus macaque primate.