Abstract
Speech pathologists are often the first professionals to identify signs of a cricopharyngeal (CP) dysfunction and make recommendations for further care. There are many care options for patients with CP dysfunction, but it is unclear how certain interventions are used in practice. A paper-based survey employing two clinical cases involving suspected CP dysfunction (Case 1 with adequate pharyngeal strength and Case 2 with coexisting pharyngeal weakness) were sent to members of American Speech-Language Hearing Associations Special Interest Group 13. Respondents ranked the order of management approaches (swallowing therapy, further evaluation, and referral to another medical professional) and selected specific interventions under each approach that they would recommend for each case. Completed surveys from 206 respondents were entered into analysis. The majority of the respondents recommended swallowing therapy as a first approach for each case (Case 1: 64%; Case 2: 88%). The most prevalent swallowing exercises recommended were the Shaker (73%), effortful swallow (62%), and Mendelsohn maneuver (53%) for Case 1 and the effortful swallow (92%), Shaker (84%), and tongue-hold swallow (73%) for Case 2. Seventy-six percent of respondents recommended a referral for Case 1, while 38% recommended the same for Case 2. Respondents with access to more types of evaluative tools were more likely to recommend further evaluation, and those with access only to videofluoroscopy were less likely to recommend further evaluation. However, the high degree of variability in recommendations reflects the need for best practice guidelines for patients with signs of CP dysfunction.
Keywords: deglutition, deglutition disorders, cricopharyngeal dysfunction, practice patterns, swallowing treatment, swallowing evaluation, speech pathologist
Introduction
The unique features of the cricopharyngeus muscle make it a challenge to evaluate and treat in swallowing dysfunction. An interdisciplinary body of research has endeavored to define the muscle's complex role as guardian of the upper aerodigestive tract. Vital responses at the cricopharyngeus serve not only during swallowing, but also in sleep, phonation and respiration (1-9).
Classically identified during videofluoroscopic swallowing evaluation, cricopharyngeal (CP) dysfunction is defined by its radiographic obstructive appearance (“cricopharyngeal bar”) and any related inefficiency of bolus transit through the UES (10, 11). CP dysfunction is not common, with incidence reported at 6.3% of VFSS performed (12). Schultz (13) proposed a clinical protocol for patients with CP dysfunction, though did not report on its justification. No clinical standard with a strong evidence base exists to more specifically evaluate or define CP dysfunction, though observed features of outflow obstruction on imaging and manometry have been described (14-18). Further complicating its evaluation are multiple etiologies for CP dysfunction. Failure of cricopharyngeal muscular compliance during bolus transit may relate to inflammatory myopathy, degenerative tissue changes, or increased connective tissue formation (19, 20). Conversely, failure of the CP to relax during swallowing relates to neurogenic etiology, including brainstem stroke, brain injury or Parkinson's disease (21, 22). Cook (18) defined a second class of CP dysfunction, functional CP disorders, in which the failed relaxation of the CP was accompanied by ineffective pharyngeal propulsion.
A speech pathologist is most likely to identify signs of CP dysfunction during videofluoroscopic evaluation and bear the initial onus of directing intervention planning. Given the lack of evaluative or intervention standards for CP dysfunction, it is unclear how speech pathologists have approached treatment planning. The state of the collective literature on behavioral swallowing treatment outcomes is sparse and with limited generalization (39). Even so, comparison to other dysphagia etiologies (e.g., stroke or Parkinson's disease), literature on swallowing therapy options specific to CP dysfunction is insubstantial (40-43). This may result in discrepancies in treatment planning and thus clinical outcomes, potentially influenced by the speech pathologist's training, resources, or access to other medical health professionals. A national survey of speech pathologists was conducted in order to examine clinical practice patterns for evaluating and treating suspected CP dysfunction. It was hypothesized that there would be considerable variability in evaluation and treatment planning recommendations, with both patient and provider characteristics influencing this variability. The purpose of this investigative survey was to characterize current speech pathology practice patterns when CP dysfunction is diagnosed and explore possible influences upon decision-making.
Methods
Participants
The 1,000 potential respondents were randomly selected from the American Speech-Language Hearing Association Member list, from those who are members of Special Interest Group 13, Swallowing and Swallowing Disorders. Inclusion criteria, as outlined in a cover letter, specified that the respondents must have 1) been practicing as a speech-language pathologist for at least 1 year (including the clinical fellowship); 2) performed at least 1 videofluoroscopic swallow study (VFSS) per week; and 3) must work partly or mostly with adult patients. The survey was conducted under approval from the Institutional Review Board of the University of Wisconsin-Madison.
