Abstract
Asymmetries in the nervous system have been observed throughout the animal kingdom. Deviations of brain asymmetries are associated with a variety of neurodevelopmental disorders; however, there has been limited progress in determining how normal asymmetry is established in vertebrates. In the C. elegans chemosensory system, two pairs of morphologically symmetrical neurons exhibit molecular and functional asymmetries. This review focuses on the development of antisymmetry of the pair of AWC olfactory neurons, from transcriptional regulation of general cell identity, establishment of asymmetry through neural network formation and calcium signaling, to the maintenance of asymmetry throughout the life of the animal. Many of the factors that are involved in AWC development have homologs in vertebrates, which may potentially function in the development of vertebrate brain asymmetry.
Keywords: AWC neurons, left-right asymmetry, stochasticity, gap junctions, calcium signaling, nematode
INTRODUCTION
At first glance, the human brain appears fairly symmetric across the left-right axis. However, there are several functional and anatomical asymmetries that have been observed (Sun and Walsh, 2006). A number of neurological diseases have been associated with disruption of asymmetry in the brain, including dyslexia and schizophrenia (Oertel-Knochel and Linden, 2011; Renteria, 2012). However, the mechanisms used to establish asymmetry are not very well understood due to the complexity of the vertebrate nervous system.
As in humans, the C. elegans nervous system is largely symmetric (Hobert et al., 2002; White et al., 1986), but upon closer inspection, at least two pairs of head sensory neurons of the nematode display molecular and functional asymmetries. Like other developmental asymmetries, C. elegans left-right neuronal asymmetries are either directional or random. Directional asymmetries have stereotypical asymmetric features on one particular side of an animal; while random asymmetries or antisymmetries have asymmetric features randomly distributed on either the left or right side within a population. The left and right amphid single cilliary ending (ASE) taste neurons develop a directional asymmetry in the expression patterns of chemosensory receptors, which is initiated by Notch signaling in early embryos and is established through transcriptional regulation cascades of microRNAs and transcription factors (Alqadah et al., 2013; Cochella and Hobert, 2012; Hobert, 2014; Hobert et al., 2002). Unlike ASE neurons, the left and right amphid wing “C” (AWC) olfactory neurons exhibit antisymmetric/stochastic expression patterns of chemosensory receptors (Fig. 1a) via a transient gap junction neural network and a downstream calcium-regulated MAPK cascade in late embryogenesis (Fig. 2,3) (Chuang and Bargmann, 2005; Chuang et al., 2007; Sagasti et al., 2001; Troemel et al., 1999). ASE and AWC asymmetries are regulated by two completely distinct mechanisms at different stages of development. A recent study identified the zinc finger transcription factor die-1 as a molecular link between the two completely different kinds of asymmetries (Cochella et al., 2014). Both ASE and AWC asymmetries allow the animal to distinguish between different chemical cues in the environment (Ortiz et al., 2009; Pierce-Shimomura et al., 2001; Wes and Bargmann, 2001). To the best of our knowledge, the pairs of AWC and ASE neurons are the only examples known to date in which molecules that are asymmetrically expressed in the nervous system are clearly correlated with functional asymmetries.
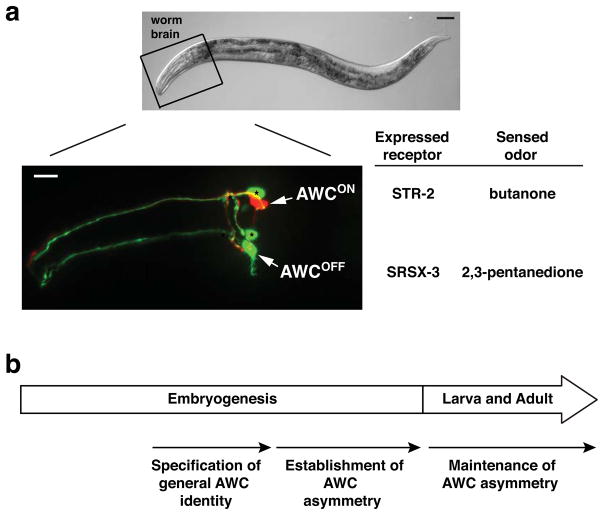
FIG. 1. The C. elegans left and right AWC olfactory neurons differentiate asymmetrically at molecular and functional levels.
(a) Top panel: DIC image of an adult C. elegans with anterior to the left and dorsal to the top. Scale bar, 50 μm. Bottom panel: Fluorescent micrograph image of the AWCON neuron expressing str-2p::TagRFP and the AWCOFF neuron expressing srsx-3p::GFP, taken from the head region outlined in the top panel. Arrows indicate the cell body of AWC neurons. Asterisks indicate AWB neurons. Scale bar, 10 μm.
(b) Developmental timeline of AWC asymmetry.
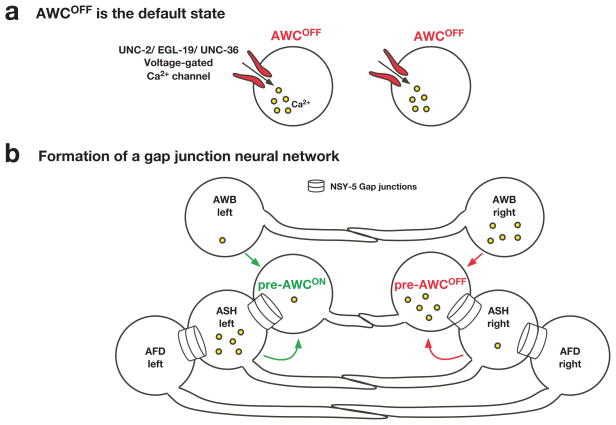
FIG. 2. A transient embryonic gap junction neural network coordinates stochastic AWC asymmetry.
