Abstract
Polydactyly occurs in some chicken breeds, but the molecular mechanism remains incompletely understood. Combined genome-wide linkage analysis and association study (GWAS) for chicken polydactyly helps identify loci or candidate genes for the trait and potentially provides further mechanistic understanding of this phenotype in chickens and perhaps other species. The linkage analysis and GWAS for polydactyly was conducted using an F2 population derived from Beijing-You chickens and commercial broilers. The results identified two QTLs through linkage analysis and seven single-nucleotide polymorphisms (SNPs) through GWAS, associated with the polydactyly trait. One QTL located at 35 cM on the GGA2 was significant at the 1% genome-wise level and another QTL at the 1% chromosome-wide significance level was detected at 39 cM on GGA19. A total of seven SNPs, four of 5% genome-wide significance (P < 2.98 × 10−6) and three of suggestive significance (5.96 × 10−5) were identified, including two SNPs (GGaluGA132178 and Gga_rs14135036) in the QTL on GGA2. Of the identified SNPs, the eight nearest genes were sonic hedgehog (SHH), limb region 1 homolog (mouse) (LMBR1), dipeptidyl-peptidase 6, transcript variant 3 (DPP6), thyroid-stimulating hormone, beta (TSHB), sal-like 4 (Drosophila) (SALL4), par-6 partitioning defective 6 homolog beta (Caenorhabditis elegans) (PARD6B), coenzyme Q5 (COQ5), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, etapolypeptide (YWHAH). The GWAS supports earlier reports of the importance of SHH and LMBR1 as regulating genes for polydactyly in chickens and other species, and identified others, most of which have not previously been associated with limb development. The genes and associated SNPs revealed here provide detailed information for further exploring the molecular and developmental mechanisms underlying polydactyly.
Keywords: chicken, polydactyly, linkage analysis, GWAS, candidate genes
Polydactyly is a common limb malformation in human, mouse, chicken, and other vertebrates characterized by supernumerary digits. In chickens, where the usual number is four, polydactyly is the phenotype with a fifth digit on one or both feet (Warren 1944). Five digits is a breed characteristic in some chickens, including Beijing-You, Silkies, Dorking, Houdan, and Sultan. The chicken polydactyly locus was mapped to chromosome 2 (GGA2) through linkage analysis, in a region homologous to human Chr7q36 and mouse Chr5 (Pitel et al. 2000). This region is known to be important for human and mouse polydactyly traits and other limb malformation (Dundar et al. 2001; Dobbs et al. 2005; Lettice and Hill 2005). Huang et al. (2006) found that the T1254C polymorphism in exon 13 of LMBR1 was strongly associated with polydactyly in chickens. Another study has shown complete association of this same single-nucleotide polymorphism (SNP; ss161109890) with the polydactyly phenotype in a manner that was concordant with the dominant and incompletely penetrant nature of this trait (Dorshorst et al. 2010). This study also searched the highly conserved zone of polarizing activity regulatory sequence (ZRS) for other SNPs that might explain this trait and found none. Several polydactylous breeds were compared, with the Silkie and Sultan breeds both having this SNP whereas three other breeds with polydactyly did not (Faverolle, Houdan, and Dorking). Due to the history of these breeds, the authors suggested there may be more than one allele or locus controlling polydactyly in the chicken, although in all of these breeds polydactyly still behaves as a single gene trait (Dorshorst et al. 2010).
The causality of the ss161109890 SNP located within the ZRS was subsequently demonstrated using SHH allelic imbalance and electroporation of mutant ZRS experiments. The ectopic expression of SHH in the anterior zone of polarizing activity is consistent with known causes of polydactyly in other species (Dunn et al. 2011; Maas et al. 2011).