Materials and Procedures
The paper-based survey included demographic questions on age, sex, years of practice in speech-language pathology, years of practice with dysphagia, types of dysphagia education, work settings, availability of swallowing diagnostic tools, and frequency of performing or recommending swallowing diagnostic tests. Responses were collected anonymously. Two hypothetical cases were presented, both with signs of CP dysfunction. A brief history was given for each case, along with written results from a VFSS. Case 1 represented CP bar without concomitant pharyngeal weakness and Case 2 represented presumed CP dysfunction in the setting of other components pharyngeal dysphagia, though none of this language was provided with the survey. Case descriptions are provided in Table 1. The respondent was asked to indicate primary and subsequent management approaches given the resources currently at his/her disposal: 1) swallowing therapy; 2) further evaluation; and 3) referral to a physician for possible surgical management. Under each management approach was a checklist of specific interventions (e.g., exercises or types of evaluation), of which the respondent could select as many as desired. See Appendix for a transcript of the survey.
Table 1.
Case descriptions.
| Case 1 |
|---|
| History |
| You saw an 81 year old female with complaints of food sticking in her throat and needing a long time to eat meals. She is eating a diet consisting of soft solids and thin liquids. She has no history of recent pneumonias and has had a gradual weight loss of 15 lbs over 5 years. She is cognitively intact, has no significant neurological history, and has no history of gastroesophageal reflux. |
| Videofluoroscopic Swallow Study Results |
A videofluoroscopic swallow study (VFSS) revealed a swallow pattern characterized by:
|
| Neither postural changes (e.g., head turn, chin tuck) nor swallow maneuvers (e.g., Mendelsohn) reduced the degree of stasis or penetration or the amount of material that cleared through the UES on the initial swallow |
| Case 2 |
|
|
| History |
| You saw a 70 year old male who is 1 year status-post a fall that resulted in a right subdural hematoma and has been discharged from a rehab facility for 2 months. An oral mechanism exam revealed decreased lingual range of motion and strength (though his speech is intelligible), but no other significant findings. He is currently NPO, expectorating saliva/secretions instead of swallowing, and is getting nutrition/hydration via PEG tube. He is cognitively intact and hoping to return to an oral diet soon. |
| Videofluoroscopic Swallow Study Results |
A videofluoroscopic swallow study (VFSS) revealed a swallow pattern characterized by:
|
| A left head turn mildly improved the amount of material that cleared through the UES on the initial swallow but did not reduce the degree of stasis or aspiration. |
Survey questions were prepared under guidance from the University of Wisconsin Survey Center. Hypothetical cases were generated with clinical expertise of the authors. A paper-based survey with written VFSS summary was chosen in lieu of an online survey with a video example of a VFSS in order to prevent the confound of differences in skill of analyzing VFSS. Further, it is common for a clinician to receive written results of a VFSS and use only that to generate a plan of care.
Data Analysis
Responses were coded and entered into a digital spreadsheet (Microsoft Excel, Redmond, WA) and imported into Statistical Analysis Software (SAS Analytics, Cary, NC) for statistical analysis. Partitioned chi-square comparisons were used to associate respondents' demographic information with their management approach recommendations for each case. Where assumptions for Chi-square comparisons were not met, Fisher's Exact Tests were used. Where demographic variables were continuous (e.g., age), the least squares means method was used to fit a general linear model. A Bonferroni adjusted α criterion level of 0.002 was used to measure statistical significance.
Results
Survey Respondents
A total of 206 respondents returned completed surveys, for a 20.6% response rate. Respondent characteristics, including gender, age, years of practice, education, work setting, and diagnostic tools available can be found in Table 2.
Table 2.
Demographic characteristics of respondents, presented as number of respondents (Percentage) with mean [Standard Deviation] where applicable. VFSS: Videofluoroscopic Swallowing Study; FEES: Fiberoptic Endoscopic Evaluation of Swallowing.
