Summary
Stress is a significant risk factor in the development of drug addictions and in addiction relapse susceptibility. This hypothesis-driven study was designed to determine if specific SNPs in genes related to stress response are associated with heroin and/or cocaine addiction in African Americans. The analysis included 27 genes (124 SNPs) and was performed independently for each addiction. The sample consisted of former heroin addicts in methadone maintenance treatment (n = 314), cocaine addicts (n = 281), and controls (n = 208). Fourteen SNPs showed nominally significant association with heroin addiction (p < 0.05), including the African-specific, missense SNP rs5376 (Asn334Ser) in the galanin receptor type 1 gene (GALR1) and the functional FKBP5 intronic SNP rs1360780. Thirteen SNPs showed association with cocaine addiction, including the synonymous SNPs rs237902, in the oxytocin receptor gene (OXTR), and rs5374 in GALR1. No signal remained significant after correction for multiple testing. Four additional SNPs (GALR1 rs2717162, AVP rs2282018, CRHBP rs1875999, and NR3C2 rs1040288) were associated with both addictions and may indicate common liability. The study provides preliminary evidence for novel association of variants in several stress related genes with heroin and/or cocaine addictions and may enhance the understanding of the interaction between stress and addictions.
Keywords: heroin addiction, cocaine addiction, African Americans, stress, HPA axis, GALR1, FKBP5, NR3C2, OXTR, AVP
INTRODUCTION
Addiction to heroin or cocaine, as well as illicit abuse of prescription opioids causes high personal, social and economic burdens. Drug addiction is a chronic disease affected by a combination of genetic, environmental and drug-induced factors (Kreek et al., 2012; Kreek et al., 2005). Stress is one of the factors that may predispose individuals to drug addiction and perpetuate the cycle of addiction, including initiation, maintenance and relapse (Koob and Kreek 2007; Kreek and Koob 1998; Sinha 2008). Similar neural circuitry is essential for stress response and drug reward (Koob et al., 2013). Cocaine stimulates many systems that are activated by stress and cocaine dependent subjects display abnormal patterns of HPA axis activity (Sinha et al., 2006). Stress exposure as well as drugs of abuse activates the hypothalamus-pituitary-adrenal (HPA) axis. Consequently, corticotropin-releasing-hormone (CRH, CRF) and arginine-vasopressin (AVP) are released from the hypothalamus paraventricular nucleus (PVN) and stimulate adrenocorticotrophic hormone (ACTH) secretion, which in turn stimulates glucocorticoids (GCs) synthesis and release from the adrenal cortex. GCs regulate the activity of the HPA axis through negative feedback.
Despite the many similarities between cocaine and heroin addiction, there are differences in the behavioral and neurobiological mechanisms underlying cocaine and heroin addiction (Badiani et al., 2011; Peters et al., 2013). A twin study demonstrated that heroin addiction has the largest unique genetic influences compared with other drug groups (Tsuang et al., 1998). In this study, we have analyzed cocaine addiction (CA) and heroin addiction (HA) separately, in order to identify SNPs that may have a selective drug-specific effect. Nevertheless, this approach also allows the identification of SNPs that have general (non-drug-specific) addiction disease liability.
Except for our previous report (Levran et al., 2009), we are not aware of reports of association between polymorphisms in stress-related genes and specific drug addictions in populations of African ancestry. There are several reports of such associations in cohorts of European ancestry, including the AVPR1A SNP and non-specific drug use disorders (Maher et al., 2011), CRHBP in alcohol use disorder (Enoch et al., 2008; Ray 2011), CRHR1 SNPs and alcoholism (Enoch 2011; Kranzler et al., 2011; Ray et al., 2013), GAL SNPs and opioid addiction (Beer et al., 2013; Levran et al., 2008) and SNPs of NPY1R and NPY2R with nicotine, amphetamine, alcohol, and cocaine dependence (Okahisa et al., 2009; Wei et al., 2012; Wetherill et al., 2008). In another study from our laboratory, an MC2R SNP was associated with heroin addiction in Hispanics (Proudnikov et al., 2008).
Here, we report the results of a case-control hypothesis-driven association study of 124 SNPs from 27 genes related to the stress response with HA and/or CA, in a sample of 803 American subjects of predominantly African ancestry. The study is an expansion of our previous study of HA (Levran et al., 2009) to which a second addiction (cocaine), 481 samples, and 12 stress-related genes were added. This study also employed more stringent inclusion criteria for ancestry, based on biographic ancestry scores obtained by STRUCTURE analysis of 155 ancestry informative markers (AIMs).
