Abstract
HIV-infected women are at increased risk of invasive cervical cancer, however screening rates remain low. The objectives of this study were to analyze a quality improvement intervention to increase cervical cancer screening rates in an urban academic HIV clinic and to identify factors associated with inadequate screening. Barriers to screening were identified by a multi-disciplinary quality improvement committee at the Washington University Infectious Diseases clinic. Several strategies were developed to address these barriers. The years pre- and post-implementation were analyzed to examine the clinical impact of the intervention. A total of 422 women were seen in both the pre-implementation and post-implementation periods. In the pre-implementation period, 222 women (53%) underwent cervical cancer screening in the form of Papanicolaou (Pap) testing. In the post-implementation period, 318 women (75.3%) underwent cervical cancer screening (p<0.01). Factors associated with lack of screening included fewer visits attended (pre: 4.2 ± 1.5; post: 3.4 ± 1.4; p<0.01). A multidisciplinary quality improvement intervention was successful in overcoming barriers and increasing cervical cancer screening rates in an urban academic HIV clinic.
Keywords: Quality Improvement, cervical cancer screening, health maintenance, HIV/AIDS
Introduction
There are currently over 1.2 million people living with human immunodeficiency virus (HIV) or Acquired Immune Deficiency Syndrome (AIDS) and ≥ 48,000 new diagnoses of HIV in the U.S. each year (Centers for Disease Control and Prevention, 2011). Women are one of the fastest growing populations with HIV, currently comprising 27% of AIDS cases compared to only 7% in 1985 (Mofenson et al., 2006). As of 2003, there were approximately 300,000 women in the United States living with HIV (Glynn & Rhodes, 2005). The introduction of highly active antiretroviral therapy (HAART) has decreased HIV/AIDS morbidity and mortality dramatically (Effros et al., 2008). Life expectancy of those with HIV now approaches that of the HIV negative population (The Antiretroviral Therapy Cohort Collaboration, 2008; van Sighem, Gras, Reiss, Brinkman, & de Wolf, 2010). Much of the focus in providing care to patients with HIV has shifted to routine health maintenance, such as management of cardiovascular disease, diabetes and cancer screening.
HIV-infected women are at increased risk for developing dysplasia and invasive cervical cancer (Massad et al., 2008). Cervical intraepithelial neoplasia (CIN) and invasive cervical cancer are seen at higher rates in HIV-infected women when compared with non-infected women (Pantanowitz & Michelow, 2010). Cervical dysplasia is seen in 20–40% of all women with HIV (Leece, Kendall, Touchie, Pottie, Angel, & Jaffey, 2010). Invasive cervical cancer is largely preventable with adequate screening through the use of the Papanicolaou (Pap) test (Kitchener, Castle, & Cox, 2006). Multiple HIV guidelines including those from the Centers for Disease Control and Prevention (CDC), the Infectious Diseases Society of America, and the National Institutes of Health recommend cervical cancer screening tests twice in the first year after HIV diagnosis and annually thereafter (Aberg et al., 2009; Panel on Opportunistic Infections in HIV-infected Adults and Adolescents, 2008). Despite these guidelines, 19–23% of HIV-infected women do not receive appropriate testing in accordance with these guidelines (Oster, Sullivan, & Blair, 2009; Stein et al., 2001).
Multiple risk factors for deficient cervical cancer screening in women without HIV have been identified including older age, obesity, lower income and educational level, lack of health insurance, and unmarried status (Datta, Colditz, Kawachi, Subramanian, Palmer, & Rosenberg, 2006; Hewitt, Devesa, & Breen, 2004). Risk factors for deficient screening in the HIV-infected population are less well-known, but lack of insurance, injection drug use, cigarette smoking, lower education, obesity, and advanced HIV may be risk factors for not receiving adequate screening (Baranoski, Horsburgh, Cupples, Aschengrau, & Stier, 2011). There are few studies detailing the impact of quality improvement interventions aimed at increasing screening. Increasing screening rates should be a priority of HIV care providers given its significant benefits in preventing invasive cervical cancer.
A quality improvement intervention was implemented in July 2010 at the Washington University Infectious Diseases (WUID) to improve cervical cancer screening rates in HIV-infected women attending the clinic. The following describes the impact of this multidisciplinary intervention to improve screening rates and provides insight in to the risk factors for failure to receive appropriate cervical cancer screening in the HIV-infected population.