The related genes for polydactyly were identified to better understand the molecular and developmental mechanisms underlying polydactyly. Genes related to hedgehog signaling, sonic hedgehog (SHH), and limb region 1 homolog (mouse) (LMBR1) are important regulatory genes for limb development (Lettice et al. 2012) and polydactyly traits in human, mouse, and chicken (Clark et al. 2001; Maas and Fallon 2004; Sagai et al. 2004, 2005; Huang et al. 2006; Albuisson et al. 2010; Dunn et al. 2011). Other candidate genes (e.g., EN2, HOXA10-13, GLI3, WNT2, and WNT16) for the polydactyly trait were also found in different species (Gorbach et al. 2010). Quinn et al. (2012) identified a previously unrecognized role for Zic3 in regulating limb digit number via its modifying effect on GLI3 and SHH expression levels in mice. Kalsoom et al. 2013 have recently identified a novel zinc-finger gene (ZNF141) associated with autosomal-recessive postaxial polydactyly type A through whole-genome sequencing combined with homozygosity mapping and array comparative genomic hybridization analysis. These studies found the gene for one polydactyly allele/locus, but the molecular mechanism and other polydactyly alleles/loci remain incompletely understood (Robb and Delany 2012).
Both linkage analysis and genome-wide association studies (GWAS) can be used to map the quantitative trait loci (QTL) underlying complex traits. The GWAS, based on high-throughput SNP genotyping technologies, has revealed loci underlying a variety of interesting traits in domestic animals (Pearson and Manolio 2008) and it is more powerful than traditional linkage analysis in detecting genetic variants. In chickens, GWAS have been performed for growth and meat quality (Gu et al. 2011; Xie et al. 2012; Liu et al. 2013; Sun et al. 2013), egg production and quality (Liu et al. 2011), and other traits.
In the present study, both linkage analysis and GWAS were performed for the polydactyly trait using the Chinese Academy of Agricultural Science (CAAS) chicken F2 resource population derived from the cross between a local Chinese chicken breed (Beijing-You; BJY) and a commercial broiler line (Cobb-Vantress; CB) (Sun et al. 2013) to identify and provide information for allele/locus for polydactyly in chickens.
Materials and Methods
Animals and phenotypes
This research complied with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). The CAAS chicken F2 resource population was derived from a cross between BJY and CB chickens. The BJY is a Chinese indigenous breed with a 25−31% incidence of five digits on one or both feet. The CB (Cobb-Vantress) is a commercial broiler breed with four digits on each foot. Six BJY males were each mated to 12 CB females. Six males and 20 females of their offspring, the F1 generation, then produced the F2 chickens via artificial insemination and only nonrelated females to avoid inbreeding. The chickens from the three generations were raised in stair-step cages under the same routine conditions of nutrition and management at the conservation farm of the Institute of Animal Sciences (Sun et al. 2013).
A total of 400 chickens from three generation (including 367 F2 chickens from 20 full-sib families in five batches) were used. Blood was collected into acid citrate dextrose anticoagulant tubes from the brachial vein on d 56. The number of digits, four or five on one or both feet, was recorded. Two of six BJY males in the F0 generation had polydactyly. The pedigrees with polydactyly traits are shown in Supporting Information, Table S1.
Genotyping and SNP quality
Genomic DNA was extracted from blood samples using the phenol-chloroform method. Genotyping was performed with 50 ng/µL genomic DNA using the Illumina 60K Chicken SNP Beadchip by DNA LandMarks Inc. (Saint-Jean-sur-Richelieur, PQ, Canada). A total of 39 were excluded due to sample call rate <90%. A total of 13,985 SNPs were removed for failing to meet one or more of the following conditions: SNP call rate <90%, minor allele frequency <3%, Hardy-Weinberg test P of < 10-6 and SNPs with no assigned chromosome or linkage group. After quality control measures, 42,585 SNPs distributed among 29 chromosomes (including the Z chromosome) and one linkage group (LGE22) remained (all SNPs information see File S1). The average physical distance between two neighboring SNPs was approximately 20.4 kb (Sun et al. 2013).
Linkage analysis
The genetic linkage map was constructed using 19 full-sib families (six males and 12 females from F0, five males and 20 females from F1, and 148 males and 152 females from F2) and 42,585 SNPs with the improved version of CRI-MAP (2.503a, run in a 64-bit Unix system). This method has been described in previous studies (Groenen et al. 2009; Elferink et al. 2010). In genetic map construction, one full-family was excluded due to a computational problem.