(a) Prior to cell-cell communication, both AWC neurons have high intracellular calcium levels and symmetrically exist in the default AWCOFF state.
(b) AWC, ASH, AFD, and AWB sensory neurons are part of a transient embryonic neural network connected by NSY-5 gap junctions and contribute to the decision making of stochastic AWC asymmetry. Differences in calcium levels between left and right sides of neuronal pairs promote the AWCON or AWCOFF subtype, depending on the cellular context. AWC asymmetry is stochastic, and this figure illustrates the case when AWCON is on the left and AWCOFF is on the right.
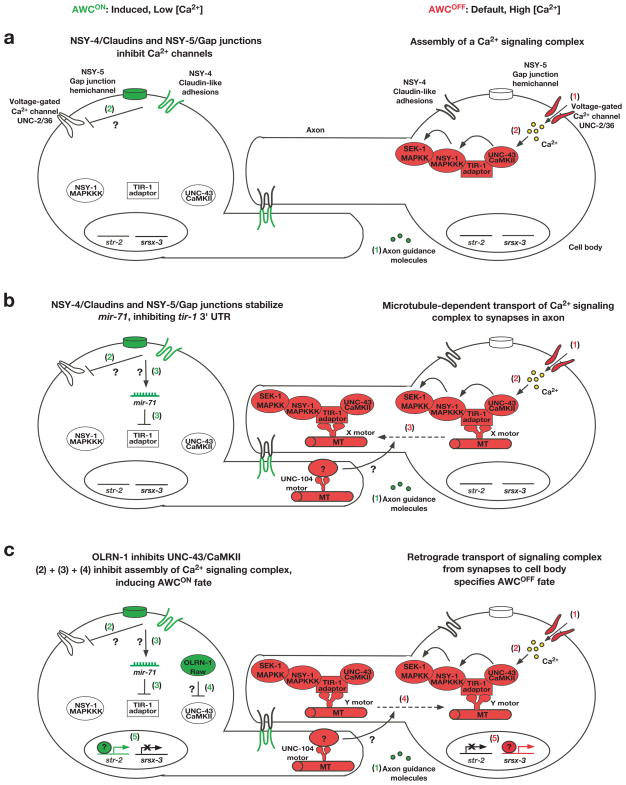
FIG. 3. Establishment of AWC asymmetry.
(a–c) AWC asymmetry is stochastic, and this figure illustrates the case when AWCON is on the left and AWCOFF is on the right. Molecules in red represent AWCOFF promoting, those in green represent AWCON promoting, and those in white indicate inactive or less active molecules. Question marks represent steps in which the molecular mechanism is unknown. Steps in the AWCOFF neuron are proposed to take place sequentially, however the steps in AWCON may not occur in the sequence proposed in the illustration, as the sequence of events has not yet been determined.
Default AWCOFF (right): (a) 1. Calcium enters the cell through voltage-gated calcium channels. 2. Calcium influx stimulates UNC-43 (CaMKII), allowing the assembly of a calcium-signaling complex consisting of UNC-43 (CaMKII), the TIR-1 (Sarm1) adaptor protein, and NSY-1 (MAPKKK). The assembly of the calcium-signaling complex brings these molecules in close proximity of each other, so that UNC-43 (CaMKII) phosphorylates NSY-1 (MAPKKK) and then NSY-1 (MAPKKK) phosphorylates SEK-1 (MAPKK). (b) 3. Microtubules transport the UNC-43 (CaMKII)/TIR-1 (Sarm1)/NSY-1 (MAPKKK) calcium-signaling complex to synapses in AWC axons via an unidentified “X” motor protein. The UNC-104 kinesin motor protein in the contralateral AWC transports an unknown molecule, which is required for the transport of the TIR-1 signaling complex in the AWCOFF cell to specify the AWCOFF subtype. (c) 4. Proposed retrograde signaling, mediated by an unidentified “Y” motor protein, may convey the lateral signaling between the two AWC cells from the synapses to regulate gene expression in the cell body. 5. The AWCOFF marker srsx-3 is transcribed, and the expression of the AWCON marker str-2 is suppressed.
Induced AWCON (left): (a) 1. Axon guidance molecules contribute to AWC axon outgrowth, allowing chemical synapse formation and communication between the two cells. 2. NSY-5 gap junctions and NSY-4 claudin-like adhesions act in parallel to inhibit voltage-gated calcium channels, resulting in a low level of intracellular calcium. (b) 3. NSY-5 and NSY-4 stabilize mature mir-71 miRNA, which inhibits calcium signaling through targeting the 3′ TUR of tir-1/Sarm1. (c) 4. OLRN-1 Raw repeat protein inhibits UNC-43 (CaMKII). 5. The AWCON marker str-2 is expressed, and the AWCOFF marker srsx-3 is inhibited.
The specification of asymmetric AWC terminal fates is regulated by three developmental events, including the specification of general AWC identity, asymmetric differentiation of the two distinct AWC subtypes, and the maintenance of the two AWC subtypes (Fig. 1b). In this review, we focus on the current understanding of the developmental mechanisms by which general AWC identity is specified as well as how stochastic AWC asymmetry is established and maintained.
