Abstract
Today, dilated cardiomyopathy (DCM) represents the main cause of severe heart failure and disability in younger adults and thus is a challenge for public health. About 30% of DCM cases are genetic in origin; however, the large majority of cases are sporadic, and a viral or immune pathogenesis is suspected. Following the established postulates for pathogenesis of autoimmune diseases, here we provide direct evidence that an autoimmune attack directed against the cardiac β1-adrenergic receptor may play a causal role in DCM. First, we immunized inbred rats against the second extracellular β1-receptor loop (β1-ECII; 100% sequence identity between human and rat) every month. All these rats developed first, receptor-stimulating anti–β1-ECII Ab’s and then, after 9 months, progressive severe left ventricular dilatation and dysfunction. Second, we transferred sera from anti–β1-ECII–positive and Ab-negative animals every month to healthy rats of the same strain. Strikingly, all anti–β1-ECII–transferred rats also developed a similar cardiomyopathic phenotype within a similar time frame, underlining the pathogenic potential of these receptor Ab’s. As a consequence, β1-adrenergic receptor–targeted autoimmune DCM should now be categorized with other known receptor Ab-mediated autoimmune diseases, such as Graves disease or myasthenia gravis. Although carried out in an experimental animal model, our findings should further encourage the development of therapeutic strategies that combat harmful anti–β1-ECII in receptor Ab–positive DCM patients.
Introduction
Heart muscle disease characterized by progressive dilatation and loss of cardiac function in the absence of known causes has been termed idiopathic dilated cardiomyopathy (DCM) (1, 2). Today, in Western countries DCM represents the main cause for severe heart failure and disability in younger adults (3). Several mutations in genes encoding for myocyte structural proteins (4, 5) and certain cardiotoxic substances (i.e., alcohol, anthracyclines) (6) account for about 30–40% of DCM cases; the etiology of the remaining 60–70%, however, is poorly understood. Current hypotheses regarding exogenous causes of DCM focus on chronic viral myocarditis (7) and/or on primary abnormalities in the immune system, including cytokine- or Ab-mediated tissue injury (8–10). In both cases the development of heart-specific autoantibodies has been reported (11–13). Recent clinical and experimental data suggest that among these Ab’s those directed against the cardiac β1-adrenergic receptor (β1-AR), in particular Ab’s that target the rather short but functionally important second extracellular receptor loop (β1-ECII), might play a key role in the pathogenesis of DCM (13, 14). Anti–β1-ECII Ab’s have been shown to activate the β1-AR–signaling cascade in vitro (14–17), and in vivo they have been found to be associated with significantly poorer left ventricular function (18), a higher prevalence of serious ventricular arrhythmias (19), and a higher incidence of sudden cardiac death (20). It is still unclear, however, whether patients develop heart disease because they possess harmful anti–β1-AR Ab’s or whether they develop anti–β1-AR Ab’s as a result of cardiac tissue injury (13).
Following Witebsky’s postulates, indirect evidence for the autoimmune etiology of a disease requires (a) a corresponding self-antigen to be identified and (b) an analogous immune response to be induced in an experimental animal, which, finally, must also develop a similar disease (21, 22). Direct evidence, however, requires reproduction of the disease by transfer of homologous pathogenic Ab’s or pathogenic T cells, that is Ab’s or autoreactive T cells from one to another of the same species. Although it has been shown that β1-ECII represents a potent autoantigen (23, 24) and that intraperitoneal injection of blood lymphocytes from Ab-positive DCM patients into immunodeficient mice (to avoid the expected immune reaction against human non-self proteins) may lead to an early stage of heart dilatation (25), a cause-and-effect relation between anti–β1-ECII Ab’s and DCM has not yet been demonstrated. To accomplish the above-mentioned stringent criteria for autoimmune diseases, here we attempted to create experimental immune cardiomyopathy by immunizing inbred rats against β1-ECII (indirect evidence) and then to reproduce the disease in healthy rats of the same strain by transfer of the generated anti–β1-ECII Ab’s, thus mimicking autoantibodies (direct evidence).
Methods
Generation and characterization of anti–β1-AR Ab’s.
Fusion-proteins between glutathione-S-transferase (GST) and the second extracellular loop of the human β1-AR (β1-ECII; amino acids [AA] 195–225) (26) were prepared as described previously (27). In accordance with institutional guidelines (Government of Unterfranken, Wuerzburg, Germany), we subcutaneously injected 35 inbred Lewis/CrlBR rats monthly over a period of 14 months either with β1-ECII/GST fusion proteins (n = 15), with GST alone (n = 10), or with 0.9% NaCl (n = 10). Serum IgG was prepared by caprylic acid precipitation and assayed for reactivity by the following: (a) ELISA with peptides corresponding to selected domains of the human β1-AR or β2-AR (N terminus [AA 1-59/1-35], C terminus [AA 381-477/330-414], and ECII domain [AA 195-225/169-200], respectively) (14); (b) Western blot analysis with lysates of Sf9 cells transiently expressing β1-AR, β2-AR, or the WT virus (negative control); and (c) immunofluorescence microscopy (IFM) with intact Sf9 cells spotted onto glass slides as previously described (14). β1-AR specificity of the generated rat Ab’s was confirmed by colocalization experiments (using IFM) carried out on human embryonal kidney (HEK) 293 cells expressing β1-AR, β2-AR, or the angiotensin AT1a receptor (DYKDDD flag-tagged) (28). Previously characterized domain- and subtype-specific rabbit anti–β-AR Ab’s (27) or monoclonal mouse M1 (anti-flag) Ab’s (28) served to simultaneously immunostain the receptors. Bound Ab’s were detected with appropriate species-specific secondary Ab’s (anti-rabbit, anti-mouse, or anti-rat Fab2; Dianova GmbH, Hamburg, Germany) conjugated to Cy2 or Cy3 (green or red epifluorescence), respectively. For all our experiments, calibrated rat IgG served to quantify specific IgG Ab’s by ELISA (Dianova GmbH). In addition, the levels of TNF-α, IL-2, and IL-6 were measured in all rat sera (R&D Systems Inc., Wiesbaden, Germany).
To analyze the effects of rat anti–β1-ECII on β1-AR–mediated signaling we incubated stably transfected Chinese hamster fibroblasts expressing 100–120 fmol/mg human β1-AR (CHW-β1 cells) (14) with 100 ∝g/ml rat IgG (2 hours, 37°C, 0.5 mM isobutylmethylxanthine [IBMX] added) in the presence or absence of 5 ∝M bisoprolol (Merck KGaA, Darmstadt, Germany); the cells were then either exposed or not exposed to 10 ∝M (–)isoprenaline (15 minutes, 37°C), and cellular cAMP was quantified as previously described by 125I-labeled radioimmunoassay (Immunotech, Freiburg, Germany) (14).
The cAMP-dependent protein kinase (protein kinase A or PKA) activity was determined in the lysates of the same CHW-β1 cells after incubation with 100 ∝g/ml rat IgG (1 hour, 37°C, no IBMX added) and exposure to (–)isoproterenol, as described for the cAMP assay, using [γ-32P]ATP (NEN Life Science Products, Zaventem, Belgium) and 100 ∝M kemptide (LRRASLG) as substrate (29).
Transfer of anti–β1-AR Ab’s.