To reduce the effect of linkage disequilibrium (LD) on the results, 6518 independent SNPs were acquired in all autosomal chromosomes using the indep-pairwise option, with a window size of 25 SNPs, a step of 5 SNPs, and r2 threshold of 0.2 (Sun et al. 2013). QTL analysis for the polydactyly trait used these same SNPs and web-based GridQTL software (Allen et al. 2012). A least-squares regression model was used for single QTL analysis, including the fixed effects of sex, hatch, and family, along with additive and dominance coefficients for the putative QTL. Significance thresholds were calculated using a permutation test (Churchill and Doerge 1994). A total of 10,000 permutations were computed to determine 5% and 1% chromosome-wide significance levels. 5% and 1% genome-wide significance levels were calculated following the Bonferroni correction:
Where Ga is the length genetic map of each chromosome and Gc is the length of the genetic map of all autosomal chromosomes. Confidence intervals for QTL positions were estimated by bootstrapping, as presented by Visscher et al. (1996).
Statistical analysis for GWAS
The population structure was assessed by multidimensional scaling (MDS) analysis using PLINK software 1.07 (Purcell et al. 2007), as described previously in detail (Gu et al. 2011 and Sun et al. 2013). Pairwise identity-by-state distances were calculated between all individuals using the 6518 independent SNP markers, and MDS components were obtained using the mds-plot option based on the identity-by-state matrix.
The GWAS for polydactyly with 328 chickens from the F2 generation, and 42,585 SNPs were performed using the compressed mixed linear model (Zhang et al. 2010) and carried out using Tassel 3.0 software (Bradbury et al. 2007). The statistical model was:
where Yijklmn are phenotypic values, µi is the common mean, C1j is the effect of the first principal component, Sk is the effect of sex, Bl is the effect of batch of hatching (l = 1 – 5), Gm is the effect of the SNP, Kn is the random effect of the relative kinship matrix, which was constructed by matrix simple matching coefficients based on the independent SNPs, and this step was followed by compression (Zhang et al. 2010), and eijklmn is the random residual effect.
The genome-wide significance P-value threshold was determined by the “LD adjusted’’ Bonferroni method (Duggal et al. 2008), which was calculated on the basis of the estimated number of independent markers and LD blocks in all chromosomes. The F2 population was estimated to have 16,760 “independent” tests, based on the “solid spine of LD” algorithm with a minimum D′ value of 0.8 calculated by Haploview 4.1 (Barrett et al. 2005). The two threshold P-values were established as 5.96 × 10-5 (1/16,760) for suggestive significance, and 2.98 × 10-6 (0.05/16,760) for 5% genome-wide significance. The Manhattan plot, showing genome-wide P-values of GWAS, was produced using R 2.13.2 software with the ‘‘gap’’ package (Zhao 2007).
Results
Linkage analysis
Before linkage analysis, the genetic linkage map was constructed using the CAAS chicken F2 population. A total of 42,585 SNP markers (each marker had 4−603 informative meiosis, average of 254), about 74% of all markers on the SNP beadchip, and 337 individuals from three generation and 19 full-sib families were used to construct the linkage map. As shown in Table 1, the total length of the sex-average map was 3040.8 cM, and there was no significant difference in total length between the male and female sex-specific maps (2929.2 and 2930.3 cM). The recombination rate of the map was 2.9 cM/Mb. The detailed information for the map is shown in Table S2.
Table 1. Linkage map lengths and recombination rates.