Methods
In 2010 the Washington University in St. Louis Infectious Diseases clinic provided comprehensive care to 1,571 HIV-infected individuals, including 501 women. Routine quality data revealed that only 56% of HIV-infected women seen in the clinic underwent cervical cancer screening in 2009. A multi-disciplinary quality improvement intervention was implemented in July 2010 with the goal of improving cervical cancer screening rates. This study examines the clinical impact of this intervention by comparing cervical cancer screening rates in the pre-implementation (7/1/09 – 6/30/10) and post-implementation (7/1/10 – 6/30/11) periods. Risk factors for inadequate screening are also identified.
Quality Improvement Intervention
WUID clinic serves as the primary HIV training site for approximately 15 infectious disease fellows, internal medicine trainees, and rotating medical students. Care is also provided by several nurse practitioners with extensive HIV experience. All care is provided under the supervision of physicians with extensive HIV training. The clinic maintains a Core Quality Improvement (QI) Team that represents all members of the care team and is dedicated to improving quality of care. During routine quality metric evaluation, deficiencies in cervical cancer screening were identified as a target for practice improvement. Multiple strategies were used to identify barriers to cervical cancer screening. Value stream analysis was used to identify current and ideal practices. Front-line providers including medical assistants, infectious disease fellows and nurse practitioners were surveyed until saturation to identify barriers. Additionally, patient input was obtained by surveying a community advisory board consisting of women with HIV. HIV providers utilized brainstorming, a quality improvement technique to identify a large number of potential methods to increase cervical cancer screening rates. All potential solutions were grouped by theme in order to identify a refined set of potential interventions.
Participants and Data Collection
All HIV-infected women seen in the Washington University in St. Louis Infectious Diseases Clinic during both pre- (July 2009 to June 2010) and post-intervention (July 2010 to June 2011) time periods were included in the retrospective analysis. Data from the time of the pre-implementation period were collected by chart review. Sociodemographic data including race, marital status, employment status and education level were collected. We alco collected risk behaviors including tobacco use, alcohol use and current or prior substance abuse. HIV-related clinical and medication data including duration of infection, type and duration of all antiretroviral therapy, history of opportunistic infections, current CD4+ T cell count and HIV viral load were collected. Opportunistic infections were defined as previously described (Castro et al., 1992). We also collected data on comorbid medical conditions including depression, anxiety, bipolar disorder and schizophrenia and history of sexually transmitted infections (STI). We collected a detailed gynecologic history during pre- and post-implementation periods. Atypical cervical cytology was defined as pap test results including low-grade squamous intraepithelial neoplasia (LSIL), high-grade squamous intraepithelial neoplasia (HSIL), atypical squamous cells of undetermined significance (ASCUS), atypical glandular cells, cervical biopsies read as grade 1, 2, or 3 cervical intraepithelial neoplasia (CIN), or adenocarcinoma in situ (CIN 2 or more severe). Human Papilloma Virus (HPV) types associated with cervical cancer were considered high-risk (Musa et al., 2013).
Statistical Methods
Descriptive statistics were used for baseline demographics of all women engaged in care. Changes in rate of Pap testing pre- and post-implementation were analyzed using McNemar’s Test. Women who were seen in both pre- and post-implementation periods were included in the analysis. Predictors for lack of screening were determined using pre-implementation data comparing those women who received screening to those who did not in the pre-implementation period. Chi-square, Fisher’s Exact and Wilcoxon Rank Sums Tests were used to determine predictors for lack of screening where appropriate.
Results
Pre-Intervention Phase
Several barriers to cervical cancer screening were identified. Provider-specific barriers included lack of provider training and comfort in performing cervical cancer screening tests as well as lack of knowledge of supplies needed. Providers had difficulty identifying when a patient was due for a screening test at the time of the clinic appointment. Performing cervical cancer screening increased visit length, causing providers to fall behind schedule and increasing patient wait times. Providers were not given feedback on their cervical cancer screening completion rates. Several structural barriers were identified including lack of space in the exam rooms to perform cervical cancer screening tests; the lack of chaperones for male providers; and lack of available supplies in the exam rooms to perform the tests. Patients identified barriers including lack of knowledge about need for scheduled cervical cancer screening and clinical importance of the exam. Finally, patients indicated they lacked a tangible incentive.