Four weeks after the last immunization all rats were bled; within treatment groups, sera were pooled and stored at –20°C. Absence of the injected antigens of the sera was ascertained by ELISA using pre-viously characterized rabbit anti–β1-ECII and monoclonal mouse anti-GST Ab’s (14). The final pool of anti–β1-ECII-positive sera from the immunization experiment augmented to 15 ∞ 9 ml = 135 ml (containing 250 ± 0.03 ∝g/ml anti–β1-ECII). In the second part of our study, over 12 months we intra-venously transferred the entire available serum pool in portions of 0.8–1.4 ml, corresponding to 0.7 ∝g anti–β1-ECII per gram of body weight (BW), at four weekly intervals to ten healthy rats of the same strain (anti–β1-ECII transfer group). In parallel, we transferred a corresponding amount of pooled sera from the GST- and NaCl-injected groups to a set of control rats (GST- or NaCl-transfer group, each n = 5). Another set of naive control rats (n = 6) served to monitor the acute effects of both subcutaneous injection of the different antigens and intravenous transfer of the different sera. For this purpose the animals were lightly anesthetized (30 mg/kg ketamine-HCl and 5 mg/kg xylazine intraperitoneally) and a 1.8 F high-fidelity catheter (Millar Instruments Inc., Houston, Texas, USA) was inserted in the right carotid artery and into the left ventricle. Left ventricular (LV) pressure tracings were recorded digitally over 4–5 minutes before (baseline) and 7–8 minutes after successive administration of each of the antigens (subcutaneously: β1-ECII/GST, GST, NaCl) or each of the pooled sera (intravenously: anti–β1-ECII, anti-GST, NaCl-control) and analyzed off-line (PowerLab; AD Instruments, Castle Hill, Australia).
Echocardiography.
In both parts of our study transthoracic echo-Doppler examinations were performed by a single, experienced echocardiographer (R. Jahns) who was blinded to the treatment of the animals examined. The rats were lightly anesthetized as described above, shaved (chest only), and placed prone on a specially designed apparatus. Echocardiograms were obtained by a commercially available echocardiographic system (Vivid Five; GE Vingmed Ultrasound, Horten, Norway) equipped with a 10-MHz phased array transducer. M-mode tracings were recorded at baseline (before immunization or serum transfer), and subsequently every 3 months in the parasternal long- and short-axis views through the aortic valve at the base of the aortic leaflets and through the anterior and posterior LV wall at the level of the papillary muscles. Wall thickness and LV internal dimensions were measured directly on the screen (online) according to the guidelines of the American Society for Echocardiography (30). Pulsed-wave Doppler spectra of mitral inflow and LV outflow were recorded from the apical four- and five-chamber views, respectively. The velocity–time integral (VTI) of LV outflow served to calculate cardiac output (CO) (CO [ml/min] = aortic VTI ∞ (π [LV outflow tract diameter/2]2) ∞ heart rate). LV mass was assessed by using a modified cube formula equation (30). To determine reproducibility of rat echocardiography, all baseline examinations (before immunization or serum transfer) were repeated within 24 hours. To evaluate accuracy of our M-mode and Doppler (online) measurements, throughout the study a second set of nonmarked M-mode and Doppler images from each rat was stored digitally. Computer aided off-line analysis of these images was performed in a blinded manner by the echocardiographer (R. Jahns, intraobserver variability) and by another independent observer (S. Triebel, interobserver variability). In addition, all our final echocardiograms (immunization and serum-transfer experiments) were anatomically validated.
Hemodynamic measurements and histology.
Forty-eight to 72 hours after the final echo-Doppler examinations, the rats underwent left heart cathe-terization as described above. LV pressure tracings were recorded digitally over 15 minutes and analyzed off-line (PowerLab; AD Instruments). After blood sampling, the rats received additional deep anesthesia (70 mg/kg sodium pentobarbital intraperitoneally). The hearts were removed, weighed, and placed in relaxing buffer (5% dextrose, 25 mM KCl in PBS). The apex was cut, frozen in isopentane (–56°C), and stored at –80°C (binding studies); the remaining three-fourths were fixed in 10% PBS-buffered formalin (24–28 hours). Cross-sections of 600 ∝m were cut exactly perpendicular to the septum (Vibratome; TPI, St. Louis, Missouri, USA) and embedded in paraffin. Cavity and wall dimensions of each heart, as well as the diameter and perimeter of 100 myocytes and corresponding nuclei per section were determined by computer-aided analysis of H&E-stained midventricular 1-∝m sections. The collagen I-III content of each heart was determined densitometrically after collagen-specific staining (Sirius red F3BA/Fast green FCF; Sigma-Aldrich, Deisenhofen, Germany) of 1-∝m sections and elution of the dye as previously described (31).
Binding studies.
Apical cardiac segments were homogenized in ice-cold buffer (10 mM HEPES, 2 mM EDTA, 10 mM MgCl2, 0.4 M KCl) containing various protease inhibitors (5 ∝g/ml leupeptine, 1.5 mM benzamidine, 200 U/ml aprotinine, 2 ∝g/ml pepstatine A). To ascertain detachment of membrane-bound receptor Ab’s the homogenization procedure (including three extensive washing steps) was repeated two times. Washed membrane protein (35 ∝g) was incubated (1.5 hours, 25°C) with 200 pM of the nonselective 125I-labeled β-AR antagonist cyanopindolol ([125I]-CYP; 2,200 Ci/mM; NEN Life Science Products) (32). Non-specific binding was determined with 5 ∝M unlabeled L-propranolol. To analyze the proportion of β1-AR/β2-AR subtypes, incubations with 70 pM [125I]-CYP were supplemented with 0.1 ∞ 10–9 to 10 ∞ 10–3 M unlabeled β1-selective antagonist CGP 20712A (Sigma-Aldrich). The reactions were stopped by adding ice-cold TRIS buffer and rapid filtration (Whatman GF/C). Filter-bound radioactivity was measured by γ counting, and ligand-binding curves were fitted to the data by computer-aided nonlinear regression analysis.
Catecholamine determination.
Catecholamine levels were determined from urine samples collected over a 24-hour period at the beginning and the end of each experiment (immunization or serum transfer). Epinephrine and norepinephrine were quantified by HPLC combined with electrochemical detection (33) as previously described.
Statistics.
Data are given as mean plus or minus SEM. Functional assays (cAMP, PKA) were analyzed by two-factor ANOVA. Significance between the treatment groups was analyzed by Scheffé F test. Comparisons between the hemodynamic short-term effects upon injection of the different antigens and/or sera and comparisons between echocardiographic parameters (long-term follow-up) were done by repeated measures ANOVA accompanied by a Bonferroni post hoc test. Agreement between the echocardiographic measurements (intraobserver and interobserver variability) was assessed according to Bland and Altmann (34). Hemodynamic and morphometric parameters of Ab-positive and corresponding control rats were compared by (unpaired) Student t tests. Correlation coefficients (echocardiography versus anatomy) were obtained using the method of least squares.
Results
Properties of rat anti–β1-ECII Ab’s and experimental strategy.