| Demographic Variable | Responses |
|---|---|
|
|
|
| Gender | |
| Female | 192 (93.2%) |
| Male | 12 (5.8%) |
| No Response | 2 (1.0%) |
| Age (years) | 39.6 [10.6] |
| Years of Practice | |
| Total | 13.6 [10.0] |
| With dysphagia | 12.4 [8.6] |
| Experience with VFSS | 9.8 [7.8] |
| Dysphagia Education | |
| Graduate School Course | 160 (77.7%) |
| Other Course | 42 (20.5%) |
| Clinical Fellowship | 135 (65.5%) |
| Mentorship | 87 (42.4%) |
| Professional Conference | 172 (83.5%) |
| Seminar/Webinar | 117 (57.1%) |
| Journal Club | 61 (29.8%) |
| Self-Study | 136 (66.3%) |
| Work Setting | |
| Acute Care | 171 (83%) |
| Long-term Acute Care | 35 (17.1%) |
| Subacute Rehabilitation | 34 (16.5%) |
| Skilled Nursing Facility | 50 (24.3%) |
| Home Health | 28 (13.7%) |
| Outpatient Clinic | 85 (41.5%) |
| Private Practice | 4 (2%) |
| Patient Population | |
| Adults | 167 (81.5%) |
| Adults and Pediatrics | 39 (18.5%) |
| Diagnostic Tools Available | |
| VFSS | 206 (100%) |
| FEES | 83 (40.3%) |
| Conventional Manometry: (1-6 sensors) | 15 (7.3%) |
| High-Resolution Manometry: (20 + sensors) | 9 (4.4%) |
| Intraluminal Impedance | 4 (2%) |
| Electromyography | 33 (16.1%) |
| Electroglottography | 10 (4.9%) |
| Number of Evaluations Performed or Recommended in One Week | |
| Clinical Swallowing | 195 (94.7%); 10.8 |
| Evaluation | [8.6] |
| VFSS | 206 (100%); 3.8 [3.5] |
| FEES | 61 (29.8%); 1.7 [2.5] |
| Conventional Manometry: (1-6 sensors) | 4 (2%); 0.5 [0.4] |
| High-Resolution Manometry: (20+ sensors) | 3 (1.5%); 1 [0.9] |
| Intraluminal Impedance | 2 (1%); 0.18 [0.1] |
| Electromyography | 14 (6.8%); 1.8 [1.6] |
| Electroglottography | 1 (0.5%); 0.25 |
Behavioral Therapy Recommendations
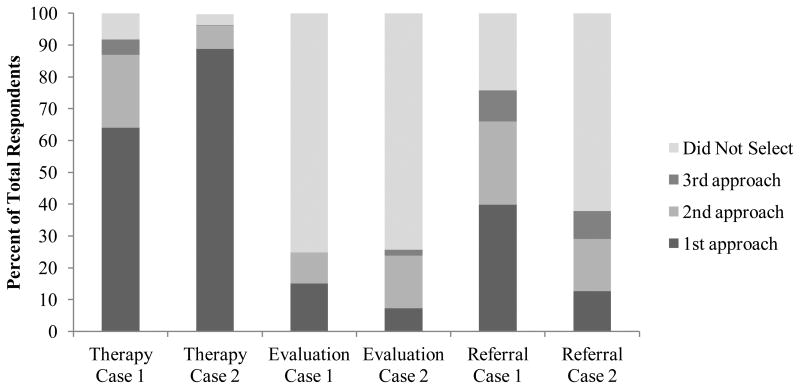
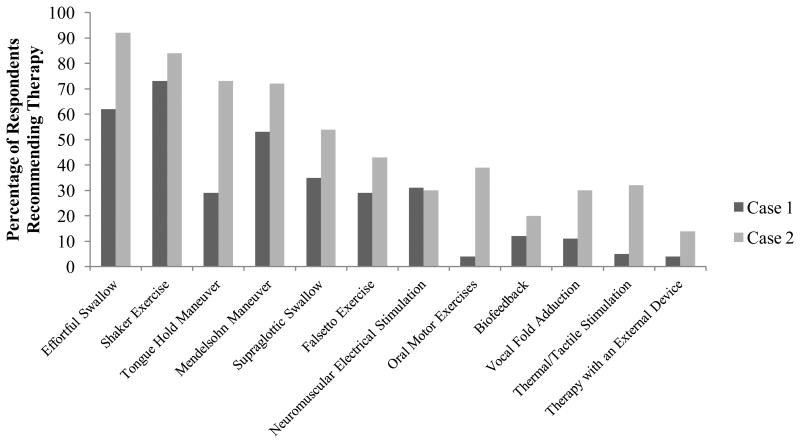
One hundred ninety respondents (92.2%) recommended swallowing therapy for Case 1 and 199 respondents (96.6%) recommended swallowing therapy for Case 2. The distribution of respondents who selected therapy as a first, second, or third approach is in Figure 1. Each respondent recommended an average of 3.4 ± 1.5 and 5.5 ± 2 types of therapeutic exercises for Case 1 and Case 2, respectively. Figure 2 shows the types of swallowing exercises selected for each case.
Figure 1.
Percentage of respondents who recommended swallowing therapy, further evaluation, and referral to a physician as a first, second, or third approach in each clinical case.
Figure 2.
Specific therapeutic exercises recommended, by case. Percentages are taken from all who recommended therapy; 92.2% for Case 1 and 96.6% for Case 2.