Intervention Phase
The QI team developed several strategies to address the identified barriers (Table 1). A one hour educational training session was implemented in July 2010 for ID fellows to improve clinical skills in performing cervical cancer screening tests. These sessions were led by providers with extensive experience in performing pelvic exams. All trainees were given the opportunity to practice the exam using anatomical models. Daily email reminders were sent to providers with a list of patients due for cervical cancer screening. Finally, monthly completion rates were disseminated to the providers, with a prize given to the clinic session with the highest screening rates. Strategies to address patient-specific barriers were also implemented. A trained HIV educator called women prior to their appointments to remind them that they were due for cervical cancer screening. During these calls, patients received education about screening. Gift bags were provided to women upon completion of the screening. Structural barriers were addressed by rearranging the exam rooms to optimize space for exam materials. Chaperones were made available for male providers. Additionally, exam rooms were prepped with supplies by the medical assistants and restocked daily. To facilitate clinic flow, medical assistants instructed the women to change into a gown before the provider entered the exam room. The interventions were implemented in July of 2010.
Table 1.
Barriers to screening and interventions
| Barriers to Screening | Interventions |
|---|---|
Training
|
|
Preparedness
|
|
Environment
|
|
Equipment
|
|
Provider incentives
|
|
Patient factors
|
|
Time
|
|
Post-Intervention Results
A total of 422 women were seen in both the pre- and post-implementation periods. Demographics are shown in Table 2. The majority of women were African American (79%) and single. Half of the women had a history of atypical cervical cytology and almost half had a history of high-risk HPV and sexually transmitted infections (STI).
Table 2.
Baseline Demographics
| Demographics | Frequency (n=422) |
|---|---|
|
| |
| Race | |
| African American (%) | 333 (79) |
| White (%) | 63 (15) |
| Hispanic (%) | 4 (1) |
| Other1 (%) | 22 (5) |
|
| |
| Median age, years (IQR)2 | 40 (33–48) |
|
| |
| Marital status | |
| Married (%) | 83 (20) |
| Single (%) | 257 (61) |
| Divorced (%) | 52 (12) |
| Widowed (%) | 30 (7) |
|
| |
| Employed | |
| Yes (%) | 163 (39) |
| No (%) | 217 (51) |
| Not documented (%) | 42 (10) |
|
| |
| History of substance abuse (%) | 181 (43) |
|
| |
| Current tobacco use (%) | 189 (45) |
|
| |
| Depression (%) | 203 (48) |
|
| |
| Mental illness3 (%) | 74 (18) |
|
| |
| History of atypical cytology (%) | 231 (55) |
|
| |
| History of any STI4 (%) | 204 (48) |
|
| |
| History of high-risk HPV5 (%) | 149 (35) |
|
| |
| On HAART (%) | 314 (74) |
| Viral load <50 copies/mL (%) | 199 (63) |
|
| |
| Current median CD4 T cell count, cells/μL (IQR) | 450 (264–627) |
|
| |
| History of opportunistic infection (%) | 84 (20) |
|
| |
| Pap smear performed, n (%) | |
| Pre-implementation | 222 (53) |
| Post-implementation | 318 (75) |
Other: includes Asian-Pacific, Eastern European, African
IQR: Interquartile Range
Mental illness: Bipolar disorder, Schizophrenia, Generalized anxiety disorder
STI: Sexually transmitted infection
HPV: Human Papilloma Virus
In the pre-implementation period, 222 women (53%) underwent cervical cancer screening. In the post-implementation period, 318 women (75.3%) underwent screening (43% increase) (p<0.01). The number of cervical cancer screening tests performed by ID fellows increased significantly from 8 (3.6%) in the pre-implementation period to 49 (13.4%) in the post-implementation period (p<0.01). The number of screening exams performed by nurse practitioners also increased from 100 (45%) in the pre-implementation period compared to 167 (46%) tests in post-implementation period (p<0.01). The number of screening exams performed by medical students, residents, or attending physicians was similar during both periods (Figure 1).
Figure 1.