MATERIALS AND METHODS
Subjects
The study sample (n = 803, 41% females) is part of a larger cohort recruited by the Kreek Laboratory at the Rockefeller University for the study of the genetics of specific drug addictions. The subjects were selected based on phenotype (history of severe heroin addiction, cocaine addiction or normal controls) and African ancestry. Ancestry was verified by family history questionnaire and STRUCTURE analysis (see below) and specific inclusion criteria were employed to obtain relative homogeneity and to limit population stratification. To be included in the study, an individual had to show > 50% African ancestry contribution by STRUCTURE analysis of AIMs, except for self-identified Hispanics that were not included even if they had African ancestry >0.5.
The study was divided into three cohorts: controls (normal volunteers, n = 208), subjects with heroin addiction (HA, n = 314) and subjects with cocaine addiction (CA, n = 281). The HA study is an expansion of our previous study (Levran et al., 2009). The current study included 178 HA subjects and 144 controls from the original study and the rest of the 481 subjects were new.
Ascertainment was made by extensive personal interview, using several instruments: the Addiction Severity Index (McLellan et al., 1992), the Kreek-McHugh-Schluger-Kellogg Scale (KMSK)(Kellogg et al., 2003) and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). The following exclusion criteria from the healthy control category were used: (1) at least one instance of drinking to intoxication or any illicit drug use in the previous 30 days; (2) a history of alcohol drinking to intoxication or illicit drug use, more than twice a week, for more than 6 consecutive months and (3) cannabis use for more than 12 days in the previous 30 days or past cannabis use for more than twice a week for more than 4 years. The HA subjects were former heroin addicts with a history of at least 1 year of daily multiple uses of heroin, treated at a methadone maintenance treatment program at the time of recruitment. Of the subjects with HA as a major dependency, 56% also had CA but cocaine was not their preferred drug. According to our classification, none of the CA subjects also had HA.
Subjects for the HA group were recruited at the Rockefeller University Hospital, the Manhattan Campus of the VA NY Harbor Health Care System and the Dr. Miriam and Sheldon G. Adelson Clinic for Drug Abuse Treatment and Research in Las Vegas. Subjects for the CA group were recruited at the Rockefeller University Hospital. The Institutional Review Boards of the Rockefeller University Hospital and the VA New York Harbor Healthcare System approved the study. The Rockefeller University IRB also reviews the Adelson Clinic, LV. All subjects signed informed consent for genetic studies.
Genes/SNPs selection and array design
Twenty-seven genes were selected based on their known function in the response to stress (Table 1, Table S1). In addition to the genes included on the original hypothesis-driven “addiction” array (GS0007064-OPA; Illumina, San Diego, CA, USA)(Hodgkinson et al., 2008) that was used in our original association studies (Levran et al., 2008; Levran et al., 2009), we have added 12 stress-related genes to the current array (GS0013101-OPA). Of the 68 new SNPs from stress-related genes that were selected for the new array, based on previous reports of potential functionality and/or association with stress response, 13 SNPs were not technically suitable for this platform based on the Illumina Assay Design Tool, and 55 SNPs were added to the new array. A total of 143 SNPs from these stress-related genes were included in the array, of which 88 SNPs were from the original “addiction” array (Table S1).
Table 1.