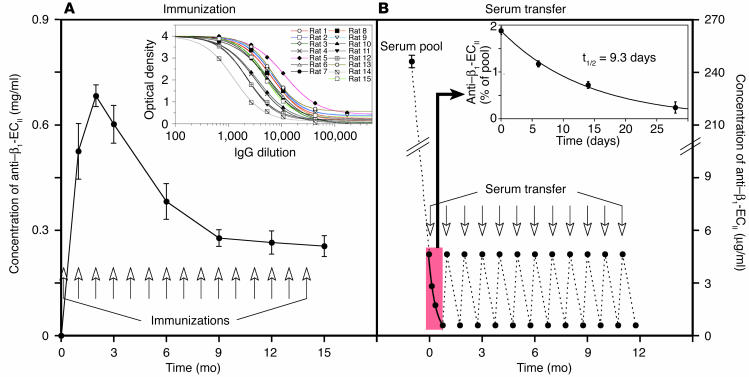
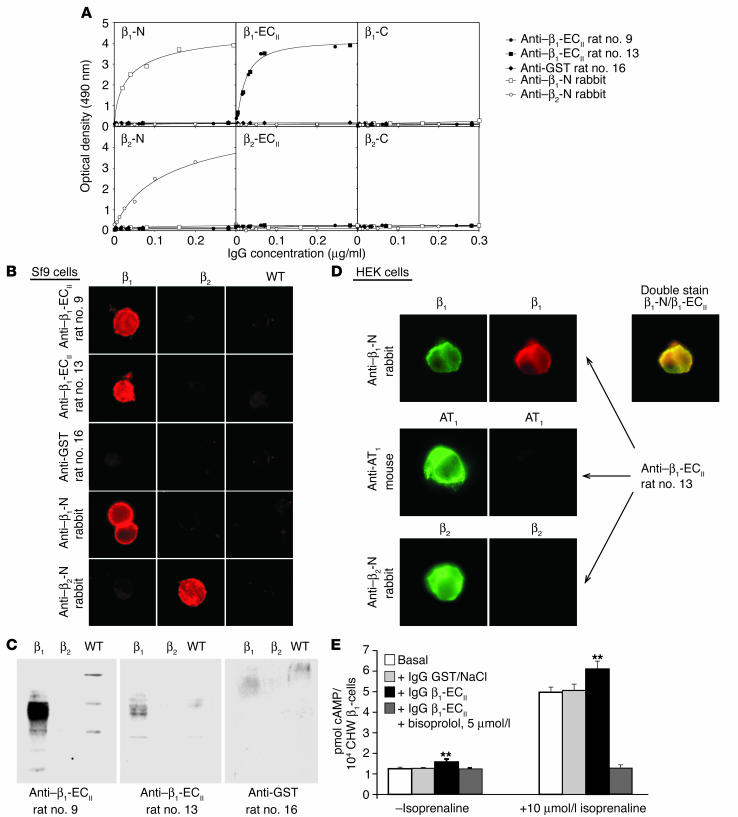
In the first part of our study all 15 rats immunized with β1-ECII/GST fusion proteins produced high and uniform levels of anti–β1-ECII Ab’s of the IgG subclass (Figure 1A, inset), reaching a peak concentration of 0.68 ± 0.03 mg/ml 8 weeks after the first immunization, which decreased to 0.25 ± 0.03 mg/ml at the end of the study (as determined by immunopurified anti–rat IgG secondary Ab’s; Figure 1A). After preparation of the serum IgG fraction (caprylic acid precipitation), domain specificity of the generated Ab’s was checked by ELISA with selected peptides corresponding to the N terminus, C terminus, and ECII domain of the β1-AR or β2-AR (Figure 2A). Using IFM we could demonstrate that all of these Ab’s recognized native β1-AR expressed either in Sf9 insect cells or in HEK 293 eukaryotic cells (Figure 2, B and D), whereas nontransfected WT cells (devoid of β1-AR and β2-AR) were not stained at all. Importantly, these experiments revealed also that our rat anti–β1-ECII cross-reacts with neither the very closely related β2-AR (expressed in Sf9 or HEK cells; Figure 2, B, and D) nor with another widely distributed cardiovascular receptor, that is, the angiotensin AT1a receptor (Figure 2D). In addition, we could demonstrate that the rat anti–β1-ECII colocalized with N-immunolabeled β1-AR in the membrane of HEK 293 cells (Figure 2D, double stain: rabbit anti–β1-N/rat anti–β1-ECII). Only about 70% (n = 11/15) of the fluorescence-positive rat anti–β1-ECII, however, also stains SDS-denatured β1-AR in Western blots of Sf9 cell lysates with the same intensity (compare Figure 2, B and C), confirming earlier data indicating a requirement for a specific native con-formation of the receptor being preferentially recognized by anti–β1-ECII Ab’s (14, 18).
Figure 1.
Study protocol and anti–β1-AR Ab’s. (A) The plotted curve indicates mean titers plus or minus SEM (error bars) of specific rat anti–β1-ECII (IgG subclass) during the immunization experiment. Time points of subcutaneous immunizations are indicated by arrows. The inset depicts the immunoreactivities of IgG prepared from all 15 β1-ECII–injected rats 3 months after the first immunization determined by ELISA. (B) Scheme depicting concentrations of rat anti–β1-ECII (serum pool and transferred amounts) during the transfer experiment. Time points of intravenous serum transfer are indicated by arrows. The inset shows representative mean titers plus or minus SEM after transfer of 0.7 ∝g anti–β1-ECII/g BW over a 4-week period and the derived half-life for rat anti–β1-ECII.
Figure 2.
Immunological and functional properties of rat anti–β1-ECII. (A) ELISA immunoreactivities of different rat and rabbit Ab’s (see key) with peptide antigens corresponding to selected domains of the β1- or β2-AR (N terminus (N); C terminus (C); and ECII-domain (ECII)) (14). (B) IFM with unfixed (IgG diluted 1:200) or (C) Western blots (IgG diluted 1:2,000) with lysates of Sf9 insect cells expressing recombinant human β1-AR, β2-AR, or the WT vector. (D) IFM colocalization experiments with HEK 293 cells transiently expressing β1-AR, β2-AR, or flag-tagged AT1a receptors (IgG diluted 1:200). Examples given are representative for IgG from β1-ECII–injected rats and a GST-injected rat (control); domain and β1-AR/β2-AR subtype-specific rabbit Ab’s (27) and a monoclonal mouse anti–AT1a flag Ab (28) served to immunostain the corresponding receptors (β1-AR, β2-AR, and AT1a receptor). (E) Increases in basal (–isoprenaline) or isoprenaline-stimulated (+10 ∝M/l isoprenaline) cAMP levels in Chinese hamster fibroblasts expressing human β1-AR (CHW-β1 cells) upon incubation with rat IgG (100 ∝g/ml). Columns represent amount of accumulated cAMP plus or minus SEM (error bars) obtained without (white, control) or in the presence of IgG from rats negative (light gray, GST/NaCl injected) or positive for functionally active anti–β1-ECII (black, β1-ECII injected). The stimulatory effects of anti–β1-ECII were blocked by 5 ∝M bisoprolol, a β1-selective receptor antagonist (dark gray). **P < 0.001 (ANOVA and Scheff– F post hoc test).