Evaluation Recommendations
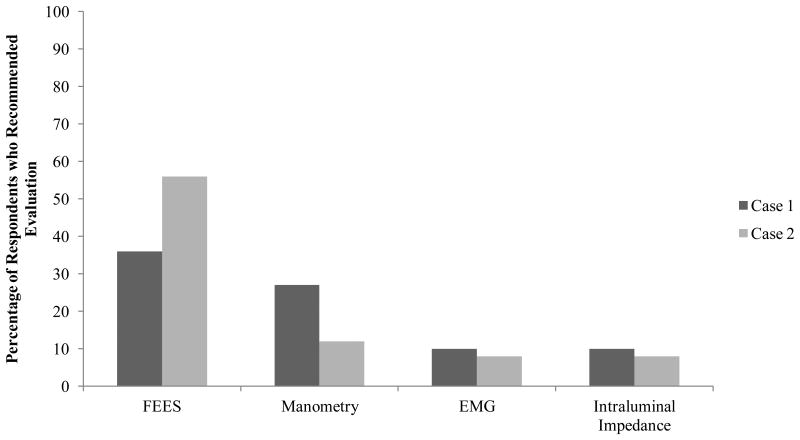
Fifty-one respondents (24.8%) recommended further swallowing evaluation for Case 1 and 53 respondents (25.7%) recommended such for Case 2. The distribution of respondents who selected further evaluation as a first, second, or third approach is in Figure 1. For each case, the respondents who recommended further evaluation proposed 1.2 ± 0.5 evaluation modalities. Sixteen percent (13/83) of those who reported having access to FEES recommended it for Case 1 and 21.7% (18/83) recommended it for Case 2. Of those reporting access to either conventional or high-resolution manometry, 53% (9/17) and 35.3% (6/17) recommended it for Case 1 and Case 2, respectively. Of respondents with access to EMG, 11.8% (4/34) and 8.8% (3/34) recommended it for Case 1 and Case 2, respectively. Fifty percent (2/4) of those who reported having access to impedance recommended it for Case 1 and 25% (1/4) recommended it for Case 2. Figure 3 presents the types of evaluative studies selected for each case from those who recommended further evaluation.
Figure 3.
Specific evaluative modalities recommended, by case. Percentages are taken from all who recommended evaluation; 24.8% for Case 1 and 25.7% for Case 2. FEES: Fiberoptic Endoscopic Evaluation of Swallowing; EMG: electromyography.
Respondents who reportedly have access to 3 or more evaluative tools were more likely to recommend further evaluation as a primary, secondary, or tertiary approach in Case 1 ( 2(1, N = 206) = 26.45, p < 0.001), and respondents who have access to 2 or more evaluative tools were more likely to further evaluation as a primary, secondary, or tertiary approach in Case 2 ( 2(1, N = 206) = 36.92, p < 0.001). Thus, those respondents who only have access to videofluoroscopy are less likely to recommend further evaluation in either case.
Referral Recommendations
One hundred fifty-six respondents (75.7%) and 78 respondents (37.9%) recommended a referral to a physician for possible surgical management for Case 1 and Case 2, respectively. The distribution of respondents who selected referral as a first, second, or third approach is in Figure 1.
Recommendation Differences Between Cases
Respondents prescribed swallowing therapy as a first approach significantly more often for Case 2 than for Case 1 ( 2(1, N = 412) = 35.07, p < 0.001) and as a second approach significantly more often for Case 2 ( 2(1, N = 412) = 19.44, p < 0.001). Significantly more respondents selected referral as a first approach for Case 1 than for Case 2 ( 2(1, N = 412) = 39.35, p < 0.001).
Discussion
This survey provides first of its kind information about speech pathologists' practice patterns concerning evaluation and management of patients with suspected CP dysfunction. Speech pathologists are most likely to recommend some form of swallowing therapy when signs of CP dysfunction have been identified on videofluoroscopic evaluation. While this recommendation may not be surprising in Case #2, given the description of accompanying pharyngeal dysfunction, a majority of respondents (64%) still advised behavioral therapy as a primary approach to CP dysfunction in Case #1, where a more isolated CP bar was described. Overall, the trends in that study and the present study may be related to the lack of best practice guidance concerning this population.
Available resources at a speech pathologist's disposal may influence the medical care that patients receive. The only statistically significant relationship between respondents' demographic information collected and their recommendations for management of the cases was the respondents' access to evaluative tools. That more respondents chose referral over further evaluation in both cases could reflect lack of access to or discomfort with using evaluative techniques other than videofluoroscopy for patients with suspected CP dysfunction. A survey study completed by Regan, et al. (44) reported only 17.9% of speech pathologists were satisfied with evaluating CP function. Despite advocacy for manometric workup prior to creating a management plan in the setting of CP dysfunction (14, 18, 21, 33, 34, 45-47), only 3.5% of respondents reported having access to conventional or high-resolution manometry systems. Although Regan, et al. (44) recorded that 13.9% of speech pathologist respondents have access to pharyngeal manometry, they documented the lack of resources and equipment as the highest-reported challenge when evaluating CP function. Although respondents with access to more evaluative tools were more likely to recommend evaluation in either case, those with access to a specific tool did not always recommend its use. No more than 53% of respondents with access to a specific evaluative tool recommended it to be used, despite the case. Discrepancies in access to and use of evaluative tools seen here warrants inquiry in future surveys of practice patterns.
A further explanation of the variability in responses could be the lack of education on CP dysfunction and/or the lack of awareness of evaluative resources, such as high-resolution manometry. Although level of education was not related to any of the current survey responses, no information on the content of the further education was collected. Regan, et al. (44) commented that many respondents were not satisfied with their level of education and that many of them lacked certified training in instrumental evaluations. While the onus of continuing education lies on the speech pathologist, there are currently very few continuing education courses available that propose to center on UES function and dysfunction. If a speech pathologist is not comfortable with his/her level of training or the evaluative resources available, the responsibility is with him/her to refer to another specialist that can provide appropriate diagnostic services and medical care. Adequate treatment for CP dysfunction relies on adequate evaluation and diagnosis.