| Chromosome | Length, Mba | Sex-Average, cM | Sex-Specific, cM | Recombination Rate, cM/Mb | |
|---|---|---|---|---|---|
| Female | Male | ||||
| GGA1 | 200.9 | 450.2 | 460.5 | 440.8 | 2.2 |
| GGA2 | 154.8 | 308.4 | 328.7 | 288.5 | 2.0 |
| GGA3 | 113.6 | 253.4 | 269.9 | 237.3 | 2.2 |
| GGA4 | 94.2 | 199.9 | 211.2 | 188.7 | 2.1 |
| GGA5 | 62.2 | 155.6 | 163.3 | 148.6 | 2.5 |
| GGA6 | 37.3 | 102.7 | 110.2 | 95.3 | 2.8 |
| GGA7 | 38.3 | 103.9 | 102.8 | 105.2 | 2.7 |
| GGA8 | 30.6 | 90.9 | 92.5 | 89.4 | 3.0 |
| GGA9 | 25.5 | 98.8 | 107.1 | 90.1 | 3.9 |
| GGA10 | 22.5 | 82.2 | 86.2 | 78.3 | 3.7 |
| GGA11 | 21.9 | 63.9 | 68.2 | 59.6 | 2.9 |
| GGA12 | 20.5 | 75.1 | 79.5 | 70.7 | 3.7 |
| GGA13 | 18.9 | 60.5 | 66.3 | 55.6 | 3.2 |
| GGA14 | 15.8 | 67.8 | 71.3 | 64.4 | 4.3 |
| GGA15 | 13 | 53.7 | 58.1 | 49.4 | 4.1 |
| GGA16 | 0.4 | 0.0 | 0.0 | 0.0 | − |
| GGA17 | 11.2 | 49.8 | 51.5 | 48.2 | 4.4 |
| GGA18 | 10.9 | 48.0 | 48.7 | 47.4 | 4.4 |
| GGA19 | 9.9 | 51.4 | 55.9 | 46.8 | 5.2 |
| GGA20 | 13.9 | 54.3 | 56.1 | 52.5 | 3.9 |
| GGA21 | 6.9 | 48.5 | 48.4 | 48.7 | 7.0 |
| GGA22 | 3.9 | 51.6 | 52.0 | 51.3 | 13.2 |
| GGA23 | 6 | 51.5 | 48.9 | 54.1 | 8.6 |
| GGA24 | 6.4 | 47.9 | 44.3 | 51.5 | 7.5 |
| GGA25 | 2 | 58.4 | 63.6 | 54.1 | 29.2 |
| GGA26 | 5.1 | 48.3 | 47.5 | 49.2 | 9.5 |
| GGA27 | 4.7 | 49.6 | 47.6 | 51.6 | 10.5 |
| GGA28 | 4.5 | 48.7 | 53.1 | 44.4 | 10.8 |
| LGE22 | 0.9 | 37.6 | 35.5 | 40.5 | 41.8 |
| GGZ | 74.6 | 228.3 | - | 228.3 | − |
| Total autosomal | 956.7 | 2812.5 | 2929.2 | 2702.0 | 2.9 |
| Total length | 1031.3 | 3040.8 | 2929.2 | 2930.3 | 2.9 |
Physical length of the chromosome was based on the position of the last marker in the WASHUC2 build (2006).
A total of three hundred chickens from the F2 generation and 6518 independent SNPs (Table S3) in autosomal chromosomes were used in the linkage analysis for polydactyly. Quantitative trait loci locations and effects for the polydactyly trait are shown in Table 2. One QTL located at 35 cM (confidence interval from 23 to 63 cM) on GGA2 was significant at the 1% genome-wise level (Figure S1); it explained 9.34% of the phenotypic variation. Another QTL at the 1% chromosome-wide significance level was detected at 39 cM on GGA19 and it explained 5.35% of the phenotypic variation.
Table 2. Polydactyly trait loci mapped in the chicken CAAS F2 resource population.
| Location, cM/Mb | Confidence Interval, cM/Mb | F Ratios | A ± SE | D ± SE | PV (%) |
|---|---|---|---|---|---|
| GGA2 (35/0.8) | 23.0−63.0/0.5−2.2 | 15.88** | 0.17 ± 0.03 | 0.05 ± 0.04 | 9.34 |
| GGA19 (39/0.5) | 22.0−45.0/0.3−0.7 | 9.06* | 0.03 ± 0.03 | 0.18 ± 0.06 | 5.35 |
CAAS, Chinese Academy of Agricultural Science; A, additive; D, dominance; PV, percent of phenotypic variance explained by the QTL; PV%, [(MSR − MSF)/MSR] × 100 where MSR is residual mean square in the reduced model and MSF is the residual mean square in the full model; QTL, quantitative trait loci.