As has been shown previously for human anti–β1-ECII (18), all of the generated rat anti–β1-ECII increased both the basal (1.25-fold ± 0.17-fold, P < 0.001) and the isoprenaline-stimulated cAMP-levels in CHW-β1 cells (1.23-fold ± 0.09-fold, P < 0.001). Both effects were blocked by preincubation with 5 ∝M of the β1-selective antagonist bisoprolol (Figure 2E), whereas maximum cAMP levels after direct stimulation of the G proteins with NaF did not change, excluding a postreceptor effect (not shown). Ab effects on PKA activity yielded substantially the same results (increase of basal and stimulated PKA activity by 1.23-fold ± 0.18-fold and 1.21-fold ± 0.06-fold, respectively; P < 0.01, not shown). Sera from both series of control animals (GST/NaCl) reacted with neither the β1-ECII peptide (ELISA) nor with β1-AR expressed in Sf9 or in CHW cells (Western blot, IFM, and cAMP assay; Figure 2).The clearly defined and uniform immunological as well as functional properties of the generated rat anti–β1-ECII taken together with the use of an inbred rat strain (immunization experiment) made these Ab’s ideal candidates for a transfer experiment. For this purpose, we intravenously transferred the entire available Ab pool from the immunization study at monthly intervals to healthy rats of the same strain (12 injections of 0.7 ∝g/g BW anti–β1-ECII, Figure 1B). The respective peak concentrations of free anti–β1-ECII (4.7 ± 0.2 ∝g/ml) were about 50% lower than expected (i.e., approximately 11 ∝g/ml, considering a blood volume of 20 ml/300 g BW and 220 ∝g of transferred anti–β1-ECII), indicating significant tissue redistribution of the transferred Ab’s. Within 4 weeks (injection intervals) the concentration of transferred specific Ab’s further decreased to 0.6 ± 0.1 ∝g/ml, giving a calculated half-life for anti–β1-ECII of 9.3 days (Figure 1B, inset). Repetitive measurements of anti–β1-ECII levels at various time points after each serum transfer indicated that the Ab’s did not accumulate over time.
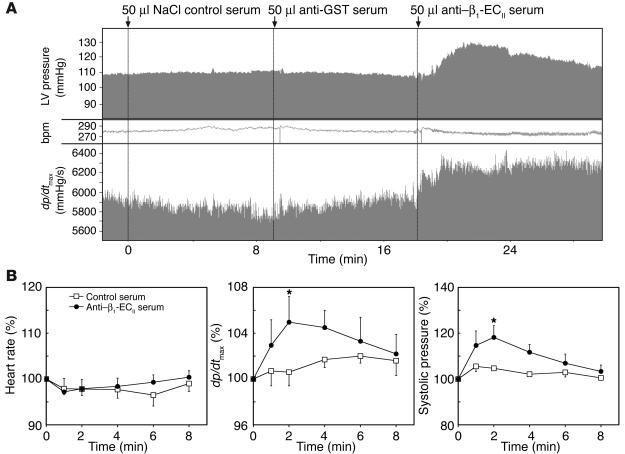
The acute effects of subcutaneous injection of the antigens and intravenous administration of the different sera (anti–β1-ECII, anti-GST, and NaCl control) were studied in naive rats of the same strain by invasively monitoring the responses in heart rate, blood pressure, and LV contractility (dp/dtmax [peak change in LV pressure per time (interval)]) over time. Whereas subcutaneous injection of the antigens did not influence any of the latter hemodynamic parameters (not shown), the transferred anti–β1-ECII Ab’s appeared to behave as mild β1 agonists by inducing a transient slight but significant increase in LV contractility (dp/dtmax) accompanied by a transient significant increase in systolic blood pressure. These changes were observed only with anti–β1-ECII-containing sera, excluding a volume effect (Figure 3). Interestingly, the heart rate remained almost stable during the different injections, which is in perfect agreement with our data from long-term echocardiographic follow-up. There were no significant differences in heart rate between anti–β1-ECII-positive and Ab-negative animals over time, supporting the concept that, in the rat, cardiac β1-AR might have a greater impact on parameters of LV contractility and relaxation than on chronotropy (35, 36).
Figure 3.
In vivo short-term effects of rat anti–β1-ECII. (A) Single representative tracing and (B) panels depicting the time course of systolic LV pressure, heart rate, and dp/dtmax from naive Lewis/CrlBR rats after i.v. application of pooled sera from NaCl/GST-injected (control) or β1-ECII–injected rats (50 ∝l each). Arrows in A indicate time points of i.v. serum application. Error bars in B indicate mean plus or minus SEM of the indicated parameters normalized to the baseline (100%) at 0, 1, 2, 4, 6, and 8 minutes after serum application. *P < 0.05 (ANOVA and Bonferroni post hoc test). bpm; beats per minute.
Echocardiography and hemodynamic measurements.
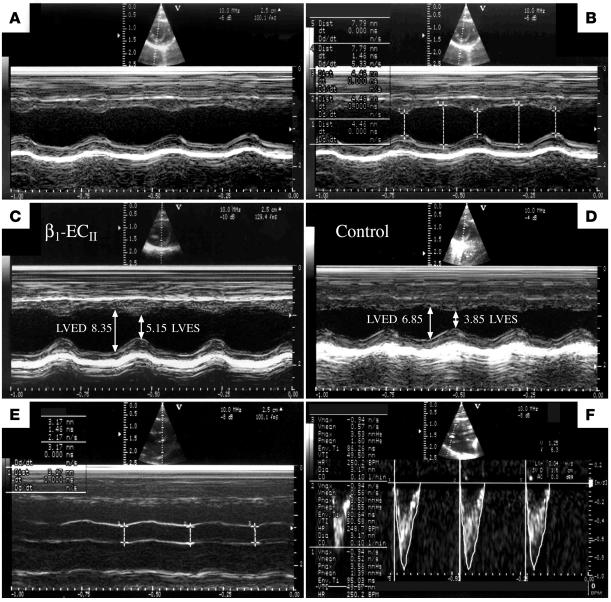
The echocardiographic system used in our study provided digitized images of high quality, which were routinely stored for computer-aided off-line analysis (Figure 4, A, C, and D). In addition, the system allowed for an analysis of M-mode and Doppler tracings directly on the screen (online) with an acceptable reproducibility at a high resolution (M-mode, 0.01 mm; Figure 4, B, E, and F). Evaluation of the accuracy of our echo-Doppler measurements yielded rather low intraobserver (1.7% M-mode and 2.1% Doppler, online and off-line measurements) and interobserver variabilities (3.2% M-mode and 4.5% Doppler, off-line measurements only), which are perhaps best explained by the recent progress in imaging technology.
Figure 4.
Echocardiography of rat hearts. Digitally stored representative M-mode and Doppler images demonstrating (A) nonmarked and (B) online marked M-mode tracings from the same animal, off-line measured end-diastolic and end-systolic LV diameters (LVED/LVES) from (C) an anti–β1-ECII-positive and (D) a corresponding control animal (immunization experiment, month 15), and on-line determination of (E) LV outflow tract diameter (LVOT; M-mode tracings) and (F) aortic VTI in a same animal (Doppler tracings).