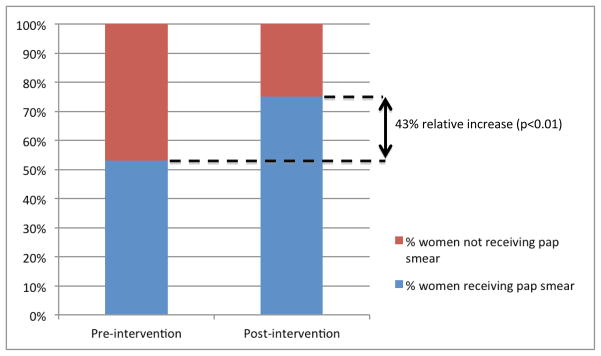
Women receiving pap smears in pre-intervention and post-intervention periods
Univariate associations with deficient cervical cancer screening are shown in Table 3. Women who attended more clinic visits were more likely to receive cervical cancer screening exams. Women who received screening had a mean of 4.2 visits in the pre-implementation period, whereas women who did not receive testing had a mean of 3.4 visits in the 1-year time period (p< 0.01). Prior atypical cervical cytology was a significant positive predictor for receiving screening. There were 138 women who had prior atypical cytology who received screening (62%) compared to 93 with prior atypical cytology who did not receive screening (47%) (p<0.01).
Table 3.
Association between predictors and receipt of pap test
| Characteristic | Received Screening (n= 222) | Did Not Receive Screening (n= 200) | P-value |
|---|---|---|---|
|
| |||
| Age, years median (IQR)1 | 40 (33–47) | 40 (33–50) | 0.09 |
|
| |||
| Race, no. (%) | 0.20 | ||
| Caucasian | 34 (15) | 29 (14.5) | |
| African-American | 178 (80) | 155 (77.5) | |
| Hispanic | 0 | 4 (2) | |
| Other | 10 (4.5) | 12 (6) | |
|
| |||
| Unemployed, no. (%) (N=380) | 108 (55) | 99 (58) | 0.45 |
|
| |||
| History of OI2, no. (%) | 43 (19) | 41 (21) | 0.79 |
|
| |||
| Prior atypical cytology, no. (%) | 138 (62) | 93 (47) | <0.01 |
|
| |||
| History of high-risk HPV3, no. (%) | 86 (39) | 63 (32) | 0.14 |
|
| |||
| Depression, no. (%) | 106 (48) | 97 (49) | 0.63 |
|
| |||
| Other mental illness, no. (%) | 36 (16) | 38 (19) | 0.43 |
|
| |||
| Current tobacco use, no. (%) | 96 (43) | 93 (47) | 0.614 |
|
| |||
| History of substance abuse, no. | 69 (31) | 76 (38) | 0.24 |
|
| |||
| Receipt of HAART4, no. (%) | 176 (79) | 144 (72) | 0.15 |
|
| |||
| Viral load <50 copies, no. (%) | 124 (56) | 100 (50) | 0.28 |
|
| |||
| CD4 count, cells/μL, Median (IQR) | 445 (282–618) | 453 (249–654) | 0.58 |
|
| |||
| History of STI5, no. (%) | 106 (48) | 98 (49) | 0.80 |
|
| |||
| Canceled visits (Mean ± SD6) | 1.27 ± 1.43 | 1.11 ± 1.29 | 0.22 |
|
| |||
| No-shows (Mean ± SD) | 1.18 ± 1.39 | 1.42 ± 1.45 | 0.08 |
|
| |||
| Clinic visits (Mean ± SD) | 4.2 ± 1.5 | 3.42 ± 1.43 | <0.01 |
IQR: Interquartile Range
OI: Opportunistic Infection
HPV: Human Papilloma Virus
HAART: Highly Active Anti-Retroviral Therapy
STI: Sexually Transmitted Infection
SD: Standard Deviation
Discussion
This is the first study evaluating the impact of strategies to increase cervical cancer screening rates in HIV-infected women in an urban academic center. Despite multiple barriers to care, we demonstrated a 43% increase in cervical cancer screening rates in an HIV-infected population with a multi-disciplinary quality improvement intervention. We identified that women with fewer clinic appointments were less likely to have screening for cervical cancer.
In the general population, efforts have been made to increase cervical cancer screening. Sadler et al. suggested that younger women had low awareness of the purpose of screening (Sadler, Albrow, Shelton, Kitchener, & Brabin, 2012). One-on-one education has been shown to be effective for the general population. A systematic review by Baron et al. evaluated five studies with one-on-one education and found an overall 8.1% increase in cervical cancer screening rates (Baron et al., 2008). Valanis et al. compared usual care to education consisting of a letter and telephone call to women who were overdue for cervical cancer screening over a 14-month study period. There was a significant increase from 19% screening rates in the usual care group to 39% in the education group (Valanis et al., 2002).