1% chromosome-wide significance.
1% genome-wide significance.
GWAS results
After 39 samples were excluded because of low sample call rate and one lost phenotypic record, 327 chickens (286 with four digits, and 41 with five digits) from the F2 generation were used for further analysis.
The MDS analysis of the 16,760 SNPs using the first two MDS components showed that the chickens within each full-sib family were clustered together (Sun et al. 2013). To correct for population stratification, the first MDS component was used as a covariate in the model as suggested (Price et al. 2006; Gu et al. 2011; Sun et al. 2013).
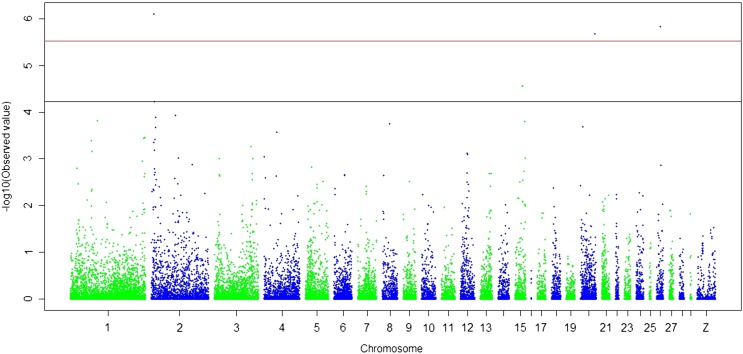
As shown in Figure 1 and Table 3, four SNPs associated with polydactyly were of genome-wide significance, and three SNPs had suggestive significance. These SNPs explained 6−9% of the phenotypic variation.
Figure 1.
Manhattan plot showing the association of all single-nucleotide polymorphisms with the polydactyly trait. Single-nucleotide polymorphisms are plotted on the x-axis according to their position on each chromosome against association with these traits on the y-axis (shown as -log10 P-value). The black line indicates genome-wise significance of suggestive association (P = 5.96 × 10−5), and the red line indicates genome-wise 5% significance with a P-value threshold of 2.98 × 10−6.
Table 3. Significant SNPs associated with chicken polydactyly.
| Chromosome | SNPs | Position, bp | P Valuea | P Adjustb | MAF | Rb | Nearest Gene |
|---|---|---|---|---|---|---|---|
| 2 | GGaluGA132178 | 6641213 | 7.94E-07 | 0.01 | C/A-0.10 | 0.09 | DPP6 D 9.5 kb |
| 2 | Gga_rs14135036 | 7628179 | 5.96E-05 | 1.00 | A/G-0.31 | 0.06 | SHH D 113.9 kb; LMBR1 U 240.7 kb |
| 15 | Gga_rs15023766 | 9240112 | 2.74E-05 | 0.46 | C/A-0.17 | 0.07 | COQ5 U 22.9 kb |
| 15 | Gga_rs14093104 | 8755174 | 2.74E-05 | 0.46 | G/A-0.17 | 0.07 | YWHAH U 2.8 kb |
| 20 | GGaluGA181512 | 12887215 | 2.08E-06 | 0.03 | C/A-0.19 | 0.09 | SALL4 U 176.3 kb |
| 20 | Gga_rs16175635 | 12900524 | 2.08E-06 | 0.03 | A/G-0.19 | 0.09 | PARD6B D 176.4 kb |
| 26 | Gga_rs13606164 | 2579119 | 1.47E-06 | 0.02 | A/G-0.14 | 0.09 | TSHB U 35.6 kb |
SNP, single-nucleotide polymorphism; MAF, minor allele frequency of the first allele; R2, the proportion of phenotypic variation explained by the SNP; U, the SNP is upstream of the gene and D is downstream.
P value from mixed linear model models.
LD-adjusted Bonferroni P value.
The most significant SNP, GGaluGA132178, was located on GGA2 at 6641.2 kb (P = 7.94 × 10-7), 9.5 kb downstream of dipeptidyl-peptidase 6, transcript variant 3 (DPP6). Another SNP, Gga_rs14135036, located on GGA2 at 7628.2 kb was suggestively associated with polydactyly (P = 5.96 × 10-5). This SNP was in close proximity to other candidate genes for polydactyly, 113.9 kb downstream of sonic hedgehog (SSH) and 240.7 kb upstream of limb region 1 homolog (mouse) (LMBR1).