TRANSCRIPTIONAL SPECIFICATION OF GENERAL AWC IDENTITY
Terminal selector genes encode transcription factors that control expression of genes specific to a single type of postmitotic neuron through cis-regulatory elements called terminal selector motifs (Hobert, 2008). ceh-36, which encodes an OTX/OTD transcription factor, has been suggested to be the terminal selector gene for AWC neurons (Kim et al., 2010; Lanjuin et al., 2003). ceh-36 mutants fail to express the AWC-specific terminal identity genes including the guanylyl cyclase gene odr-1 and lose the ability to chemotax to odors detected by AWC (Lanjuin et al., 2003). In addition, a motif regulated by ceh-36 was identified in the odr-1 promoter region, suggesting that ceh-36 likely activates terminal differentiation of AWC directly (Kim et al., 2010).
Mutations in mls-2, which encodes a HMX/NKX homeodomain protein, lead to the loss of ceh-36 expression in AWC neurons (Kim et al., 2010). Furthermore, mls-2 is transiently expressed in AWC; MLS-2 recognizes a DNA sequence in the ceh-36 promoter that is required for ceh-36 expression in the AWC neuron (Kim et al., 2010). This suggests that mls-2 is an inducer of the AWC terminal selector gene ceh-36. However, mls-2 mutants display incomplete penetrance in losing the expression of ceh-36 and odr-1 in AWC neurons, suggesting that other transcription factor(s) may be required for regulating ceh-36 expression in the AWC neurons.
ESTABLISHMENT OF STOCHASTIC AWC ASYMMETRY
AWC neurons differentiate asymmetrically into two distinct AWCOFF and AWCON subtypes in a stochastic manner
The pair of AWC neurons, like other chemosensory neurons, appears symmetric anatomically and morphologically. However, AWC neurons display both molecular and functional asymmetries. One of the AWC neurons expresses the putative chemoreceptor gene str-2 and is defined as the AWCON neuron. The other AWC neuron in the pair that does not express str-2 but expresses an alternative chemoreceptor gene srsx-3 is called the AWCOFF neuron (Fig. 1a). The two AWC subtypes also have different functions, as AWCON senses butanone, while AWCOFF detects 2,3-pentanedione (Fig. 1a). Wild-type animals have one AWCON and one AWCOFF neurons. However, AWC asymmetry has random sidedness of the AWCON and AWCOFF neurons, such that the left AWC neuron becomes the AWCON subtype in 50% of the animals in a population, while the right AWC neuron becomes AWCON in the other 50%. The stochastic nature of AWC asymmetry provides a unique model for understanding the underlying mechanisms of antisymmetry, one of the common biological phenomena (such as paw preference and handedness) across the animal kingdom (Palmer, 2004).
The cell bodies of the left and right AWC neurons are distant from each other, but their axons have direct contact and form chemical synapses with each other (White et al., 1986). When one of the AWC precursor cells is ablated in early embryos, the surviving contralateral AWC neuron always becomes AWCOFF (Troemel et al., 1999). In addition, AWCON is not specified in mutants that are defective in axon guidance (Troemel et al., 1999). Furthermore, AWC asymmetry is established during the time when the two AWC neurons form synapses on each other in late embryogenesis (Chuang and Bargmann, 2005; Troemel et al., 1999). Together, these findings indicate that the default AWC state is AWCOFF (Fig. 2a) and that communication between the two AWC neurons is required to induce the AWCON subtype and establish AWC asymmetry (Troemel et al., 1999).
AWC asymmetry is reminiscent of other developmental events in which groups of equivalent cells interact to generate distinct cell types through lateral inhibition [also referred to as lateral specification (Greenwald, 2012)]. In many systems, including C. elegans, lateral inhibition is mediated by the Delta-Notch signaling pathway. While the involvement of Notch in this process is still presently unclear (Troemel et al., 1999), a number of alternative mechanisms have been shown to regulate AWC asymmetry. Forward genetic screens have been the major driving force of elucidating the molecular mechanisms used to establish AWC asymmetry. The neuronal symmetry (nsy) mutants identified from the screens have 2 AWCOFF neurons (2AWCOFF phenotype) or 2 AWCON neurons (2AWCON phenotype) (Chuang and Bargmann, 2005; Chuang et al., 2007; Lesch and Bargmann, 2010; Lesch et al., 2009; Sagasti et al., 2001; Troemel et al., 1999; Vanhoven et al., 2006). Genetic analysis of the nsy mutants and molecular characterization of the nsy genes define a transient, embryonic gap junction neural network (Fig. 2b) and a novel calcium-regulated Ca2+/calmodulin dependent protein kinase (CaMKII)-MAP kinase pathway (Fig. 3) for the establishment of AWC asymmetry.
A calcium- and microtubule- dependent MAP kinase pathway specifies the default AWCOFF identity
In the default AWCOFF state, calcium influx through UNC-2/EGL-19/UNC-36 voltage-gated calcium channels activates UNC-43 (CaMKII) (Fig. 3a, right cell, steps 1,2) (Troemel et al., 1999). Upon UNC-43 (CaMKII) activation, the TIR-1 (Sarm1) adaptor protein assembles a calcium-signaling complex that includes UNC-43 (CaMKII) and NSY-1 MAP kinase kinase kinase (MAPKKK) (Fig. 3a, right cell, steps 2) (Chuang and Bargmann, 2005). NSY-1/MAPKKK physically binds to and phosphorylates SEK-1/MAPKK (Tanaka-Hino et al., 2002), but the MAPK involved in the signaling cascade has yet to be identified.