The practice patterns reported in this study bear potential implications for patient care. Recommendations for swallowing exercises were made without a strong evidence base (e.g., neuromuscular electrical stimulation) or without clear clinical indication (e.g., oral motor exercises recommended for Case 1, who was without oral dysphagia). This may lead to time wasted on the patient's and clinician's part, a feeling of burden with a long list of unnecessary swallowing exercises, and a feeling of failure if swallowing function is not improved. Lack of access to evaluative tools, such as manometry, may lead to a recommendation for exercise without clear targets for strengthening (e.g., recommendations for effortful swallow and the tongue hold maneuver for Case 1 despite adequate tongue base to posterior pharyngeal wall retraction). The recommendation of FEES for both cases without substantive evidence for its utility for evaluating UES function may lead to unnecessary and expensive medical procedures. The number of responses that did not include referral as a secondary or tertiary approach may leave patients with no alternative if a course of swallowing exercises does not remediate their symptoms. Providing the highest quality care includes services that have a strong evidence base, are medically necessary, and are efficiently delivered (49).
Collaborative treatment planning may allow etiologic factors to be considered in pursuit of improved treatment outcomes. Favorable outcomes with medical and surgical options, including CP dilation, botulinum toxin (Botox) injection, or CP myotomy have been described (23-30). Such treatment options have been shown to require discretion in patient selection to achieve desired medical or surgical outcomes (15, 23, 31-35). Risks inherent to each intervention must also be assessed by a qualified surgeon (30, 36-38).
This survey presents several limitations which must be considered. The clinicians were only presented a case description, but no imaging was reviewed to represent swallowing function. It is possible that such representation may change responses. However, this modality was chosen so as to avoid response differences based on the respondents' ability to analyze a videofluoroscopic study. Future practice patterns surveys could include this component, as well as an option for the respondent to provide an overall impression of function. Further, it is fairly common in practice for the treating speech pathologist to not perform the videofluoroscopic swallow study (e.g., when the patient transfers facilities or if there is not a radiology department at the facility), but rather receive a written report and generate a plan from the results. There was no personal or professional connection with the hypothetical cases, with patient factors such as motivation impossible to consider. The respondents may have felt external pressure to provide what they believe to be the best responses, instead of based on their current recourses and caseload. Since these were not real scenarios, the responses provided are theoretical at best. There could have been a potential bias in the survey, with the option to refer to a physician for surgical management. In future studies, it would be prudent to present multiple options for physician referral. As with any survey presenting a list of options, there is also the power of suggestion and any perceived biases influencing responses.
Given the variability in responses between cases and amongst options for treatment and interdisciplinary consultation, more systematic research, along with high-quality continuing education programs for speech pathologists, is needed to establish a cross-disciplinary standard of care for CP dysfunction. Interdisciplinary best practice guidelines would provide an equalizing force amongst speech pathology practitioners regardless of care setting, reducing reliance upon available tools or experience.
Acknowledgments
This study was supported by National Institutes of Health grant number R21 DC011130A from the National Institute on Deafness and other Communicative Disorders and by the Diane M. Bless Chair in Otolaryngology at University of Wisconsin-Madison. The authors would like to acknowledge Department of Surgery biostatistician Glen Leverson, Ph.D. for his assistance with statistical analysis.
Appendix
Transcript of Survey
Case #1: You saw an 81 year old female with complaints of food sticking in her throat and needing a long time to eat meals. She is eating a diet consisting of soft solids and thin liquids. She has no history of recent pneumonias and has had a gradual weight loss of 15 lbs over 5 years. She is cognitively intact, has no significant neurological history, and has no history of gastroesophageal reflux. A videofluoroscopic swallow study (VFSS) revealed a swallow pattern characterized by:
functional oral phase
adequate tongue base to posterior pharyngeal wall retraction and bolus propulsion
time pharyngeal swallow trigger for age
reduced hyolaryngeal excursion
narrow UES opening with a CP prominence needing multiple swallows to clear bolus
stasis in the valleculae and pyriform sinuses
penetration of thin liquid to the vocal folds without a cough response
no aspiration events
Neither postural changes (e.g., head turn, chin tuck) nor swallow maneuvers (e.g., Mendelsohn) reduced the degree of stasis or penetration or the amount of material that cleared through the UES on the initial swallow.