Stress-related genes list
| Gene symbol | Gene name |
|---|---|
| AVP | arginine vasopressin |
| AVPR1A | arginine vasopressin receptor 1A |
| AVPR1B | arginine vasopressin receptor 1B |
| CARTPT | CART prepropeptide |
| CCK | cholecystokinin |
| CRH | corticotropin releasing hormone |
| CRHBP | corticotropin releasing hormone binding protein |
| CRHR1 | corticotropin releasing hormone receptor 1 |
| CRHR2 | corticotropin releasing hormone receptor 2 |
| FKBP5 | FK506-binding protein 51 |
| GAL | galanin |
| GALR1 | galanin receptor 1 |
| GLRA1 | glycine receptor, alpha 1 |
| HCRT | hypocretin neuropeptide precursor |
| HCRTR1 | hypocretin receptor 1 |
| HCRTR2 | hypocretin receptor 2 |
| MC2R | melanocortin 2 receptor |
| NPY | neuropeptide Y |
| NPY1R | neuropeptide Y receptor Y1 |
| NPY2R | neuropeptide Y receptor Y2 |
| NPY5R | neuropeptide Y receptor Y5 |
| NR3C1 | nuclear receptor subfamily 3, group C, member 1 |
| NR3C2 | nuclear receptor subfamily 3, group C, member 2 |
| OXT | oxytocin |
| OXTR | oxytocin receptor |
| PITX1 | paired-like homeodomain transcription factor 1 |
| SERPINA6 | corticosteroid binding globulin |
Genes are sorted by alphabetical order
Genotyping
Blood samples were taken and DNA was extracted and quantified using standard methods. DNA (700 ng) was precipitated as described (Levran et al., 2008). Genotyping was performed using an Illumina GoldenGate Custom Panel of 1536 SNPs at the Rockefeller University Genomics Resource Center according to the manufacturer’s protocol. Random samples were genotyped in duplicate. Analysis was performed with BeadStudio software v2.3.43 (Illumina). The genotype data for all SNPs were visually inspected to verify and correct automatic calling. Genotype data were filtered based on SNP call rates (>90%). Of the 143 SNPs genotyped, 14 SNPs were excluded from analysis based on low quality (Table S1).
Assessment of percentage of African Ancestry Using AIMs
Of the original 186 AIMs from the GS0007064-OPA panel (Hodgkinson et al., 2008), 171 SNPs with adequate quality were included in the new panel, and 155 AIMs with high quality score were used for analysis. Biographic Ancestry Scores (e.g., fractions of genetic affiliation of the individual in each cluster) were estimated using the program STRUCTURE 2.2 (Pritchard et al., 2000) with seven populations (k). Each subject was anchored against genotypes of 1051 samples from 51 worldwide populations represented in the Human Genome Diversity Cell Line Panel (http://www.cephb.fr/HGDP-CEPH-Panel), as described (Ducci et al., 2009).
Statistical analysis
Quality control for the SNP genotypes was carried out as follows. Exact tests for deviation from Hardy-Weinberg equilibrium (HWE) were performed with the PLINK program (Purcell et al., 2007), with SNPs to be rejected based on the recommended threshold of p ≤ 0.001 in control individuals. Pairwise linkage disequilibrium (LD) values were estimated using Haploview 4.2 (Barrett et al., 2005). Association analysis was performed for each SNP separately by logistic regression in the PLINK program, with genotype and sex as covariates, where in different analyses the genotype was coded as a linear allelic effect (genotypes AA, AB, and BB were given numerical values 0, 1, and 2, respectively), and as two groups reflecting dominant or recessive inheritance model. The multiple testing issue was addressed by assuming 105 independent tests (there are 19 highly correlated SNPs.) An uncorrected p = 0.0005 was chosen as the threshold for significance.