Microtubules also play an important role in defining the AWCOFF subtype. Disrupting microtubule polymerization using nocodazole causes a 2 AWCON phenotype, which recapitulates loss of function mutations in the calcium signaling proteins (Chang et al., 2011). Microtubules in the AWCOFF cell function to transport the UNC-43 (CaMKII)/TIR-1 (Sarm1)/NSY-1 (MAPKKK) complex to the axonal synapses via an unknown motor protein (Fig. 3b, right cell, step 3). A missense mutation within the N-terminal regulatory domain of TIR-1 in the tir-1(ky648) allele confers a gain of function phenotype in a microtubule-dependent manner (Chang et al., 2011), suggesting an uncharacterized mechanism of regulation for the calcium signaling complex. Retrograde signaling transported from the AWC axons to the cell body is proposed to suppress the expression of str-2 and allow the transcription of srsx-3, thereby defining the AWCOFF subtype (Fig. 3c, right cell, steps 4,5) (Chang et al., 2011). Additionally, the UNC-104/kinesin motor protein acts non-cell autonomously in the contralateral AWC neuron to promote AWCOFF, indicating a role of unc-104 in a feedback mechanism (Chang et al., 2011).
Intercellular calcium signaling via a gap junction neural network coordinates AWC asymmetry
During embryogenesis, a transient neural network is formed between the cell bodies of AWC neurons and adjacent neurons via NSY-5 gap junctions (Fig. 2b) (Chuang et al., 2007). The NSY-5 network is defined by the 18 pairs of nsy-5-expressing neurons that are likely to be linked by gap junctions (Chuang et al., 2007; Schumacher et al., 2012; Taylor et al., 2010). Calcium in the NSY-5 network has dual roles in AWC asymmetry: 1) an autonomous role in AWC neurons in promoting the AWCOFF subtype, 2) a non-autonomous role in non-AWC neurons of the NSY-5 network in promoting the induced AWCON subtype (Schumacher et al., 2012). nsy-5 activity functions primarily in AWC to promote the AWCON subtype, and nsy-5 activity in specific non-AWC neurons of the network promotes or inhibits the AWCON induction depending on the neuron types (Chuang et al., 2007). In addition, the levels of calcium in specific non-AWC neurons within the NSY-5 network influence side biases of AWCON induction in a manner dependent on NSY-5 gap junctions. A high calcium level in the right AWB neuron inhibits the right AWC neuron to become the AWCON (or promotes AWCOFF) subtype and a high calcium level in the left ASH promotes the AWCON subtype on the left side (Fig. 2b) (Schumacher et al., 2012). Together, these findings indicate that intercellular calcium signaling between the two AWCs and other neurons in the NSY-5 network is required for the precise 1AWCON/1AWCOFF decision (Schumacher et al., 2012).
Neurons in C. elegans form chemical synapses along their axons in order to communicate with each other (White et al., 1986). Proper axon guidance is required for precise contact and communication between the left and right sides of the neuron pairs in the NSY-5 gap junction network, and is thus important for the AWCON induction (Fig. 2b, 3a, left cell, step 1), as mutations in axon guidance molecule genes such as unc-76 (plasma-membrane associated protein) and sax-3 (ROBO) display a 2AWCOFF phenotype (Troemel et al., 1999). Differential calcium levels between the two AWC neurons establish AWC asymmetry: the AWC with a higher calcium level remains as the default AWCOFF subtype, and the AWC with a lower calcium level becomes the induced AWCON subtype (Fig. 3) (Schumacher et al., 2012). Several mechanisms downstream of axon outgrowth and guidance have been identified to inhibit the calcium-regulated UNC-43 (UNC-43)/TIR-1 (Sarm1)/NSY-1 (MAPKKK) signaling pathway in the future AWCON neuron (Fig. 3, left cell). 1) NSY-5 gap junctions and NSY-4 claudin-like adhesions function in parallel to inhibit voltage-gated calcium channels, which results in a low intracellular calcium concentration and repression of the UNC-43/CaMKII mediated signaling cascade (Fig. 3a, left cell, step 2) (Chuang et al., 2007; Vanhoven et al., 2006). 2) microRNAs have been shown to play crucial roles in various aspects of neural development, including neuronal asymmetry (Alqadah et al., 2013). The microRNA mir-71 inhibits the expression of the TIR-1/Sarm1 adaptor protein by binding to its 3′ UTR (Fig. 3b, left cell, step 3) (Hsieh et al., 2012). nsy-5 and nsy-4 positively regulate the stabilization of mature mir-71 via an unidentified mechanism (Fig. 3b, left cell, step 3) (Hsieh et al., 2012). 3) The Raw repeat protein OLRN-1 represses UNC-43 (CaMKII) (Fig. 3c, left cell, step 4) (Bauer Huang et al., 2007). Taken together, these events ensure the repression of the calcium-regulated CaMKII-MAPK signaling cascade in the future AWCON neuron, leading to the expression of the AWCON marker str-2 and repression of the AWCOFF marker srsx-3 (Fig. 3c, left cell, step 5).