The SNP Gga_rs13606164, located at 2579.1 kb on GGA26, is 35.6 kb downstream of the thyroid stimulating hormone, beta (TSHB) gene, had highly significant association with the polydactyly (P < 2.98 × 10-6). Two other SNPs (GGaluGA181512 and Gga_rs16175635), located within a 13.3-kb segment on GGA20, were of genome-wide significance (P < 2.98 × 10-6); one was 176.3 kb upstream of the Sal-like 4 (Drosophila) (SALL4) gene and the other was 176.4 kb from the Par-6 partitioning defective 6 homolog beta (C. elegans), (PARD6B) gene.
Two SNPs (Gga_rs15023766 and Gga_rs14093104) with suggestive association (both p-values were 2.74 × 10−5) with polydactyly were located within a 484.99-kb segment on GGA15; one was 22.9 kb downstream of coenzyme Q5 (COQ5), and the other was 2.8 kb upstream of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, etapolypeptide, (YWHAH).
Discussion
In the present study, the QTLs and candidate genes for polydactyly were identified in chickens by the use of both linkage analysis and GWAS. A high-density genetic linkage map is the basis for linkage analysis. The current map is comprised of 29 chromosomes and one linkage group, with a total size of 3040.8 cM. The map size was similar with the high-resolution chicken genetic map using 13340 SNPs and 1619 chickens (Elferink et al. 2010). The present map ensures accuracy and precision of linkage analysis. In the present study, two QTLs (one located at 35 cM on GGA2 and another located at 39 cM on GGA19) were identified through linkage analysis. The QTL on GGA2 was also found in previous studies in chicken (Dunn et al. 2011; Yang et al. 2013) and other species.
For GWAS analysis, the compressed mixed linear model was one of the most effective methods for controlling false-positive results in GWAS (Huang et al. 2010; Zhang et al. 2010) and ensured reliability of the results. The population-based GWAS has more power than traditional family-based linkage analysis (Klein 2007) and it can detect more SNPs of interest that are in or near potential candidate genes. The GWAS results obtained here were in accord with the linkage analysis on GGA2 and obtained more detail of relevant SNPs. The GWAS also found additional potential loci on other chromosomes. Seven significant SNPs were identified near the potential candidate genes or the important regulatory genes for polydactyly. The genes, SHH and LMBR1 on GGA2, already known to be important for polydactyly in chickens and other species, were identified again here. Variations of LMBR1 were found to influence digits numbers in chickens (Huang et al. 2006; Hang et al. 2007). Both LMBR1 and SHH, were shown to be essential for normal development of the chicken limb (Lettice et al. 2003; Lettice and Hill 2005; Dunn et al. 2011). The SHH and LMBR1 were also in the QTL region identified here by linkage analysis. Another gene DPP6, near the QTL peak in GGA2, was also identified here. Mutations of DPP6 produce human disease and cause movement disorders (Tanaka et al. 2011).
The gene SALL4 encodes a putative zinc finger transcription factor and plays a role in modulating embryonic stem cell pluripotency and early embryonic development (Zhang et al. 2006). Variations of SALL4 are associated with upper limb abnormality in humans (Kohlhase et al. 2002; Paradisi and Arias 2007). Given what is known of the roles of this gene in limb development, the present GWAS findings suggest that it is a potential candidate gene for polydactyly in chickens.
Four other genes, TSHB, PARD6B, COQ5, and YWHAH, are involved in growth and development, cell differentiation, or signal transduction (Dibrov et al. 1997; Phillips 2004; Nolan et al. 2008; Grover et al. 2009; Alarcon 2010), but there has been no suggestion that they are involved in limb development. The present results indicate that they might be potential candidate genes for polydactyly and so they are deserving of further study in this regard.