RESULTS
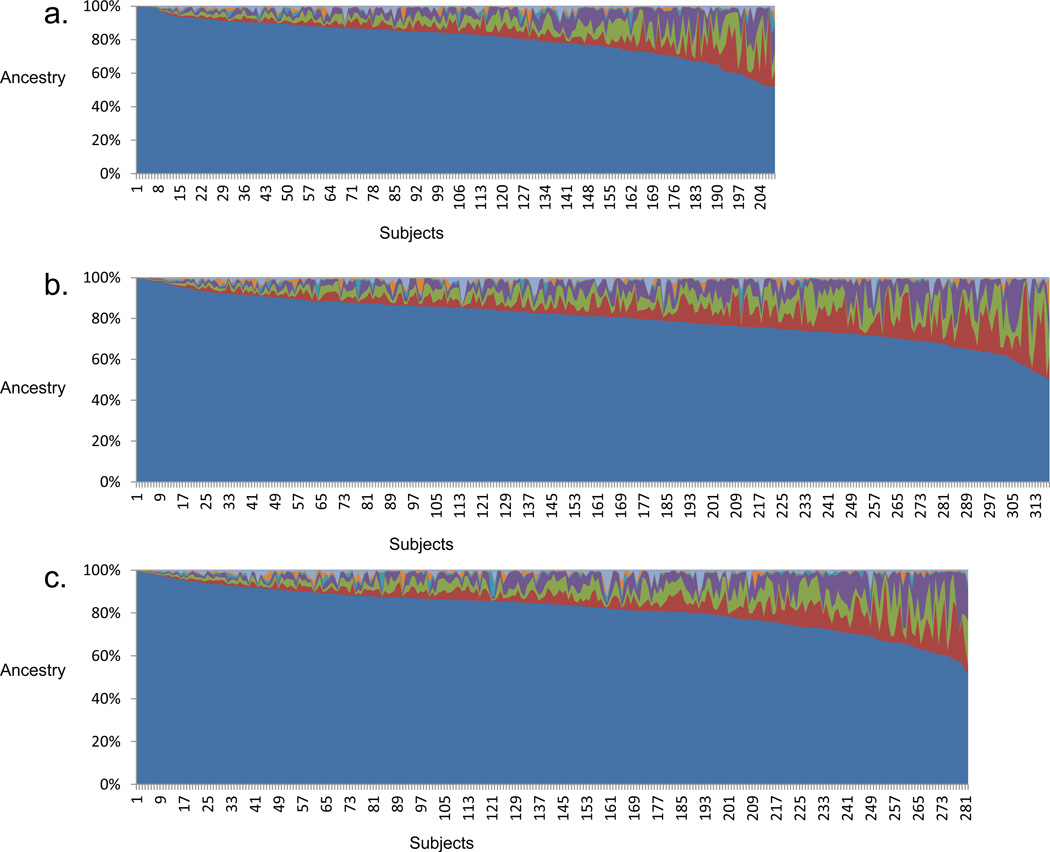
A total of 803 African American subjects (314 HA, 281 CA, and 208 controls) were included in the two independent case-control association analyses for HA and CA. All subjects had at least 50% African ancestry based on STRUCTURE analysis of 155 AIMs, and were non-Hispanics based on self-report. From the original sample of self-identified African Americans (AA), 31 subjects were excluded because they did not meet the inclusion criteria of >50% African ancestry contribution and 15 subjects were excluded based on major conflict between their self-identified ethnicity (AA) and STRUCTURE results. Fifty-eight subjects with ambiguous self-identified ancestry and 14 subjects who self-identified as Caribbean African (non-Hispanic), European, or Native American were included based on >50% African ancestry contribution. Cases and controls had similar combinations of African and other ancestries with an average of 81% African ancestry and 6% European ancestry (Fig. 1).
Figure 1.
Schematic representation of the individual admixture estimates based on STRUCTURE analysis using k=7. a. controls; b. Heroin addicts; c. cocaine addicts. Each vertical line (X axis) represents one individual, and subjects are displayed according to their predominant cluster contribution (see Methods). The Y axis represents percentage of ancestry. The clusters correspond to the geographical regions based on the HGDP sample. Color code: Africa (blue), Europe (red), Middle East (green), Central Asia (purple), Far East Asia (cyan), Oceania (amber), and America (light blue).
Quality control resulted in the exclusion of 14 SNPs with low quality and four SNPs based on minor allele frequency (MAF) < 0.05 (Table S1). No SNP showed deviation from HWE in controls (p < 0.001). LD analysis of all SNPs, in the control sample, revealed that 20 SNP pairs are in moderate to strong LD (r2 > 0.5), including two AVPR1A SNPs that are in complete LD (Table S2). One of these AVPR1A SNPs was excluded from analyses.
Genotypes of 124 SNPs from 27 stress-related genes were analyzed for association with HA or CA (Table 1, Table S1). Fourteen SNPs, in ten genes, showed nominally significant association of genotype with HA (p < 0.05) (Table 2A), of which the two FKBP5 signals are not independent, based on LD analysis (Fig. S1). One SNP (GALR1 rs5376) is non-synonymous, two SNPs are from the 3' UTR and the rest are intronic or intergenic. Thirteen SNPs in 11 genes showed nominally significant association of genotype with CA (p < 0.05) (Table 2B). Two SNPs (OXTR rs237902 and GALR1 rs5374) are synonymous, one SNP is from the 3' UTR, and the rest are intronic or intergenic. Four SNPs (AVP rs2282018, CRHBP rs1875999, GALR1 rs2717162, and NR3C2 rs1040288) were associated with the two addictions. The strongest association was observed at GALR1 SNP rs5374 for CD (p = 0.001) but no signal remained significant after correction for multiple testing.
Table 2.