Case #2: You saw a 70 year old male who is 1 year status-post a fall that resulted in a right subdural hematoma and has been discharged from a rehab facility for 2 months. An oral mechanism exam revealed decreased lingual range of motion and strength (though his speech is intelligible), but no other significant findings. He is currently NPO, expectorating saliva/secretions instead of swallowing, and is getting nutrition/hydration via PEG tube. He is cognitively intact and hoping to return to an oral diet soon. A videofluoroscopic swallow study (VFSS) revealed a swallow pattern characterized by:
functional oral phase
dilated pharynx with poor tongue base propulsion and reduced pharyngeal constriction
delay of pharyngeal swallow to the valleculae with thin liquids
reduced hyolaryngeal excursion
narrow UES opening needing multiple swallows to clear bolus
stasis in the pyriform sinuses
aspiration of thin liquid from stasis in the pyriform sinus without a cough response
A left head turn mildly improved the amount of material that cleared through the UES on the initial swallow but did not reduce the degree of stasis or aspiration.
After each case, respondents were given the following options:
Given the resources currently at your disposal, please indicate your primary and subsequent management approach(es) (e.g., 1, 2, etc.) and mark the specific interventions you would pursue under each approach.
-
___Swallowing therapy
Oral motor exercises (range-of-motion and/or resistance)
Supraglottic and/or super-supraglottic swallow
Vocal fold adduction exercises
Falsetto exercise
Effortful swallow
Masako maneuver (tongue-hold swallow)
Mendelsohn maneuver
Shaker (head lift) exercise
Therapy with external device (e.g., IOPI)
Biofeedback therapy (e.g., submental surface EMG)
Thermal-tactile stimulation
Neuromuscular Electrical Stimulation (NMES)
-
___Further evaluation
FEES
Conventional manometry (1-6 pressure transducers)
High-resolution manometry (20+ pressure transducers)
Intraluminal impedance
EMG
EGG
___Consult physician for surgical management
Footnotes
Conflict Of Interest: The authors declare that they have no conflict of interest associated with this manuscript.
References
- 1.Shaker R, Lang IM. Reflex mediated airway protective mechanisms against retrograde aspiration. Am J Med. 1997;103:64S–73S. doi: 10.1016/s0002-9343(97)00326-4. [DOI] [PubMed] [Google Scholar]
- 2.Szczesniak MM, Fuentealba SE, Burnett A, Cook IJ. Differential relaxation and contractile responses of the human upper esophageal sphincter mediated by interplay of mucosal and deep mechanoreceptor activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G982–988. doi: 10.1152/ajpgi.00496.2007. [DOI] [PubMed] [Google Scholar]
- 3.Chernichenko N, Woo JS, Hundal JS, Sasaki CT. Response of cricopharyngeus muscle to esophageal stimulation by mechanical distension and acid and bile perfusion. Ann Otol Rhinol Laryngol. 2011;120:137–142. doi: 10.1177/000348941112000211. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;291:G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 5.Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of Age, Gender, Bolus Condition, Viscosity, and Volume on Pharyngeal and Upper Esophageal Sphincter Pressure and Temporal Measurements During Swallowing. Journal of Speech Language and Hearing Research. 2009;52:240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 6.Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol. 1992;262:G621–628. doi: 10.1152/ajpgi.1992.262.4.G621. [DOI] [PubMed] [Google Scholar]
- 7.Perera L, Kern M, Hofmann C, Tatro L, Chai K, Kuribayashi S, Lawal A, Shaker R. Manometric evidence for a phonation-induced UES contractile reflex. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294:G885–G891. doi: 10.1152/ajpgi.00470.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Dodds WJ, Dent J, Haeberle B, Hogan WJ, Arndorfer RC. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology. 1987;92:466–471. doi: 10.1016/0016-5085(87)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Pasricha PJ. Effect of sleep on gastroesophageal physiology and airway protective mechanisms. Am J Med. 2003;115(Suppl 3A):114S–118S. doi: 10.1016/s0002-9343(03)00208-0. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg O, Nylander G. Dysfunction of the cricopharyngeal muscle: A cineradiographic study of patients with dysphagia. Radiology. 1982;143:481–486. doi: 10.1148/radiology.143.2.7071351. [DOI] [PubMed] [Google Scholar]
- 11.Curtis DJ, Cruess DF, Berg T. The cricopharyngeal muscle: a videorecording review. AJR Am J Roentgenol. 1984;142:497–500. doi: 10.2214/ajr.142.3.497. [DOI] [PubMed] [Google Scholar]