In conclusion, two QTLs and eight genes (SHH, LMBR1, DPP6, TSHB, SALL4, PARD6B, COQ5, and YWHAH) were identified through combined linkage analysis and GWAS for the polydactyly phenotype in chickens. In addition to confirming earlier studies in chickens and other species that SHH and LMBR1 are important regulating genes for polydactyly, the other genes identified here provide additional information for potentially exposing the molecular mechanisms and developmental biology underlying polydactyly in chickens. As new candidate genes, especially the SALL gene, they will be targets for future work.
Supplementary Material
Acknowledgements
We acknowledge W. Bruce Currie (Emeritus Professor, Cornell University) for his suggestions on presentation. The research was supported by grants: National Key Technology R&D Program (2011BAD28B03), National High-tech R&D Program (2011AA100301), the Agricultural Science and Technology Innovation Program (ASTIP-IAS04), and Longyan University Scientific Research Fund for the Doctoral Young Scholars.
Footnotes
Communicating editor: D.- J. De Koning
Literature Cited
- Alarcon V. B., 2010. Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biol. Reprod. 83: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuisson J., Isidor B., Giraud M., Pichon O., Marsaud T., et al. , 2010. Identification of two novel mutations in Shh long-range regulator associated with familial pre-axial polydactyly. Clin. Genet. 79: 371–377 [DOI] [PubMed] [Google Scholar]
- Allen, J., D. Scott, M. Illingworth, B. Dobrzelecki, D. Virdee et al., 2012 CloudQTL: evolving a bioinformatics application to the cloud. Digital Research, September 10−12, pp. 1-4, Oxford, UK. [Google Scholar]
- Barrett J., Fry B., Maller J., Daly M., 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265 [DOI] [PubMed] [Google Scholar]
- Bradbury P. J., Zhang Z., Kroon D. E., Casstevens T. M., Ramdoss Y., et al. , 2007. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23: 2633–2635 [DOI] [PubMed] [Google Scholar]
- Churchill G. A., Doerge R. W., 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. M., Marker P. C., Roessler E., Dutra A., Schimenti J. C., et al. , 2001. Reciprocal mouse and human limb phenotypes caused by gain-and loss-of-function mutations affecting Lmbr1. Genetics 159: 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibrov E., Robinson K. M., Lemire B. D., 1997. The COQ5 gene encodes a yeast mitochondrial protein necessary for ubiquinone biosynthesis and the assembly of the respiratory chain. J. Biol. Chem. 272: 9175–9181 [DOI] [PubMed] [Google Scholar]
- Dobbs M. B., Dietz F. R., Gurnett C. A., Morcuende J. A., Steyers C. M., et al. , 2005. Localization of dominantly inherited isolated triphalangeal thumb to chromosomal region 7q36. J. Orthop. Res. 18: 340–344 [DOI] [PubMed] [Google Scholar]
- Dorshorst B., Okimoto R., Ashwell C., 2010. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the Silkie chicken. J. Hered. 101: 339–350 [DOI] [PubMed] [Google Scholar]
- Duggal P., Gillanders E. M., Holmes T. N., Bailey-Wilson J. E., 2008. Establishing an adjusted p-value threshold to control the family-wide type 1 error in genome wide association studies. BMC Genomics 9: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundar M., Gordon T. M., Ozyazgan I., Oguzkaya F., Ozkul Y., et al. , 2001. A novel acropectoral syndrome maps to chromosome 7q36. J. Med. Genet. 38: 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn I. C., Paton I. R., Clelland A. K., Sebastian S., Johnson E. J., et al. , 2011. The chicken polydactyly (Po) locus causes allelic imbalance and ectopic expression of Shh during limb development. Dev. Dyn. 240: 1163–1172 [DOI] [PubMed] [Google Scholar]
- Elferink M. G., van As P., Veenendaal T., Crooijmans R. P., Groenen M. A., 2010. Regional differences in recombination hotspots between two chicken populations. BMC Genet. 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach D., Mote B., Totir L., Fernando R., Rothschild M., 2010. Polydactyl inheritance in the pig. J. Hered. 101: 469–475 [DOI] [PubMed] [Google Scholar]