Significant SNP associationsof opioid or cocaine addiction (p< 0.05)
| A. Opioid addiction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Chr. | Position | Location | Alleles1 | P | OR | Test |
| AVP | rs2282018 | 20 | 3064949 | intronic | C/T2 | 0.03 | NA | Geno |
| rs2740204 | 20 | 3062467 | 3' intergenic3 | G/T | 0.04 | 1.47 | Dom | |
| CRHBP | rs7728378 | 5 | 76259350 | intronic | C/T2 | 0.02 | 1.54 | Dom |
| rs1875999 | 5 | 76264982 | 3' UTR | T/C | 0.04 | 0.67 | Dom | |
| CRHR1 | rs81189 | 17 | 43894798 | intronic | G/C | 0.04 | 1.88 | Rec |
| FKBP5 | rs3800373 | 6 | 35542476 | 3' UTR | A/C | 0.03 | 0.65 | Dom |
| rs1360780 | 6 | 35607571 | intronic | C/T | 0.04 | 0.67 | Dom | |
| GAL | rs3136541 | 11 | 68457943 | intronic | C/T2 | 0.04 | 1.66 | Rec |
| GALR1 | rs5376 | 18 | 74980809 | Asn334Ser | A/G4 | 0.02 | 1.49 | Dom |
| rs2717162 | 18 | 74968327 | intronic | T/C | 0.04 | NA | Geno | |
| MC2R | rs1893219 | 18 | 13916387 | 5' intergenic | G/A | 0.03 | 0.52 | Rec |
| NPY5R | rs6536721 | 4 | 164496347 | 3' intergenic5 | A/G | 0.03 | 0.66 | Dom |
| NR3C1 | rs864082 | 5 | 142663939 | intronic | T/G | 0.03 | 0.62 | Add |
| NR3C2 | rs1040288 | 4 | 149048117 | intronic | G/C | 0.04 | 0.60 | Rec |
| B. Cocaine addiction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Chr. | Position | Location | Alleles1 | P | OR | Test |
| AVP | rs2282018 | 20 | 3064949 | intronic | C/T2 | 0.027 | 0.59 | Rec |
| CCK | rs754635 | 3 | 42305131 | intronic | G/C | 0.040 | 1.47 | Dom |
| rs2029127 | 3 | 42311742 | 5' intergenic | G/A4 | 0.049 | 1.47 | Dom | |
| CRHBP | rs1875999 | 5 | 76264982 | 3' UTR | T/C | 0.038 | 0.76 | Add |
| CRHR1 | rs8072451 | 17 | 43893716 | intronic | C/T6 | 0.045 | 0.59 | Dom |
| CRHR2 | rs3779250 | 7 | 30694260 | intronic | G/A6 | 0.022 | 0.09 | Rec |
| GALR1 | rs5374 | 18 | 74962645 | Gly47Gly | T/C2 | 0.001 | 1.53 | Add |
| rs2717162 | 18 | 74968327 | intronic | T/C | 0.03 | 0.66 | Dom | |
| GLRA1 | rs1428157 | 5 | 151306610 | 5' intergenic | G/T | 0.046 | 0.51 | Rec |
| NPY5R | rs4632602 | 4 | 164267129 | intronic | T/C | 0.015 | 2.74 | Rec |
| NR3C1 | rs10482672 | 5 | 142692533 | intronic | C/T | 0.035 | 0.70 | Add |
| NR3C2 | rs1040288 | 4 | 149048117 | intronic | G/C | 0.038 | 0.76 | Add |
| OXTR | rs237902 | 3 | 8809184 | Asn230Asn | G/A | 0.037 | 1.36 | Add |
Chr, chromosome; Geno, genotype; Dom, dominant; Rec, recessive; Add, additive. SNPs are sorted alphabetically by gene symbol. SNPs in bold were associated with HA and CA.
Major/minor in populations with African ancestry (forward strand).
The minor allele in African populations is the major allele in European populations.
This SNP is located in the intergenicregionbetween OXT and AVP and is listed as OXT SNP in some studies.ASNP listed under each gene may influence the other gene as well.
The minor allele is absent in European populations.
NPY1R and NPY5R are located 8 kb apart such that SNP listed under each gene may influence the other gene as well.
This SNP is very rare in YRI.