Patient reminders such as letters and telephone calls that also include patient education have been shown to be effective in increasing cervical cancer screening in HIV-negative women. Vogt et al. noted an increase from 16% to 27% when women received telephone reminders (Vogt, Glass, Glasgow, La Chance, & Lichtenstein, 2003). As part of our intervention, women received a reminder phone call prior to their appointment. During this interaction, women were reminded of the need for yearly cervical cancer screening and provided education. This intervention required a minimal amount of time and resources. Although not formally measured, based on the data above, this simple, low cost intervention likely had a significant role in increasing screening rates.
Studies have not evaluated patient incentives alone excluding other interventions. Women in our study received a gift bag containing cosmetics and toiletries after undergoing cervical cancer screening. HIV-infected women are often marginalized and of lower socioeconomic status (Aziz & Smith, 2011). A nominal gift that provides positive reinforcement for the performance of screening may have beneficial effects for populations disproportionately affected by poverty.
Reminders to general practitioners have been shown to improve cervical cancer screening rates in the general population. Computerized reminders to providers resulted in a 3% increase in screening among women overdue for cervical cancer screening (McDowell, Newell, & Rosser, 1989). Providers in our clinic had difficulty finding results from prior screening exams and were often unaware which women were due for cervical cancer screening. Prior to the clinic session, providers received an automated email message including all women due for screening during that session.
Additionally, regular feedback to providers has resulted in increased screening rates in studies among HIV-negative women (Community Preventive Services Task Force, 2012; McPhee, Bird, Jenkins, & Fordham, 1989; Sabatino et al., 2012). Fleming et al reported the findings of an audit conducted as part of continuing medical education for 38 different primary care practices; the auditors highlighted the need for improvement in areas such as cervical cancer screening and tobacco use education. Results were then presented to physicians, resulting in a significant increase in cervical cancer screening, especially in younger women (Fleming & Lawrence 1983). In our intervention, providers were provided monthly feedback on their performance. In addition to seeing their own performance, providers were also provided with blinded data on their peers for comparison.
During pre-implementation surveys, a lack of comfort with performing cervical cancer screening was frequently identified by ID fellows. Educational sessions were designed to address deficiencies in training and improve confidence in performing the exam. Much of the improvement seen during our intervention was driven by a significant increase in the number of tests performed by ID fellows.
Structural barriers to cervical cancer screening included lack of space for exam equipment in patient rooms, increase in time spent by providers to prepare the exam rooms, and lack of chaperones for male providers. Simple, low-cost interventions were devised to address these barriers. Furniture and medical cabinets in the exam rooms were rearranged to optimize available space. The implementation of standard work for the medical assistants to stock the exam rooms with supplies and also prepare the room prior to the patient visit greatly decreased the amount of time needed to perform the screening test. Chaperones consisting of case managers and social workers were also “on call” and available within minutes when needed. A longitudinal multi-year study in underserved women including several interventions to reduce structural barriers (eg. inventory of exam room equipment and establishment of clinic procedures to streamline the screening activities), demonstrated an increase in screening rates (Bastani et al., 2002).
Our study demonstrates that a multidisciplinary quality improvement intervention can be successful in overcoming barriers and increasing cervical cancer screening rates in a busy urban academic HIV clinic. Several strategies were concurrently implemented, and it is impossible to determine if one activity was more instrumental in increasing screening over another activity. It is important to note that these interventions were low-cost and minimally time-consuming. Lack of cervical cancer screening is likely multi-factorial and involves patient-, provider-, staff- and system-related factors. Our multi-dimensional approach was the catalyst in achieving our success.
Multiple risk factors for lack of screening have been suggested in prior studies; we did not have similar findings. In our patient population, only fewer clinic visits were significant predictors for lack of screening. Age, race, education level, employment status, mental illness, substance use, or more advanced HIV infection were not associated with lower screening rates. We did show that simple low-cost interventions increased screening rates, even though patients miss clinic visits (Christopoulos et al., 2013). Routine health maintenance including cancer screening is becoming more important in the care of HIV infected patients as they continue to age. Poor clinic retention and adherence is a significant barrier in providing quality care to these patients and should be a priority for improving, not only for cervical cancer screening but for other cancer screening and general health issues.