MAINTENANCE OF AWC ASYMMETRY
AWC asymmetry is established using transient signaling during embryogenesis and is maintained using distinct mechanisms throughout the life of an animal (Troemel et al., 1999). Forward genetic screens identified three pathways required for the maintenance of AWC asymmetry: olfactory signaling, transcriptional regulation, and TGF-β signaling (Fig. 4) (Lesch and Bargmann, 2010; Lesch et al., 2009; Troemel et al., 1999). Mutations in the maintenance pathways lead to the loss of AWC asymmetry after the first larval stage.
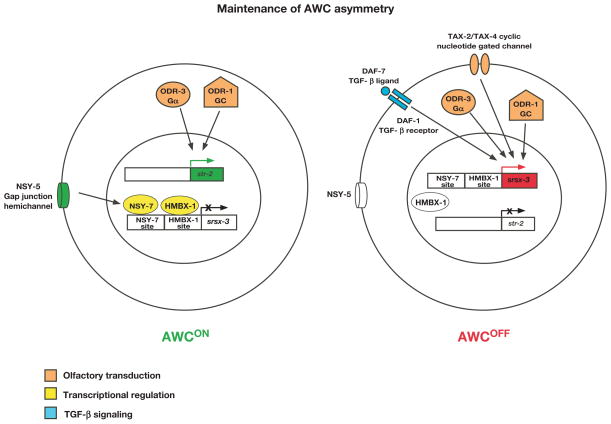
FIG. 4. Maintenance of AWC asymmetry.
AWC asymmetry is stochastic, and this figure illustrates the case when AWCON is on the left and AWCOFF is on the right. Molecules in red represent AWCOFF promoting, those in green represent AWCON promoting, and those in white indicate inactive or less active molecules. Molecules in orange, yellow, and blue represent the three distinct mechanisms used for the maintenance of AWC asymmetry. GC, guanylyl cyclase. Left: The AWCON subtype is maintained using two mechanisms: olfactory transduction (ODR-1 and ODR-3 represented in orange maintain str-2 expression), and transcriptional regulation (HMBX-1 and NSY-7 represented in yellow repress srsx-3 expression). Right: The AWCOFF subtype (srsx-3 expression) is maintained using olfactory transduction molecules (TAX-4, ODR-1, and ODR-3 represented in orange) and TGF-β signaling (DAF-7 and DAF-1 represented in blue).
Olfactory signaling
An olfactory cGMP transduction pathway maintains AWCON and AWCOFF subtypes. Components in the olfactory signaling pathway required for the maintenance of AWC asymmetry include two receptor-type guanylyl cyclases (encoded by odr-1 and daf-11), a Gα subunit (encoded by odr-3), a cyclic-nucleotide gated cation channel (encoded by tax-2 and tax-4), and a cGMP-responsive protein kinase (encoded by egl-4) (Lesch and Bargmann, 2010; Lesch et al., 2009; Troemel et al., 1999). odr-1, daf-11, odr-3, and egl-4 are required to maintain both AWCON and AWCOFF subtypes, while tax-2 and tax-4 only maintains the AWCOFF subtype (Fig. 4) (Lesch and Bargmann, 2010; Lesch et al., 2009; Troemel et al., 1999).
Transcriptional regulation
Two transcription factors, NSY-7 and HMBX-1, are known to play a role in maintaining AWC asymmetry. NSY-7, a homeodomain-like transcription factor, is expressed predominantly in AWCON. NSY-7 maintains the expression of str-2 and also functions to repress srsx-3 in response to a transient embryonic signal from NSY-5 gap junctions in the AWCON neuron (Fig. 4, left cell) (Lesch et al., 2009). HMBX-1, a homolog of mammalian HMBOX1, also functions to repress srsx-3 expression in the AWCON neuron (Fig. 4, left cell) (Lesch and Bargmann, 2010).
TGF-β signaling
TGF-β signaling is also involved in maintaining AWCOFF (Fig. 4, right cell), as mutations in a TGF-β ligand (encoded by daf-7) or a TGF-β type I receptor (encoded by daf-1) causes the loss of srsx-3 expression in adulthood (Lesch and Bargmann, 2010). daf-7 inhibits the formation of dauer (Lesch and Bargmann, 2010; Ren et al., 1996), an arrested stage developed in adverse environments such as starvation, overcrowding, or high temperature. daf-7 expression is inhibited by the dauer pheromone (Lesch and Bargmann, 2010; Ren et al., 1996), a mixture of small molecules for entry into dauer. The dauer pheromone inhibits srsx-3 expression through downregulating the TGF-β pathway (Lesch and Bargmann, 2010), suggesting that dynamic environmental cues can regulate the maintenance of AWC asymmetry.