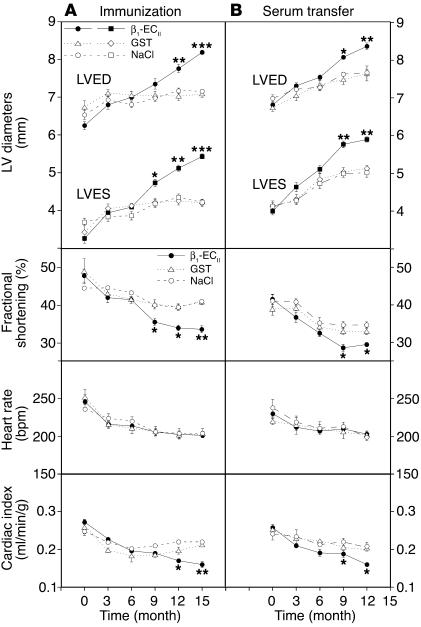
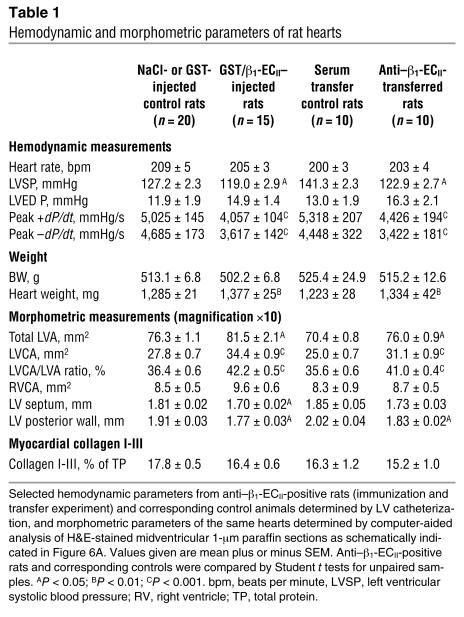
In the first part of our study we observed that LV dilatation and dysfunction in anti–β1-ECII-positive rats started about 9 months after immunization and then continuously progressed during the course of the experiment (Figure 5A; see also Figure 4, C and D). Interestingly, in the transfer experiment we obtained a similar echocardiographic pattern with an onset of cardiac dilatation and dysfunction also about 9 months after regular anti–β1-ECII-transfer (Figure 5B). At the end of each part of our study we checked the final echocardiographic findings by invasive measurements. We found that systolic LV pressure was constantly lower in Ab-positive than in corresponding control animals at a similar heart rate (Table 1). Most significantly, LV contractility (+dP/dtmax) and, in particular, LV relaxation (–dP/dtmax) were decreased to about the same extent in both β1-ECII–injected and anti–β1-ECII-transferred rats (P < 0.001 versus corresponding controls). Accordingly, LV end-diastolic pressure (LVEDP) tended to be higher in Ab-positive animals, but this failed to reach significance (Table 1). Thus, immunized rats and serum-transferred rats showed a clear functional deficit both by echocardiography and by catheterization.
Figure 5.
Echocardiographic follow-up. (A) Immunization and (B) transfer experiment. The panels depict the time course of selected echocardiographic parameters. Upper panels: LVED and end-systolic diameters (LVES). Middle panels: fractional shortening (%), derived from (LVED-LVES/LVED ∞ 100), and heart rate (bpm). Lower panels: Cardiac index corresponding to CO/BW. CO (milliliters per minute) was assessed by echocardiography (see Methods). Error bars indicate mean plus or minus SEM.*P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA and Bonferroni post hoc test).
Table 1.
Hemodynamic and morphometric parameters of rat hearts
Cardiac anatomical measurements and tissue collagen I-III.
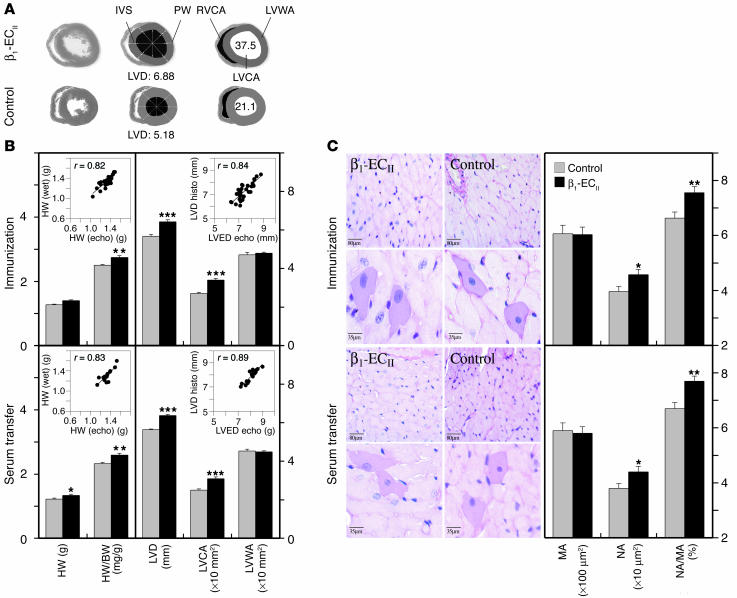
The absolute (milligrams) and relative heart mass (milligrams per gram), as well as the size of the left ventricles, were significantly increased in both β1-ECII–injected and anti–β1-ECII-transferred rats compared with corresponding control animals (Table 1 and Figure 6B, left panels). As determined from H&E-stained midventricular 1-∝m sections (Figure 6A), in both parts of our study the increases in total LV area (LVA) were due to an increase in LV cavity area (LVCA) without a significant change in LV wall area (LVWA; Table 1 and Figure 6B, right panels). In fact, the thickness of the septum and the posterior wall was significantly decreased in Ab-positive versus control animals (Table 1 and Figure 6A). In both experiments echocardiography correlated well with the anatomic measurements, that is, predicted LV mass versus heart wet weight (r = 0.82 or 0.83) and echocardiographic versus anatomic left ventricular end-diastolic diameter (LVED) (r = 0.84 or 0.89; Figure 6B, insets). By light microscopic inspection of H&E-stained 1-∝m sections of the hearts, we did not detect any obvious signs of myocardial inflammation (i.e., cellular infiltrates, using a threshold of more than seven cells per square millimeter) in anti–β1-ECII-positive versus control rats (Figure 6C, left panels). In addition, the serum cytokine levels (i.e., TNF-α, IL-2, IL-6) between Ab-positive and control animals did not differ significantly (not shown). Further morphometric analysis of the heart sections, however, revealed significantly enlarged polymorphous nuclei and an increased nucleus-to-myocyte ratio in both β1-ECII–injected and anti–β1-ECII-transferred rats, whereas the size (i.e., diameter and perimeter) of the myocytes themselves did not differ significantly (Figure 6C, right panels). With respect to fibrosis, we did not observe an increase in total myocardial collagen I-III in hearts from β1-ECII-injected or anti–β1-ECII-transferred rats (Table 1), which is in perfect agreement with a recent report on rats with low-dose isoprenaline-induced cardiomyopathy (37).
Figure 6.