Our study had several limitations that may limit its generalizability. Our preliminary analysis indicated multiple barriers that contributed to deficient cervical cancer screening among women. Since we implemented multiple changes simultaneously, the study was not designed to identify a single intervention that resulted in increased cervical cancer screening rates. However, we provide a roster of possible interventions that could be selected based on the needs and available resources at a clinical site. This study was performed at a single urban academic infectious diseases clinic and our results may not be generalizable to other settings. Additionally, data may have been incomplete or inaccurately recorded during data collection. If pap report records had not been received by an outside physician, it is possible that women were misclassified into the “no screening” category. Missing data may account for our lack of identified risk factors for failure to receive screening as have been shown in other studies (Baranoski, Horsburgh, Cupples, Aschengrau, & Stier, 2011).
Although our strategies have demonstrated that a multidisciplinary intervention can significantly improve cervical cancer screening rates, there are opportunities to further improve care and refine our improvement strategies. In order to maintain gains made, improvement strategies must be sustainable. We found that some interventions were difficult to maintain during subsequent years. Budgetary constraints required the elimination of patient gift baskets. Additionally, chaperones availability decreased after the study period concluded. However, we improved training and education for ID providers in subsequent years to further improve clinical skills and provider comfort in performing the exam. There are multiple barriers to providing appropriate care to women with HIV; they often have poor clinic retention and challenging social and financial situations. Therefore, it is even more imperative that health maintenance issues such as cervical cancer screening be a priority of the provider in order to maintain the health of HIV infected women.
Acknowledgments
Financial Support: MAL has been supported by NIH KM1CA156708 and UL1 TR000448, the Goldfarb Patient Safety & Quality Fellowship program and the Barnes-Jewish Hospital Foundation. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
References
- Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, Gallant JE. Primary care guidelines for the management of persons infected with Human Immunodeficiency Virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–81. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. The Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis. 2011;52:S231–237. doi: 10.1093/cid/ciq047. [DOI] [PubMed] [Google Scholar]
- Baranoski AS, Horsburgh CR, Cupples LA, Aschengrau A, Stier EA. Risk factors for nonadherence with pap testing in HIV-infected women. J Women’s Health. 2011;20:1–9. doi: 10.1089/jwh.2010.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RC, Rimer BK, Breslow RA, Coates RJ, Kerner J, Melillo S, Briss PA Task Force on Community Preventive Services. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening. A systematic review. Am J Prev Med. 2008;35:S34–S55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bastani R, Berman BA, Belin TR, Crane LA, Marcus AC, Nasseri K, Henneman CE. Increasing cervical cancer screening among underserved women in a large urban county health system: Can it be done? What does it take? Medical Care. 2002;40:891–907. doi: 10.1097/00005650-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Castro KG, Ward JW, Slutsker L, Buehler O, Jaffe HW, Berkelman RL, Curran JW. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Report. 1992;41(RR-17) Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) HIV in the United States. 2011 Retrieved from http://www.cdc.gov/hiv/resources/factsheets/PDF/us.pdf.