BEYOND ASYMMETRY
Stochastic cell fate decisions
The AWC neuron pair acquires two mutually exclusive subtypes and distinct functions through a stochastic, coordinated cell-signaling event. Stochastic cell fate acquisition in animal development is a conserved but only partly understood phenomenon in all species (Johnston and Desplan, 2008, 2010; Losick and Desplan, 2008). For example, only one allele of the ~1300 olfactory receptor genes is expressed per neuron probably via a stochastic, random process in the mouse olfactory system (Mombaerts, 2004; Nguyen et al., 2007; Serizawa et al., 2003). In the human retina, the M or L cone photoreceptor cell subtypes are selected randomly through stochastic cell fate choice (Nathans, 1999; Smallwood et al., 2002). In the Drosophila eye, two subtypes of ommatidia are specified randomly by stochastic opsin expression in R7 and R8 photoreceptors (Mikeladze-Dvali et al., 2005; Wernet et al., 2006). Stem cells have the ability to maintain their own populations through self-renewal and to give rise to differentiated progeny (Reya et al., 2001); the balance between self-renewal and differentiation of stem cells is regulated in a stochastic manner (Blanpain and Simons, 2013). In the immune system, B lymphocytes acquire a large number of distinct fates in response to stimulation; B-cell fate choice can be stochastic, directed, inherited, or some combination of these mechanisms (Duffy et al., 2012; Tarlinton, 2012). Although promoter selection and lateral signaling are shown to play roles in some of these processes, fundamental questions regarding these stochastic choice mechanisms still remain. AWC asymmetry is an excellent model to identify molecular mechanisms controlling stochastic cell fate specification.
Lateral inhibition
The asymmetry observed in AWC neurons reflects a novel example of lateral inhibition independent of Notch. A classic example of Notch-mediated lateral inhibition is observed in the C. elegans gonad primordium, in which two cells have equal potential to become either an anchor cell (AC) or a ventral uterine cell (VU). The two initially equivalent cells interact via the LIN-12 (Notch) receptor and the LAG-2 (Delta) ligand. During this interaction, positive and negative feedback loops amplify a small difference in Notch activity, so that the cell with lower Notch activity adopts the AC fate and the cell with higher Notch activity becomes VU (Greenwald, 2012). In the adult peripheral nervous system of Drosophila, interactions among a small proneural cluster of equivalent cells via Notch and Delta ensure that the cell with lower Notch activity becomes the sense organ precursor and the cells with higher Notch activity become epidermal (Hartenstein and Posakony, 1990; Simpson, 1990), which is analogous to the C. elegans AC/VU decision. In the developing vertebrate nervous system, neurogenesis is also controlled by Notch-dependent lateral inhibition (Formosa-Jordan et al., 2013; Kageyama et al., 2008; Kiernan, 2013).
Although AWC asymmetry could possibly be independent of Notch (Troemel et al., 1999), similarities between the Notch signaling system of lateral inhibition and that of AWC are observed. First, as in Notch signaling, cell-cell interaction between the two AWC neurons and other neurons is important for the specification of the two AWC subtypes (Chuang et al., 2007; Troemel et al., 1999). Second, in Notch lateral inhibition, high levels of Notch receptor lead to a particular fate versus another. Similarly, differential levels of calcium determine AWC asymmetry. High levels of calcium in an AWC neuron specify an AWCOFF fate, whereas low calcium levels in an AWC neuron results in induction of AWCON (Schumacher et al., 2012). Third, similar to Notch signaling, AWC lateral inhibition also displays feedback mechanisms (Chang et al., 2011; Chuang et al., 2007). AWC feedback is mediated via a network of neurons, which are connected by gap junctions to ensure precise AWCON/AWCOFF fate specification perhaps by sensing the levels of calcium (Chuang et al., 2007; Schumacher et al., 2012).
Homologs of AWC asymmetry genes and their relevant functions in vertebrates
Calcium is an important signaling molecule for AWC asymmetry. In vertebrates, calcium is also required for left-right patterning and neuronal development. Transient calcium release in the left side of vertebrates initiates asymmetric expression of the TGF-β ligand Nodal to regulate visceral and brain asymmetry (Bisgrove et al., 2003; Liang et al., 2000; McGrath and Brueckner, 2003). Spontaneous patterns of calcium spikes during embryonic CNS development are critical for the specification of transmitter expression (Borodinsky et al., 2004).
Vertebrate homologs of AWC asymmetry genes have been implicated in left-right patterning as well as nervous system development and function: 1) Claudin tight junction proteins play a role in left-right patterning of internal organs in chick and frogs (Brizuela et al., 2001; Simard et al., 2006). 2) Connexin gap junctions play critical roles in early left-right patterning in frog embryos (Levin and Mercola, 1998). Gap junctions are also involved in the differentiation of P19 embryonic carcinoma cells into neurons (Bani-Yaghoub et al., 1999). Furthermore, gap junction adhesions are required for glial-guided neuronal migration in the mouse embryonic cerebral cortex (Elias et al., 2007). 3) UNC-2/UNC-36 is an N/P-type voltage-gated calcium channel. In vertebrates, N/P-type calcium channels are important for initiation of synaptic transmission at fast synapses (Catterall, 2011). 4) The vertebrate TIR domain adaptor protein Sarm1, which is also called MyD88-5, is a homolog of C. elegans TIR-1 (Kim et al., 2007; Mink et al., 2001). Sarm1 is required for cell death of hippocampal neurons during deprivation of oxygen and glucose (Kim et al., 2007) and for activation of an injury-induced axon death pathway (Osterloh et al., 2012). The functions of Sarm1 in cell death and axon degeneration are consistent with the role of tir-1 in promoting a cell death program in C. elegans linker cells (Blum et al., 2012). In addition, Sarm1 controls syndecan-2 dependent dendritic arborization (Chen et al., 2011). 5) CaMKII is important for dendritic development, memory, and plasticity (Wayman et al., 2008). CaMKII directly phosphorylates NeuroD, a proneural transcription factor of the basic helix loop helix class, to regulate dendrite morphogenesis (Gaudilliere et al., 2004). 6) MAPK signaling is required for fear conditioning in mice and CaMKII is activated in the same hippocampal region after learning (Atkins et al., 1998).