The MAFs of the identified SNPs were compared among the control sample of this study, Americans of African ancestry in Southwest USA (HapMap ASW), and Africans from Yoruba, Nigeria (HapMap YRI)(Table S3). For the most part, the MAF data in the control sample is in concordance with that of ASW.
DISCUSSION
The goal of this study was to identify variations in genes related to the stress response that contribute to vulnerability for heroin and/or cocaine addiction in African Americans. Except for our previous report (Levran et al., 2009), we are not aware of previous reports of association between polymorphisms in stress related genes and specific drug addictions in populations of African ancestry. This study is an expansion of our previous study of heroin addiction in AA (Levran et al., 2009). In the original study we analyzed 130 genes, including 15 stress-related genes. In the current study, we have limited the analysis to 27 stress-related genes, of which 12 were not part of the original array. We have also added several SNPs in genes that were included in the original array. From the top results of the original study, only one SNP belonged to a stress-related gene (AVPR1A, rs3759292), a result that was not supported by the current study.
An important finding of this study is that variants in the genes encoding galanin (GAL) and galanin receptor 1 (GALR1) are associated with HA and CA, including a GALR1 SNP (rs2717162) that is associated with both addictions. Galanin and its receptors were shown to be involved in the behavioral and neurochemical effects of opiates and stress response and were shown to counteract opiate reward and withdrawal (Picciotto 2010). Galanin is considered a candidate for a protective factor against opiate addiction, and galanin receptor agonists have been suggested as therapeutic targets. Studies suggest that the effects of galanin result from its ability to reduce stress response through the HPA axis (Picciotto et al., 2010). Recent studies in mice showed that chronic stress increased nucleus accumbens mRNA expression of galanin and that fluoxetine reversed this elevation (Zhao et al., 2013).
From the SNPs indicated in this study, GALR1 SNP rs2717162 was previously associated with craving for tobacco (Lori et al., 2011), and with quitting success in bupropion smoking cessation trials (Gold et al., 2012). GAL haplotype (including rs3136541) was associated with alcoholism (Belfer et al., 2006). We have previously reported an association of GAL SNP rs694066 and HA in subjects of European ancestry (Levran et al., 2008).
One of the GALR1 SNPs indicated, rs5376, is a missense SNP (Asn334Ser) located at the C-terminus of the galanin receptor. It is African-specific with high MAF of the G allele (43%). This is the first report of association of this SNP with any phenotype. The second GALR1 SNP indicated, rs5374, is synonymous and is in moderate LD (r2 ~ 0.4, HapMap YRI) with several SNPs from the 5' region that may be functional. GAL SNP rs3136541 indicated in this study was also indicated in a recent study of HA in subjects of European ancestry (Levran et al. submitted) suggesting an effect that is not population-specific.
Cocaine and heroin addictions may be heterogeneous due to differences in chemical classes, primary targets, routes of administration, metabolic pathways, and psychopharmacologic effects, but may share some biological mechanisms (Badiani et al., 2011; Kendler et al., 2003; Peters et al., 2013; Tsuang et al., 1998; Vanyukov et al., 2003). A twin study demonstrated that heroin addiction has the largest unique genetic influences compared with other drug groups (Tsuang et al., 1998). Association studies of mixed addictions may be limited to the identification of general effects, and specific drug effects could be missed. We have analyzed CA and HA separately in order to identify SNPs that may have specific effects. Nevertheless, this approach also allows the identification of variants with general effects. Four SNPs (AVP rs2282018, CRHBP rs1875999, GALR1 rs2717162, and NR3C2 rs1040288) were associated with both addictions in this study and may indicate a common liability.
Although none of the associations identified in this study survived multiple testing corrections, additional support for the results is provided by the results of previous association studies of affective disorders and personality traits. These include CRHBP SNPs rs7728378 and rs1875999 (Claes et al., 2003; De Luca et al., 2010; Enoch et al., 2008; Roy et al., 2012), CRHR2 SNP rs3779250 (Ishitobi et al., 2012), OXTR SNP rs237902 (Montag et al., 2013; Stankova et al., 2012), CCK SNP rs754635 (Koefoed et al., 2010; Maron et al., 2005), NPY5R SNP rs4632602 (Wetherill et al., 2008), and FKBP5 SNPs rs1360780 and rs3800373 (Binder et al., 2004).