Anatomic measurements and histology of rat hearts. (A) The panel shows representative H&E-stained 1-∝m cross-sections of hearts from an anti–β1-ECII-positive rat and a control animal (left row, ∞10 magnification). The middle and right rows indicate schematically how LV diameter, wall thickness, and different cardiac areas were determined by computer-aided image analysis. IVS, interventricular septum; LVD, LV-diameter; PW, posterior wall; RV, right ventricular; RVCA, right ventricular cavity area. (B) Immunization and transfer experiment. Left panels: Columns represent heart wet weight (HW) and the relative heart weight (HW/BW). The insets show correlations between wet weight and echocardiographically predicted HW for all animals (Ab-positive and Ab-negative rats) in each experiment. Right panels: Columns represent LV diameter (LVD), LVCA, and LVWA. The insets show correlations between the anatomic and the echocardiographic LV diameter for all animals in each experiment. Error bars indicate mean values plus or minus SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test). (C) Immunization and transfer experiment. Left panels: H&E-stained 1-∝m sections of hearts from anti–β1-ECII-positive rats and corresponding control animals at ∞50 (upper rows) or ∞120 magnification (lower rows) with representative myocytes and corresponding nuclei. Right panels: Columns indicate the respective myocyte area (MA), nuclear area (NA), and the calculated nucleus-to-myocyte ratio (NA/MA). Error bars indicate mean values plus or minus SEM.
Cardiacβ1-ARs and catecholamines.
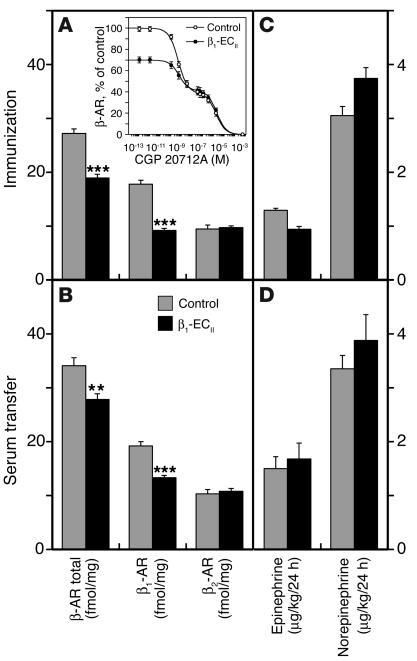
Using radioligand binding, we determined that total [125I]-CYP binding sites were significantly decreased in hearts from β1-ECII–injected as well as anti–β1-ECII-transferred rats. Although we cannot exclude that age-dependent reductions in myocardial β-AR density (32) might have partially contributed to the absolute differences obtained between immunized and transferred rats (i.e., age 18 versus 15 months), this effect was clearly more pronounced in the immunization experiment resulting in about 30% downregulation of cardiac β-AR (Figure 7A) as compared with 18% downregulation in the transfer experiment (Figure 7B). Assuming a two-site binding model, the biphasic displacement curves in the presence of unlabeled β1-AR selective antagonist CGP 20712A (Figure 7A, inset) revealed that this downregulation was primarily a function of diminished cardiac β1-AR density in both parts of our study (thus age independent), whereas the absolute number of cardiac β2-AR did not change substantially (Figure 7, A and B). When comparing the epinephrine and norepinephrine levels from urine samples collected over 24 hours at the beginning (not shown) and the end of each experiment, however, we found no significant differences between anti–β1-ECII-positive and corresponding control animals (Figure 7, C and D).
Figure 7.
β1-AR/β2-AR subtypes and urine catecholamines. (A) Immunization and (B) transfer experiment. Columns represent total β-AR density and the amount of β1-AR and β2-AR subtypes (femtomoles per milligram of protein) in cardiac membranes of β1-ECII–injected (n = 15, aged 18 months) or anti–β1-ECII-transferred rats (n = 10, aged 15 months), and corresponding control animals at the end of each experiment (n = 20 or n = 10, respectively). Error bars indicate mean values plus or minus SEM. **P < 0.01; ***P < 0.001. The inset in A depicts a representative radioligand displacement curve using the β1-selective antagonist CGP 20712A (immunization experiment). (C) Immunization and (D) transfer experiment. Columns correspond to urine catecholamine concentrations determined at study end. Error bars indicate mean values plus or minus SEM.
Discussion
Proof of a pathogenetic principle.
The two major hypotheses on the exogenous causes of idiopathic DCM are either persistent viral infection (7, 38) or autoimmune-mediated damage to myocytes (8, 10–13). Actually, the hypotheses are not mutually exclusive because a large number of autoimmune reactions to myocardial proteins appear to be virus triggered (11, 38). An important prerequisite for potentially pathogenic autoantibodies is their ability to (a) recognize their corresponding antigens in a native conformation and (b) exert pathophysiologically relevant functional effects (39). Therefore, autoantibodies that bind to and activate cardiac β1-ARs have been claimed as promising candidates involved in the pathogenesis of DCM (13, 14, 16, 18, 25). A cause-and-effect relation between anti–β1-AR autoantibodies and the disease remained to be demonstrated (13), however. Following the stringent established postulates for autoimmune diseases (22), we provide here direct evidence that an autoimmune attack to the β1-ECII may play a causal role in DCM: we immunized inbred rats against β1-ECII (100% sequence-identity between human and rat) in order to induce both the potentially harmful anti–β1-ECII Ab’s and the disease. All these rats developed receptor-stimulating anti–β1-ECII, and from the ninth immunization month onward, progressive LV dilatation and dysfunction (indirect evidence). In the following step, we mimicked autoantibodies by transferring anti–β1-ECII from immunized rats to healthy rats of the same strain. The fact that all healthy rats within a similar time frame also developed a cardiomyopathic phenotype finally delivered the direct proof of the (pathogenetic) principle.
Cardiomyopathic phenotypes induced by immunization againstβ1-ECII.
For more than a decade it has been well established that β1-ECII represents a potent autoantigen containing the T and B cell epitopes necessary for induction of an immune response (23, 24). In addition, we and others have previously shown that both synthetic peptides corresponding to β1-ECII and/or fusion proteins containing β1-ECII induce functionally active anti–β1-ECII Ab’s in rabbits (14, 40, 41). Two groups also reported on the morphological changes observed in the hearts of anti–β1-ECII-positive rabbits (post mortem examinations); the results, however, differed to some extent, demonstrating either biventricular dilatation without a change in myocardial fibrosis (40) or LV hypertrophy with an increase in fibrosis (41). None of the earlier immunization studies have addressed the in vivo functional consequences of anti–β1-ECII Ab’s, however. By echocardiographic follow-up of β1-ECII–injected rats we demonstrate here a time course of LV dilatation with an onset of anti–β1-ECII-mediated immune cardiomyopathy about 9 months after the generation of activating anti–β1-ECII. This LV dilatation and dysfunction slowly but steadily progressed during the study, resulting in physical signs of heart failure in all anti–β1-ECII-positive animals (reduced activity, intraperitoneal edema, hepatic enlargement). Because of the planned hemodynamic measurements in vivo and the planned serum transfer, however, we stopped the immunization experiment before the animals developed end-stage heart failure with an increase in mortality. Interestingly, also in the transfer experiment (in which the duration was limited by the pool of anti–β1-ECII-containing sera), cardiac dilatation and dysfunction were apparent about 9 months after regular Ab transfer, suggesting the same slow-acting pathogenetic mechanism in both parts of our study. In view of the functional effects of our anti–β1-ECII in vitro and their hemodynamic short-term effects in vivo (which are in perfect agreement with the electrophysiological and hemodynamic effects of human anti–β1-ECII observed in isolated rat cardiomyocytes) (17), the most likely explanation is that, in our model, the Ab’s acted as mild β1 agonists, which, over time, might have initiated and/or worsened the vicious circle of sympathetic overdrive and progressive heart failure. The fact that we were able to transfer the disease by relatively low amounts of anti–β1-ECII (compared with immunized animals) indicates (a) that the measured serum levels after transfer of anti–β1-ECII were not representative of the true amount of functionally relevant Ab’s acting in these animals (approximately 50% tissue redistribution) and (b) that the biological efficiency of the Ab’s was rather high, which means that in our model the absolute amount of circulating anti–β1-ECII appeared to be less important than the pathogenic potential of each receptor Ab bound. Taken together, these data demonstrate that development of anti–β1-AR-induced immune cardiomyopathy is a slow but continuously progressing process, which is perhaps best mimicked by low-dose isoprenaline-induced heart failure models (37). This notion is further supported by the fact that total myocardial collagen was not increased in either low-dose agonist–induced or in anti–β1-AR-induced cardiomyopathy. Further microstructural analysis of the hearts from Ab-positive rats revealed enlarged and polymorphous nuclei without a significant increase in myocyte size, however. This feature is frequently found in failing cardiomyopathic hearts (42, 43) and thought to reflect changes in gene expression (i.e., induction of specific members of the embryonic gene program, including the atrial natriuretic factor gene) (44) as a compensatory response to both sympathetic overdrive and hemodynamic overload, with the degree of dilatation exceeding the capacity to hypertrophy (44, 45).