CONCLUSIONS
In late embryogenesis, the C. elegans left and right AWC olfactory neurons communicate through intercellular calcium signaling across a gap junction neural network to differentiate into two distinct subtypes: default AWCOFF and induced AWCON. AWCON and AWCOFF express different odorant receptors and sense different odors. Asymmetrical differentiation of the two AWC neurons diversifies sensory repertoire of the animal. The AWCOFF subtype is specified by a calcium- and microtubule-dependent CaMKII-MAPK cascade. Lateral signaling resulting from cell-cell interaction (mediated by gap junctions and claudin-like adhesions) inhibits the calcium-CaMKII-MAPK pathway in the AWC cell that becomes AWCON. AWC asymmetry is maintained throughout the life of the animal by three distinct mechanisms: olfactory transduction, transcriptional regulation, and TGF-β signaling.
C. elegans has emerged as a powerful organism to investigate the molecular mechanism involved in establishing asymmetry in the nervous system. The organism’s genetic amenability combined with the ability to identify mutants in an unbiased manner using forward genetic screens has provided insight into the mechanisms on how asymmetry of the nervous system occurs and the rationale for evolutionary advantage in developing asymmetry. However, much remains to be elucidated on how AWC asymmetry is established. One of the most important questions that remain unaddressed is how stochastic AWC asymmetry is initiated. In addition, there are some gaps in the calcium-regulated CaMKII-MAPK pathway required for AWC asymmetry. In AWCOFF cell specification, there are several unanswered questions including the identification the microtubule-based motor protein(s) required for the transport of the TIR-1 calcium-signaling complex to the AWC synapses, the identification of downstream signaling components (such as MAPK and transcription factors) downstream of SEK-1 (MAPKK), and the retrograde signal that leads to the expression of srsx-3 and the suppression of str-2 expression. In the AWCON cell, it is still unknown how gap junctions and claudin-like adhesions result in the inhibition of voltage-gated calcium channels and the transcription factor(s) that initiate str-2 expression. Furthermore, it is possible that molecular and functional asymmetries may extend to other neuronal pairs of the NSY-5 network and this hypothesis is worth further investigation.
Many of the genes involved in AWC asymmetry are evolutionarily conserved and vertebrate homologs of these genes are required for left-right patterning and many facets of nervous system development and function. The knowledge gained from AWC asymmetry may shed light on various aspects of developmental asymmetries and neuronal development in vertebrates. Furthermore, AWC asymmetry is a unique form of lateral inhibition, which leads to stochastic cell fate specification. Pioneering work using C. elegans and Drosophila was instrumental to elucidating the Notch signaling system, which proved to be conserved in vertebrates. The novel type of AWC lateral inhibition may prove to be common in developmental decisions in other systems.
Acknowledgments
We thank Oliver Hobert for comments on the manuscript. A.A. is supported by a Choose Ohio First Scholarship, Y.-W.H. by a NIH Organogenesis Training Grant, and C.-F.C. by an Alfred P. Sloan Research Fellowship and a NIH R01GM098026 grant.
References
- Alqadah A, Hsieh YW, Chuang CF. microRNA function in left-right neuronal asymmetry: perspectives from C. elegans. Front Cell Neurosci. 2013;7:158. doi: 10.3389/fncel.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Underhill TM, Naus CC. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev Genet. 1999;24:69–81. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<69::AID-DVG8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bauer Huang SL, Saheki Y, VanHoven MK, Torayama I, Ishihara T, Katsura I, van der Linden A, Sengupta P, Bargmann CI. Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev. 2007;2:24. doi: 10.1186/1749-8104-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove BW, Morelli SH, Yost HJ. Genetics of human laterality disorders: insights from vertebrate model systems. Annu Rev Genomics Hum Genet. 2003;4:1–32. doi: 10.1146/annurev.genom.4.070802.110428. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nat Rev Mol Cell Biol. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- Blum ES, Abraham MC, Yoshimura S, Lu Y, Shaham S. Control of nonapoptotic developmental cell death in Caenorhabditis elegans by a polyglutamine-repeat protein. Science. 2012;335:970–973. doi: 10.1126/science.1215156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Dev Biol. 2001;230:217–229. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Hsieh YW, Lesch BJ, Bargmann CI, Chuang CF. Microtubule-based localization of a synaptic calcium-signaling complex is required for left-right neuronal asymmetry in C. elegans. Development. 2011;138:3509–3518. doi: 10.1242/dev.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol. 2011;193:769–784. doi: 10.1083/jcb.201008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 2005;19:270–281. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Cochella L, Hobert O. Embryonic priming of a miRNA locus predetermines postmitotic neuronal left/right asymmetry in C. elegans. Cell. 2012;151:1229–1242. doi: 10.1016/j.cell.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella L, Tursun B, Hsieh YW, Galindo S, Johnston RJ, Chuang CF, Hobert O. Two distinct types of neuronal asymmetries are controlled by the Caenorhabditis elegans zinc finger transcription factor die-1. Genes Dev. 2014;28:34–43. doi: 10.1101/gad.233643.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Wellard CJ, Markham JF, Zhou JH, Holmberg R, Hawkins ED, Hasbold J, Dowling MR, Hodgkin PD. Activation-induced B cell fates are selected by intracellular stochastic competition. Science. 2012;335:338–341. doi: 10.1126/science.1213230. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Formosa-Jordan P, Ibanes M, Ares S, Frade JM. Lateral inhibition and neurogenesis: novel aspects in motion. Int J Dev Biol. 2013;57:341–350. doi: 10.1387/ijdb.120259jf. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Greenwald I. Notch and the awesome power of genetics. Genetics. 2012;191:655–669. doi: 10.1534/genetics.112.141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, Posakony JW. A dual function of the Notch gene in Drosophila sensillum development. Dev Biol. 1990;142:13–30. doi: 10.1016/0012-1606(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. Development of left/right asymmetry in a gustatory neuron pair of the C. elegans nervous system. Genesis. 2014 doi: 10.1002/dvg.22747. [DOI] [PubMed] [Google Scholar]