FKBP5 SNP rs1360780 as well as CRHBP SNPs rs7728378 and rs1875999 were shown to interact with childhood trauma to predict depression, suicidality, aggression, or PTSD (Appel et al., 2011; Bevilacqua et al., 2012; Binder et al., 2008; Roy et al., 2010; Xie et al., 2010; Zimmermann et al., 2011). The FKBP5 SNP rs1360780 was shown to alter chromatin conformations and transcription in response to childhood trauma (Klengel et al., 2013).
Although the risk to develop heroin or cocaine addiction is similar among all ethnic groups, LD and allele frequency differences between populations of European and African ancestry may indicate that each may have some distinct genetic risk factors for heroin and/or cocaine addiction. Nevertheless, they may share some of the risk factors. Our current and previous studies (Levran et al., 2008; Levran et al., 2009) (Levran et al. submitted) support this assumption. Most of the findings were distinct between the samples of European and African ancestry, but several SNPs were associated with heroin and/or cocaine addiction in both samples (AVP rs2282018, FKBP5 rs3800373 and rs1360780, GAL rs3136541, GLRA1 rs1428157, and NR3C2 rs1040288). Notably, AVP rs2282018 and NR3C2 rs1040288 were also identified as risk factors for both cocaine and heroin addiction and may represent non drug-specific and non-population-specific liability.
The SNPs identified by this study showed only nominally significant association that may not reflect true association. However, testing a set of candidate genes increases the probability of detecting true associations and may not require as stringent a threshold for significance as a hypothesis-free study. Nevertheless, an independent study is warranted to further corroborate these findings.
In summary, the study provides novel preliminary evidence for the association of several variants in stress-related genes with heroin and/or cocaine addictions and may enhance the understanding of the interaction between stress and drug addictions. It may assist the development of pharmacological treatments targeting stress mechanisms. The study supports the presence of both shared and distinct genetic liability for heroin and/or cocaine addictions.
Supplementary Material
Acknowledgements
We thank all the clinical staff who enrolled and assessed subjects for this study, including S. Linzy, P. Casadonte, E. Ducat and B. Ray. We are grateful to P-H. Shen and D. Goldman, from the NIH/NIAAA, for STRUCTURE analysis, and C. Zhao and B. Zhang, from the Rockefeller Genomic Resource Center, for their excellent assistance in genotyping. This work was supported by the Adelson Medical Research Foundation and the Shanxi Scholarship Council of China (LY).
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1: SNPs list.
Table S2: Highest LD scores in the control group.
Table S3: Comparison of minor allele frequencies (MAF).
Figure S1: Pairwise LD between the FKBP5 SNPs.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organised for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Conflict of interest
The authors declare no conflict of interest
REFERENCES
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Volzke H, Freyberger HJ, Grabe HJ. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beer B, Erb R, Pavlic M, Ulmer H, Giacomuzzi S, Riemer Y, Oberacher H. Association of Polymorphisms in Pharmacogenetic Candidate Genes (OPRD1 GAL, ABCB1, OPRM1) with Opioid Dependence in European Population: A Case-Control Study. PLoS One. 2013;8:e75359. doi: 10.1371/journal.pone.0075359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, Albaugh B, Virkkunen M, Yuan Q, Max MB, Goldman D, Enoch MA. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry. 2006;11:301–311. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch MA. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S, Villafuerte S, Forsgren T, Sluijs S, Del-Favero J, Adolfsson R, Van Broeckhoven C. The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry. 2003;54:867–872. doi: 10.1016/s0006-3223(03)00425-6. [DOI] [PubMed] [Google Scholar]