Functional properties of anti–β1-ECII Ab’s.
We show here that anti–β1-ECII generated in rats uniformly increases both basal and agonist-stimulated cAMP levels and PKA activity in CHW-β1 cells to about the same extent as has been demonstrated for human anti–β1-ECII (15, 18). The cAMP effects were blocked by the β1-specific antagonist bisoprolol, suggesting that our stimulatory rat anti–β1-ECII acted, indeed, via the β1-AR. By contrast, anti–β1-ECII raised in rabbits exhibited inconsistent functional properties. Some rabbit anti–β1-ECII increased basal and partially inhibited isoprenaline-stimulated cAMP levels (partial agonist-like effect) (41), whereas others blocked both basal and agonist-stimulated receptor signaling (14). Accordingly, in one of the immunization studies with rabbits, cardiac β1-ARs were upregulated in the presence of receptor Ab’s (40), whereas in the other they were downregulated (41). As suggested in a recent report focusing on the role of the MHC in the elaboration of autoantibodies, some of these differences might derive from the specific epitope-dependent functional properties of the generated anti–β1-ECII (epitope defined as sequence of individually recognized AAs) (39). Allosterically blocking (inhibitory) anti–β1-ECII would be expected to upregulate cardiac β1-AR, whereas receptor-stimulating anti–β1-ECII should induce β1-AR downregulation (14, 41). The transfer experiment further highlights the importance of the functional properties of an Ab and/or autoantibody for its pathogenicity (46). Independent of an age-associated reduction in total β-AR density (32), we show here that our stimulating rat anti–β1-ECII almost selectively downregulates cardiac β1-AR, even at a 25-fold (calculated) or 50-fold (measured) dilution compared with anti–β1-ECII levels in immunized rats, suggesting a high biological activity and/or efficiency of these Ab’s.
Anti–β1-ECII-targeted autoimmune cardiomyopathy: study limitations and future perspectives.
In summary, anti–β1-AR-targeted autoimmune cardiomyopathy in the rat is characterized by the following: (a) progressive LV dilatation and dysfunction; (b) a relative decrease in LV wall thickness; and (c) downregulation of β1-AR, a feature that is also seen in human DCM (47). Our data suggest that both the induced and the transferred cardiomyopathic phenotypes must be attributed mainly to the mild but sustained receptor activation by stimulating anti–β1-ECII. This notion is further supported by the fact that serum cytokine levels were not significantly different between Ab-positive and corresponding control animals and that the selective downregulation of cardiac β1-AR in Ab-positive animals occurred in the absence of elevated catecholamines. The observation that both the induced and the transferred cardiomyopathic phenotypes developed within a similar time frame, however, remains open to speculation. Assuming a mild β1 agonistic behavior of the generated Ab’s in vivo (see Figure 3), it seems conceivable that in the first part of our experiment (immunization with β1-ECII) maximal circulating concentrations of anti–β1-ECII did not develop until 2–3 months after immunization (see Figure 1A). By contrast, in the second part of the study (transfer of anti–β1-ECII to naive animals) a lower concentration of Ab’s was active from the very beginning of the experiment. Thus, it is conceivable that differences in lag time of Ab generation and concentration between the two series result in similar time courses of disease development. For this point our study does not allow final conclusions, because echocardiograms were obtained at 3-month intervals only, making it difficult to retrospectively determine the exact time point of disease onset with one or the other protocol.
The aim of our study was to analyze the potential pathogenic significance of autoantibodies targeting cardiac β1-AR in DCM by testing the established postulates for autoimmune diseases in an animal model. Therefore, in the present work we focused on the immunological and functional characterization of the generated receptor Ab’s and on the morphological (echocardiography and anatomy) and functional characterization of the hearts from our rats, including a precise analysis of their cardiac membrane β1-AR (with experiments done at least in triplicate). After the binding experiments, only a faint amount of fresh-frozen cardiac tissue remained from some but not all of our rats, precluding further valid gene expression analyses in the frame of this study. By accomplishing the established postulates for pathogenesis of an autoimmune disease, however, the presented data here indicate that β1-AR–targeted autoimmune DCM should now be categorized with other known receptor Ab–mediated diseases such as Graves disease, myasthenia gravis, or type B insulin-resistant diabetes (48). Although carried out in an experimental animal model, our study might, therefore, also serve as a basis for more specific therapeutic approaches in Ab-positive human DCM patients. These would include pharmacological neutralization of functionally active anti–β1-AR Ab’s by β1-AR blockade and/or their elimination by nonselective (49, 50) or, perhaps more desirable, anti–β1-AR-selective immunoadsorption (51). In addition, the anti–β1-AR-producing B cells themselves might represent an interesting target for future therapies.
Acknowledgments
Our work was supported by the Deutsche Forschungsgemeinschaft (Grant Ja 706/2-1).
Footnotes
See the related Commentary beginning on page 1379.