- 12.Baredes S, Shah CS, Kaufman R. The frequency of cricopharyngeal dysfunction on videofluoroscopic swallowing studies in patients with dysphagia. American Journal of Otolaryngology. 1997;18:185–189. doi: 10.1016/s0196-0709(97)90080-6. [DOI] [PubMed] [Google Scholar]
- 13.Schultz AR, Niemtzow P, Jacobs SR, Naso F. Dysphagia associated with cricopharyngeal dysfunction. Archives of Physical Medicine and Rehabiltation. 1979;60:381–386. [PubMed] [Google Scholar]
- 14.Yip HT, Leonard R, Kendall KA. Cricopharyngeal myotomy normalizes the opening size of the upper esophageal sphincter in cricopharyngeal dysfunction. Laryngoscope. 2006;116:93–96. doi: 10.1097/01.mlg.0000184526.89256.85. [DOI] [PubMed] [Google Scholar]
- 15.Mason RJ, Bremner CG, DeMeester TR, Crookes PF, Peters JH, Hagen JA, DeMeester SR. Pharyngeal swallowing disorders: Selection for and outcome after myotomy. Annals of Surgery. 1998;228:598–607. doi: 10.1097/00000658-199810000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgursoy OB, Salassa JR. Manofluorographic and functional outcomes after endoscopic laser cricopharyngeal myotomy for cricopharyngeal bar. Otolaryngology-Head and Neck Surgery. 2010;142:735–740. doi: 10.1016/j.otohns.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Brondbo K. Treatment of cricopharyngeal dysfunction by endoscopic laser myotomy. Acta Oto-Laryngologica. 2000:222–224. [PubMed] [Google Scholar]
- 18.Cook IJ. Cricopharyngeal function and dysfunction. Dysphagia. 1993;8:244–251. doi: 10.1007/BF01354546. [DOI] [PubMed] [Google Scholar]
- 19.Kristmundsdottir F, Mahon M, Froes MM, Cumming WJ. Histomorphometric and histopathological study of the human cricopharyngeus muscle: in health and in motor neuron disease. Neuropathol Appl Neurobiol. 1990;16:461–475. doi: 10.1111/j.1365-2990.1990.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 20.Laurikainen E, Aitasalo K, Halonen P, Falck B, Kalimo H. Muscle pathology in idiopathic cricopharyngeal dysphagia. Enzyme histochemical and electron microscopic findings. Eur Arch Otorhinolaryngol. 1992;249:216–223. doi: 10.1007/BF00178473. [DOI] [PubMed] [Google Scholar]
- 21.Williams RBH, Wallace KL, Ali GN, Cook IJ. Biomechanics of failed deglutitive upper esophageal sphincter relaxation in neurogenic dysphagia. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283:G16–G26. doi: 10.1152/ajpgi.00189.2001. [DOI] [PubMed] [Google Scholar]
- 22.Alfonsi E, Versino M, Merlo IM, Pacchetti C, Martignoni E, Bertino G, Moglia A, Tassorelli C, Nappi G. Electrophysiologic patterns of oral-pharyngeal swallowing in parkinsonian syndromes. Neurology. 2007;68:583–589. doi: 10.1212/01.wnl.0000254478.46278.67. [DOI] [PubMed] [Google Scholar]
- 23.Alfonsi E, Merlo IM, Ponzio M, Montomoli C, Tassorelli C, Biancardi C, Lozza A, Martignoni E. An electrophysiological approach to the diagnosis of neurogenic dysphagia: implications for botulinum toxin treatment. Journal of Neurology Neurosurgery and Psychiatry. 2010;81:54–60. doi: 10.1136/jnnp.2009.174698. [DOI] [PubMed] [Google Scholar]
- 24.Chiu MJ, Chang YC, Hsiao TY. Prolonged effect of botulinum toxin injection in the treatment of cricopharyngeal dysphagia: Case report and literature review. Dysphagia. 2004;19:52–57. doi: 10.1007/s00455-003-0029-3. [DOI] [PubMed] [Google Scholar]
- 25.Masiero S, Briani C, Marchese-Ragona R, Giacometti P, Costantini M, Zaninotto G. Successfultreatment of long-standing post-stroke dysphagia with botulinum toxin and rehabilitation. Journal of Rehabilitation Medicine. 2006;38:201–203. doi: 10.1080/16501970500515840. [DOI] [PubMed] [Google Scholar]
- 26.Terre R, Valles M, Panades A, Mearin F. Long-lasting effect of a single botulinum toxin injection in the treatment of oropharyngeal dysphagia secondary to upper esophageal sphincter dysfunction: A pilot study. Scandinavian Journal of Gastroenterology. 2008;43:1296–1303. doi: 10.1080/00365520802245403. [DOI] [PubMed] [Google Scholar]
- 27.Allen J, White CJ, Leonard R, Belafsky PC. Effect of cricopharyngeus muscle durgery on the pharynx. Laryngoscope. 2010;120:1498–1503. doi: 10.1002/lary.21002. [DOI] [PubMed] [Google Scholar]
- 28.Murry T, Wasserman T, Carrau RL, Castillo B. Injection of botulinum toxin A for the treatment of dysfunction of the upper esophageal sphincter. American Journal of Otolaryngology. 2005;26:157–162. doi: 10.1016/j.amjoto.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Solt J, Bajor J, Moizs M, Grexa E, Horvath PO. Primary cricopharyngeal dysfunction: treatment with balloon catheter dilatation. Gastrointestinal Endoscopy. 2001;54:767–771. doi: 10.1067/mge.2001.118442. [DOI] [PubMed] [Google Scholar]