- De Luca V, Tharmalingam S, Zai C, Potapova N, Strauss J, Vincent J, Kennedy JL. Association of HPA axis genes with suicidal behaviour in schizophrenia. J Psychopharmacol. 2010;24:677–682. doi: 10.1177/0269881108097817. [DOI] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, Goldman LR, Goldman D. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166:1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, Albaugh B, Hodgkinson CA, Goldman D. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology. 2012;37:1683–1688. doi: 10.1038/npp.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitobi Y, Nakayama S, Yamaguchi K, Kanehisa M, Higuma H, Maruyama Y, Ninomiya T, Okamoto S, Tanaka Y, Tsuru J, Hanada H, Isogawa K, Akiyoshi J. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koefoed P, Woldbye DP, Hansen TO, Hansen ES, Knudsen GM, Bolwig TG, Rehfeld JF. Gene variations in the cholecystokinin system in patients with panic disorder. Psychiatr Genet. 2010;20:59–64. doi: 10.1097/YPG.0b013e32833511a8. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Nelson EC, Covault J, Anton RF, Farrer L, Gelernter J. A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:960–968. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Randesi M, Rotrosen J, Casadonte P, Linzy S, Ott J, Adelson M, Kreek MJ. Heroin addiction in African Americans: a hypothesis-driven association study. Genes Brain Behav. 2009;8:531–540. doi: 10.1111/j.1601-183X.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori A, Tang Y, O'Malley S, Picciotto MR, Wu R, Conneely KN, Cubells JF. The galanin receptor 1 gene associates with tobacco craving in smokers seeking cessation treatment. Neuropsychopharmacology. 2011;36:1412–1420. doi: 10.1038/npp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BS, Vladimirov VI, Latendresse SJ, Thiselton DL, McNamee R, Kang M, Bigdeli TB, Chen X, Riley BP, Hettema JM, Chilcoat H, Heidbreder C, Muglia P, Murrelle EL, Dick DM, Aliev F, Agrawal A, Edenberg HJ, Kramer J, Nurnberger J, Tischfield JA, Devlin B, Ferrell RE, Kirillova GP, Tarter RE, Kendler KS, Vanyukov MM. The AVPR1A Gene and Substance Use Disorders: Association, Replication, and Functional Evidence. Biol Psychiatry. 2011;70:519–527. doi: 10.1016/j.biopsych.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Nikopensius T, Koks S, Altmae S, Heinaste E, Vabrit K, Tammekivi V, Hallast P, Koido K, Kurg A, Metspalu A, Vasar E, Vasar V, Shlik J. Association study of 90 candidate gene polymorphisms in panic disorder. Psychiatr Genet. 2005;15:17–24. doi: 10.1097/00041444-200503000-00004. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann EM, Bayerl M, Rujescu D, Muller DJ, Gallinat J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case-control study. World J Biol Psychiatry. 2013;14:500–508. doi: 10.3109/15622975.2012.677547. [DOI] [PubMed] [Google Scholar]
- Okahisa Y, Ujike H, Kotaka T, Morita Y, Kodama M, Inada T, Yamada M, Iwata N, Iyo M, Sora I, Ozaki N, Kuroda S. Association between neuropeptide Y gene and its receptor Y1 gene and methamphetamine dependence. Psychiatry Clin Neurosci. 2009;63:417–422. doi: 10.1111/j.1440-1819.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Galanin and addiction. EXS. 2010;102:195–208. doi: 10.1007/978-3-0346-0228-0_14. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Res. 2010;1314:206–218. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudnikov D, Hamon S, Ott J, Kreek MJ. Association of polymorphisms in the melanocortin receptor type 2 (MC2R, ACTH receptor) gene with heroin addiction. Neurosci Lett. 2008;435:234–239. doi: 10.1016/j.neulet.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Sehl M, Bujarski S, Hutchison K, Blaine S, Enoch MA. The CRHR1 gene, trauma exposure, and alcoholism risk: a test of G × E effects. Genes Brain Behav. 2013;12:361–369. doi: 10.1111/gbb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. 2012;46:72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankova T, Eichhammer P, Langguth B, Sand PG. Sexually dimorphic effects of oxytocin receptor gene (OXTR) variants on Harm Avoidance. Biol Sex Differ. 2012;3:17. doi: 10.1186/2042-6410-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Wei J, Chu C, Wang Y, Yang Y, Wang Q, Li T, Zhang L, Ma X. Association study of 45 candidate genes in nicotine dependence in Han Chinese. Addict Behav. 2012;37:622–626. doi: 10.1016/j.addbeh.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Wetherill L, Schuckit MA, Hesselbrock V, Xuei X, Liang T, Dick DM, Kramer J, Nurnberger JI, Jr, Tischfield JA, Porjesz B, Edenberg HJ, Foroud T. Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcohol Clin Exp Res. 2008;32:2031–2040. doi: 10.1111/j.1530-0277.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Seese RR, Yun K, Peng T, Wang Z. The role of galanin system in modulating depression, anxiety, and addiction-like behaviors after chronic restraint stress. Neuroscience. 2013;246:82–93. doi: 10.1016/j.neuroscience.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, Ising M. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.