Nonstandard abbreviations used: β1-adrenergic receptor (β1-AR); β1-AR second extracellular receptor loop (β1-ECII); body weight (BW); cAMP-dependent protein kinase (PKA); cardiac output (CO); dilated cardiomyopathy (DCM); glutathione-S-transferase (GST); human embryonal kidney (HEK); immunofluorescence microscopy (IFM); 125I-labeled cyanopindolol ([125I]-CYP); isobutylmethylxanthine (IBMX); left ventricular (LV); LV end-diastolic diameter (LVED); LV end-diastolic pressure (LVEDP); LV area (LVA); LV cavity area (LVCA); LV wall area (LVWA); peak change in LV pressure per time (interval) (dp/dtmax); velocity–time integral (VTI).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Richardson P, et al. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease ControlPrevention. Changes in mortality from heart failure, United States. J. Am. Med. Assoc. 1998;280:874–875. [PubMed] [Google Scholar]
- 4.Graham RM, Owens WA. Pathogenesis of inherited forms of dilated cardiomyopathy. N. Engl. J. Med. 1999;341:1759–1762. doi: 10.1056/NEJM199912023412309. [DOI] [PubMed] [Google Scholar]
- 5.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizio L, Regan TJ. Alcoholic cardiomyopathy. Cardiovasc. Drugs Ther. 1994;8:89–94. doi: 10.1007/BF00877094. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. doi: 10.1136/hrt.75.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luppi P, et al. Idiopathic dilated cardiomyopathy. A superantigen-driven autoimmune disease. Circulation. 1998;98:777–785. doi: 10.1161/01.cir.98.8.777. [DOI] [PubMed] [Google Scholar]
- 9.Limas CJ, et al. Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur. J. Clin. Invest. 2003;33:443–448. doi: 10.1046/j.1365-2362.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson U, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat. Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 11.Caforio AL, et al. Organ-specific cardiac autoantibodies in dilated cardiomyopathy — an update. Eur. Heart J. 1995;16(Suppl. O):68–70. doi: 10.1093/eurheartj/16.suppl_o.68. [DOI] [PubMed] [Google Scholar]
- 12.Matsui S, Fu M. Characteristic distribution of circulating autoantibodies against G-protein coupled receptors in patients with idiopathic dilated and hypertrophic cardiomyopathy. Int. J. Cardiol. 1996;54:143–147. doi: 10.1016/0167-5273(96)02591-0. [DOI] [PubMed] [Google Scholar]
- 13.Limas CJ. Cardiac autoantibodies in dilated cardiomyopathy. Circulation. 1997;95:1979–1980. doi: 10.1161/01.cir.95.8.1979. [DOI] [PubMed] [Google Scholar]
- 14.Jahns R, et al. Modulation of β1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J. Am. Coll. Cardiol. 2000;36:1280–1287. doi: 10.1016/s0735-1097(00)00881-0. [DOI] [PubMed] [Google Scholar]
- 15.Limas CJ, Goldenberg IF, Limas C. Influence of anti-β-receptor antibodies on cardiac adenylate cyclase in patients with idiopathic dilated cardiomyopathy. Am. Heart J. 1990;119:1322–1328. doi: 10.1016/s0002-8703(05)80182-6. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson Å, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the β1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–2767. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 17.Christ T, et al. Autoantibodies against the β1-adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J. Mol. Cell. Cardiol. 2001;33:1515–1525. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 18.Jahns R, et al. Autoantibodies activating human β1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–654. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 19.Chiale PA, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 20.Iwata M, et al. Autoantibodies against the second extracellular loop of β1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2001;37:418–424. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 21.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J. Am. Med. Assoc. 1957;164:1439–1447. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 22.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited) Immunol. Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson Y, et al. Antigenic analysis of the second extracellular loop of the human β-adrenergic receptors. Clin. Exp. Immunol. 1989;78:42–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Mobini R, et al. Probing the immunological properties of the extracellular domains of the human β1-adrenoceptor. J. Autoimmun. 1999;13:179–186. doi: 10.1006/jaut.1999.0310. [DOI] [PubMed] [Google Scholar]
- 25.Omerovic E, et al. Induction of cardiomyopathy in severe combined immunodeficiency mice by transfer of lymphocytes from patients with idiopathic dilated caediomyopathy. Autoimmunity. 2000;32:271–280. doi: 10.3109/08916930008994101. [DOI] [PubMed] [Google Scholar]
- 26.Frielle T, et al. Cloning of the cDNA for the human β1-adrenergic receptor. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahns R, et al. Probing human β1- and β2-adrenoceptors with domain-specific fusion protein antibodies. Eur. J. Pharmacol. 1996;316:111–121. doi: 10.1016/s0014-2999(96)00654-1. [DOI] [PubMed] [Google Scholar]
- 28.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol. Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 29.Roskoski R. Assays of protein kinase. Meth. Enzymol. 1983;99:3–7. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- 30.Litwin SE, Katz SE, Morgan JP, Douglas PS. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89:345–354. doi: 10.1161/01.cir.89.1.345. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-De Leon A, Roijkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J. Histochem. Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 32.Xiao RP, et al. Age-associated reductions in cardiac β1- and β2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J. Clin. Invest. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 34.Bland JM, Altmann DG. Statistical methods for assessing agreement between two methods of clinical assessment. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 35.Xiao RP, Lakatta EG. β1-adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ. Res. 1993;73:286–300. doi: 10.1161/01.res.73.2.286. [DOI] [PubMed] [Google Scholar]
- 36.Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac β2-adrenergic signal transduction. Circ. Res. 1999;85:1092–1100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 37.Woodiwiss AJ, et al. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation. 2001;103:155–160. doi: 10.1161/01.cir.103.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Rose NR. Infection, mimics, and autoimmune disease. J. Clin. Invest. 2001;107:943–944. doi: 10.1172/JCI12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limas CJ. Autoimmunity in dilated cardiomyopathy and the major histocompatibility complex. Int. J. Cardiol. 1996;54:113–116. doi: 10.1016/0167-5273(96)02587-9. [DOI] [PubMed] [Google Scholar]
- 40.Matsui S, et al. Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunized rabbits. J. Mol. Cell Cardiol. 1997;29:641–655. doi: 10.1006/jmcc.1996.0307. [DOI] [PubMed] [Google Scholar]
- 41.Iwata M, et al. Autoimmunity against the second extracellular loop of β1-adrenergic receptors induces β-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ. Res. 2001;88:578–586. doi: 10.1161/01.res.88.6.578. [DOI] [PubMed] [Google Scholar]
- 42.Scholz D, Diener W, Schaper J. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5:127–138. [PubMed] [Google Scholar]
- 43.Yan SM, Finato N, Di Loreto C, Beltrami CA. Nuclear size of myocardial cells in end-stage cardiomyopathies. Anal. Quant. Cytol. Histol. 1999;21:174–180. [PubMed] [Google Scholar]
- 44.Chien KR, Knowlton KU, Zhu H, Cien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiological response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 45.Davies CH, Harding SE, Poole-Wilson PA. Cellular mechanisms of contractile dysfunction in human heart failure. Eur. Heart J. 1996;17:189–198. doi: 10.1093/oxfordjournals.eurheartj.a014834. [DOI] [PubMed] [Google Scholar]
- 46.Kuan AP, Zuckier L, Liao L, Factor SM, Diamond B. Immunoglobulin isotype determines pathogenicity in antibody-mediated myocarditis in naive mice. Circ. Res. 2000;86:281–285. doi: 10.1161/01.res.86.3.281. [DOI] [PubMed] [Google Scholar]
- 47.Staudt A, et al. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation. 2001;103:2681–2686. doi: 10.1161/01.cir.103.22.2681. [DOI] [PubMed] [Google Scholar]
- 48.Fraser CM, Venter JC. Anti-receptor antibodies in human disease. J. Allergy Clin. Immunol. 1984;74:661–673. doi: 10.1016/0091-6749(84)90227-6. [DOI] [PubMed] [Google Scholar]
- 49.Müller J, et al. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation. 2000;101:385–391. doi: 10.1161/01.cir.101.4.385. [DOI] [PubMed] [Google Scholar]
- 50.Felix SB, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three-month results from a randomized study. J. Am. Coll. Cardiol. 2000;35:1590–1598. doi: 10.1016/s0735-1097(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 51.Wallukat G, Müller J, Hetzer R. Specific removal of β1-adrenergic antibodies directed against cardiac proteins from patients with idiopathic dilated cardiomyopathy [letter] N. Engl. J. Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]