- 30.Kelly JH. Management of upper esophageal sphincter disorders: indications and complications of myotomy. Am J Med. 2000;108(Suppl 4a):43S–46S. doi: 10.1016/s0002-9343(99)00334-4. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs JR, Logemann J, Pajak TF, Pauloski BR, Collins S, Casiano RR, Schuller DE. Failure of cricopharyngeal myotomy to improve dysphagia following head and neck cancer surgery. Arch Otolaryngol Head Neck Surg. 1999;125:942–946. doi: 10.1001/archotol.125.9.942. [DOI] [PubMed] [Google Scholar]
- 32.Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: Clinical features, management, and clinical outcome. Am J Phys Med Rehabil. 2008;87:883–889. doi: 10.1097/PHM.0b013e31818a50e2. [DOI] [PubMed] [Google Scholar]
- 33.Ali GN, Wallace KL, Laundl TM, Hunt DR, deCarle DJ, Cook IJ. Predictors of outcome following cricopharyngeal disruption for pharyngeal dysphagia. Dysphagia. 1997;12:133–139. doi: 10.1007/PL00009527. [DOI] [PubMed] [Google Scholar]
- 34.Born LJ, Harned RH, Rikkers LF, Pfeiffer RF, Quigley EMM. Cricopharyngeal dysfunction in Parkinson's disease: Role in dysphagia and response to myotomy. Movement Disorders. 1996;11:53–58. doi: 10.1002/mds.870110110. [DOI] [PubMed] [Google Scholar]
- 35.Shama L, Connor NP, Ciucci MR, McCulloch TN. Surgical Treatment of Dysphagia. Physical Medicine and Rehabilitation Clinics of North America. 2008;19:817–+. doi: 10.1016/j.pmr.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Campbell BH, Tuominen TC, Toohill RJ. The risk and complications of aspiration following cricopharyngeal myotomy. American Journal of Medicine. 1997;103:61S–63S. doi: 10.1016/s0002-9343(97)00325-2. [DOI] [PubMed] [Google Scholar]
- 37.Takes RP, van den Hoogen FJA, Marres HAM. Endoscopic myotomy of the cricopharyngeal muscle with CO2 laser surgery. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2005;27:703–709. doi: 10.1002/hed.20201. [DOI] [PubMed] [Google Scholar]
- 38.Krause E, Schirra J, Gurkov R. Botulinum Toxin A Treatment of Cricopharyngeal Dysphagia After Subarachnoid Hemorrhage. Dysphagia. 2008;23:406–410. doi: 10.1007/s00455-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 39.Speyer R, Baijens L, Heijnen M, Zwijnenberg I. Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia. 2010;25:40–65. doi: 10.1007/s00455-009-9239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartolome G, Neumann S. Swallowing therapy in patients with neurological disorders causing cricopharyngeal dysfunction. Dysphagia. 1993;8:146–149. doi: 10.1007/BF02266995. [DOI] [PubMed] [Google Scholar]
- 41.Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG, Arndorfer RC, Hofmann C, Bonnevier J. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1997;272:G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 42.Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, Grande B, Kazandjian M, Dikeman K. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–1321. doi: 10.1053/gast.2002.32999. [DOI] [PubMed] [Google Scholar]
- 43.Logemann JA, Rademaker A, Pauloski BR, Kelly A, Stangl-McBreen C, Antinoja J, Grande B, Farquharson J, Kern M, Easterling C, Shaker R. A Randomized Study Comparing the Shaker Exercise with Traditional Therapy: A Preliminary Study. Dysphagia. 2009;24:403–411. doi: 10.1007/s00455-009-9217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regan J, Walshe M, McMahon BP. Current evaluation of upper oesophageal sphincter opening in dysphagia practice: an international SLT survey. International Journal of Language & Communication Disorders. 2012;47:156–165. doi: 10.1111/j.1460-6984.2011.00087.x. [DOI] [PubMed] [Google Scholar]
- 45.Schneider I, Pototschnig C, Thumfart WF, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: Introduction of a new, noninvasive method. Annals of Otology Rhinology and Laryngology. 1994;103:31–35. doi: 10.1177/000348949410300105. [DOI] [PubMed] [Google Scholar]
- 46.Schindler JS, Kelly JH. State of the art review: Swallowing disorders in the elderly. Laryngoscope. 2002;112:589–602. doi: 10.1097/00005537-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 47.St Guily JL, Moine A, Perie S, Bokowy C, Angelard B, Chaussade S. Role of pharyngeal propulsion as an indicator for upper esophageal sphincter myotomy. Laryngoscope. 1995;105:723–727. doi: 10.1288/00005537-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Crary MA, Groher ME. Basic concepts of surface electromyographic biofeedback in the treatment of dysphagia: A tutorial. American Journal of Speech-Language Pathology. 2000;9:116–125. [Google Scholar]
- 49.Yong PL, Saunders RS, Olsen LA, editors. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington (DC): National Academies Press (US)National Academy of Sciences; 2010. Institute of Medicine Roundtable on Evidence-Based M: The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]