- Hobert O, Johnston RJ, Jr, Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat Rev Neurosci. 2002;3:629–640. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- Hsieh YW, Chang C, Chuang CF. The microRNA mir-71 inhibits calcium signaling by targeting the TIR-1/Sarm1 adaptor protein to control stochastic L/R neuronal asymmetry in C. elegans. PLoS Genet. 2012;8:e1002864. doi: 10.1371/journal.pgen.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Jr, Desplan C. Stochastic neuronal cell fate choices. Curr Opin Neurobiol. 2008;18:20–27. doi: 10.1016/j.conb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Jr, Desplan C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu Rev Cell Dev Biol. 2010;26:689–719. doi: 10.1146/annurev-cellbio-100109-104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kiernan AE. Notch signaling during cell fate determination in the inner ear. Semin Cell Dev Biol. 2013;24:470–479. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim R, Sengupta P. The HMX/NKX homeodomain protein MLS-2 specifies the identity of the AWC sensory neuron type via regulation of the ceh-36 Otx gene in C. elegans. Development. 2010;137:963–974. doi: 10.1242/dev.044719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Zhou P, Qian L, Chuang JZ, Lee J, Li C, Iadecola C, Nathan C, Ding A. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 2007;204:2063–2074. doi: 10.1084/jem.20070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjuin A, VanHoven MK, Bargmann CI, Thompson JK, Sengupta P. Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev Cell. 2003;5:621–633. doi: 10.1016/s1534-5807(03)00293-4. [DOI] [PubMed] [Google Scholar]
- Lesch BJ, Bargmann CI. The homeodomain protein hmbx-1 maintains asymmetric gene expression in adult C. elegans olfactory neurons. Genes Dev. 2010;24:1802–1815. doi: 10.1101/gad.1932610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch BJ, Gehrke AR, Bulyk ML, Bargmann CI. Transcriptional regulation and stabilization of left-right neuronal identity in C. elegans. Genes Dev. 2009;23:345–358. doi: 10.1101/gad.1763509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Mink M, Fogelgren B, Olszewski K, Maroy P, Csiszar K. A novel human gene (SARM) at chromosome 17q11 encodes a protein with a SAM motif and structural similarity to Armadillo/beta-catenin that is conserved in mouse, Drosophila, and Caenorhabditis elegans. Genomics. 2001;74:234–244. doi: 10.1006/geno.2001.6548. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Curr Opin Neurobiol. 2004;14:31–36. doi: 10.1016/j.conb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Nguyen MQ, Zhou Z, Marks CA, Ryba NJ, Belluscio L. Prominent roles for odorant receptor coding sequences in allelic exclusion. Cell. 2007;131:1009–1017. doi: 10.1016/j.cell.2007.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Linden DE. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17:456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Ortiz CO, Faumont S, Takayama J, Ahmed HK, Goldsmith AD, Pocock R, McCormick KE, Kunimoto H, Iino Y, Lockery S, Hobert O. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306:828–833. doi: 10.1126/science.1103707. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Faumont S, Gaston MR, Pearson BJ, Lockery SR. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature. 2001;410:694–698. doi: 10.1038/35070575. [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Renteria ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 2012;15:401–413. doi: 10.1017/thg.2012.13. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, Bargmann CI. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell. 2001;105:221–232. doi: 10.1016/s0092-8674(01)00313-0. [DOI] [PubMed] [Google Scholar]
- Schumacher JA, Hsieh YW, Chen S, Pirri JK, Alkema MJ, Li WH, Chang C, Chuang CF. Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans. Development. 2012;139:4191–4201. doi: 10.1242/dev.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK. Alterations in heart looping induced by overexpression of the tight junction protein Claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev. 2006;123:210–227. doi: 10.1016/j.mod.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development. 1990;109:509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Wang Y, Nathans J. Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes. Proc Natl Acad Sci U S A. 2002;99:1008–1011. doi: 10.1073/pnas.022629799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Tanaka-Hino M, Sagasti A, Hisamoto N, Kawasaki M, Nakano S, Ninomiya-Tsuji J, Bargmann CI, Matsumoto K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002;3:56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D. B-cell lymphomas: getting in the zone! Blood. 2012;120:2158–2159. doi: 10.1182/blood-2012-07-441238. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Hsieh YW, Gamse JT, Chuang CF. Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development. 2010;137:681–691. doi: 10.1242/dev.038695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Sagasti A, Bargmann CI. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- Vanhoven MK, Bauer Huang SL, Albin SD, Bargmann CI. The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron. 2006;51:291–302. doi: 10.1016/j.neuron.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Bargmann CI. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature. 2001;410:698–701. doi: 10.1038/35070581. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]