- Christopoulos KA, Massey AD, Lopez AM, Geng EH, Johnson MO, Pilcher CD, Dawson-Rose C. “Taking a half day at a time”: Patient perspective and the HIV engagement in care continuum. AIDS Patient Care STDS. 2013;27:223–30. doi: 10.1089/apc.2012.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Community Preventive Services Task Force. Updated recommendations for client- and provider-oriented interventions to increase breast, cervical, and colorectal cancer screening. Am J Prev Med. 2012;43:92–96. doi: 10.1016/j.amepre.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Datta GD, Colditz GA, Kawachi I, Subramanian SV, Palmer JR, Rosenberg L. Individual-, neighborhood- and state-level socioeconomic predictors of cervical carcinoma screening among U.S. black women: a multi-level analysis. Cancer. 2006;106:664–669. doi: 10.1002/cncr.21660. [DOI] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, High KP. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DM, Lawrence MS. Impact of audit on preventive measures. Br Med J. 1983;287:1852–1854. doi: 10.1136/bmj.287.6408.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn MK, Rhodes P. Estimated HIV prevalence in the United States at the end of 2003 [Abstract T1-B1101]. Proceedings of the 2005 National HIV Prevention Conference; Atlanta, GA. 2005. [Google Scholar]
- Hewitt M, Devesa SS, Breen N. Cervical cancer screening among U.S. women: Analyses of the 2000 National Health Interview Survey. Prev Med. 2004;39:270–278. doi: 10.1016/j.ypmed.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24:S3/63–70. doi: 10.1016/j.vaccine.2006.05.113. [DOI] [PubMed] [Google Scholar]
- Leece P, Kendall C, Touchie C, Pottie K, Angel JB, Jaffey J. Cervical cancer screening among HIV-positive women. Retrospective cohort study from a tertiary care HIV clinic. Can Fam Physician. 2010;56:e425–31. [PMC free article] [PubMed] [Google Scholar]
- Massad LS, Seaberg EC, Wright RL, Darragh T, Lee YC, Colie C, Watts DH. Squamous cervical lesions in women with human immunodeficiency virus: Long-term follow up. Obstet Gynecol. 2008;111:1388–93. doi: 10.1097/AOG.0b013e3181744619. [DOI] [PubMed] [Google Scholar]
- McDowell I, Newell C, Rosser W. Computerized reminders to encourage cervical screening in family practice. J Fam Pract. 1989;28:420–424. [PubMed] [Google Scholar]
- McPhee SJ, Bird JA, Jenkins CN, Fordham D. Promoting cancer screening. A randomized, controlled trial of three interventions. Arch Intern Med. 1989;149:1866–1872. doi: 10.1001/archinte.149.8.1866. [DOI] [PubMed] [Google Scholar]
- Mofenson L, Taylor AW, Rogers M, Campsmith M, Ruffo NM, Clark J, Sansom S Centers for Disease Control and Prevention (CDC) Achievements in public health: reduction in perinatal transmission of HIV infection --- United States, 1985–2005. Morbidity and Mortality Weekly Report. 2006;55(21):592–597. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5521a3.htm. [PubMed] [Google Scholar]
- Musa J, Taiwo B, Achenback C, Olugbenga S, Berzins B, Sagay AS, Murphy RL. High-risk human papillomavirus among HIV-infected women with normal cervical cytology: a pilot study in Jos, Nigeria. Arch Gynecol Obstet. 2013 doi: 10.1007/s00404-013-2885-x. Epud ahead of print. [DOI] [PubMed] [Google Scholar]
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr. 2009;51:430–6. doi: 10.1097/QAI.0b013e3181acb64a. [DOI] [PubMed] [Google Scholar]
- Panel on opportunistic infections in HIV-infected adults and adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America. 2008 Retrieved from http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0.
- Pantanowitz L, Michelow P. Review of Human Immunodeficiency Virus and squamous lesions of the uterine cervix. Diagn Cytopathol. 2010;39:65–72. doi: 10.1002/dc.21364. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, Glanz K Community Preventive Services Task Force. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers. Nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Sadler L, Albrow R, Shelton R, Kitchener H, Brabin L. Development of a pre-notification leaflet to encourage uptake of cervical screening at first invitation: a qualitative study. Health Educ Res. 2012 doi: 10.1093/her/cys103. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stein MD, Cunningham WE, Nakazono T, Turner BJ, Anderson RM, Bozette SA, Shapiro MF HCSUS Consortium. Screening for cervical cancer in HIV-infected women receiving care in the United States. J Acquir Immune Defic Syndr. 2001;27:463–466. doi: 10.1097/00126334-200108150-00007. [DOI] [PubMed] [Google Scholar]
- Valanis BG, Glasgow RE, Mullooly J, Vogt TM, Whitlock EP, Boles SM, Kimes TM. Screening HMO women overdue for both mammograms and Pap tests. Prev Med. 2002;34:40–50. doi: 10.1006/pmed.2001.0949. [DOI] [PubMed] [Google Scholar]
- van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F ATHENA National Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–1535. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Glass A, Glasgow RE, La Chance PA, Lichtenstein E. The safety net: a cost-effective approach to improving breast and cervical cancer screening. J Women’s Health. 2003;12:789–798. doi: 10.1089/154099903322447756. [DOI] [PubMed] [Google Scholar]