- Groenen M. A., Wahlberg P., Foglio M., Cheng H. H., Megens H. J., et al. , 2009. A high-density SNP-based linkage map of the chicken genome reveals sequence features correlated with recombination rate. Genome Res. 19: 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover D., Verma R., Goes F. S., Mahon P. L. B., Gershon E. S., et al. , 2009. Family‐based association of YWHAH in psychotic bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150: 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Feng C., Ma L., Song C., Wang Y., et al. , 2011. Genome-wide association study of body weight in chicken F2 resource population. PLoS ONE 6: e21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang Y., Chen W., Deng X., Li N., Qiu X., et al. , 2007. The association of single nucleotide polymorphisms in chicken Lmbr1 intron with chicken polydactyl. Scientia Agricultura Sinica 40: 1254–1259 [Google Scholar]
- Huang X., Wei X., Sang T., Zhao Q., Feng Q., et al. , 2010. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42: 961–967 [DOI] [PubMed] [Google Scholar]
- Huang Y. Q., Deng X. M., Du Z. Q., Qiu X., Du X., et al. , 2006. Single nucleotide polymorphisms in the chicken Lmbr1 gene are associated with chicken polydactyly. Gene 374: 10–18 [DOI] [PubMed] [Google Scholar]
- Kalsoom U. E., Klopocki E., Wasif N., Tariq M., Khan S., et al. , 2013. Whole exome sequencing identified a novel zinc-finger gene ZNF141 associated with autosomal recessive postaxial polydactyly type A. J. Med. Genet. 50: 47–53 [DOI] [PubMed] [Google Scholar]
- Klein R. J., 2007. Power analysis for genome-wide association studies. BMC Genet. 8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J., Heinrich M., Schubert L., Liebers M., Kispert A., et al. , 2002. Okihiro syndrome is caused by SALL4 mutations. Hum. Mol. Genet. 11: 2979–2987 [DOI] [PubMed] [Google Scholar]
- Lettice L. A., Hill R. E., 2005. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr. Opin. Genet. Dev. 15: 294–300 [DOI] [PubMed] [Google Scholar]
- Lettice L. A., Heaney S. J. H., Purdie L. A., Li L., De Beer P., et al. , 2003. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12: 1725–1735 [DOI] [PubMed] [Google Scholar]
- Lettice L. A., Williamson I., Wiltshire J. H., Peluso S., Devenney P. S., et al. , 2012. Opposing functions of the ETS factor family define Shh spatial expression in limb buds and underlie polydactyly. Dev. Cell 22: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Sun Y., Zhao G., Wang F., Wu D., et al. , 2013. Genome-wide association study identifies loci and candidate genes for body composition and meat quality traits in Beijing-You chickens. PLoS ONE 8: e61172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li D., Liu J., Chen S., Qu L., et al. , 2011. A genome-wide SNP scan reveals novel loci for egg production and quality traits in white leghorn and brown-egg dwarf layers. PLoS One 6: e28600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S. A., Fallon J. F., 2004. Isolation of the chicken Lmbr1 coding sequence and characterization of its role during chick limb development. Dev. Dyn. 229: 520–528 [DOI] [PubMed] [Google Scholar]
- Maas S. A., Suzuki T., Fallon J. F., 2011. Identification of spontaneous mutations within the long‐range limb‐specific Sonic hedgehog enhancer (ZRS) that alter Sonic hedgehog expression in the chicken limb mutants oligozeugodactyly and silkie breed. Dev. Dyn. 240: 1212–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M. E., Aranda V., Lee S., Lakshmi B., Basu S., et al. , 2008. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 68: 8201–8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi I., Arias S., 2007. IVIC syndrome is caused by a c. 2607delA mutation in the SALL4 locus. Am. J. Med. Genet. A. 143: 326–332 [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Manolio T. A., 2008. How to interpret a genome-wide association study. JAMA 299: 1335–1344 [DOI] [PubMed] [Google Scholar]
- Phillips J. A., 2004. Genetics of growth retardation. J. Pediatr. Endocrinol. Metab. 17: 385–399 [PubMed] [Google Scholar]
- Pitel F., Bergé R., Coquerelle G., Crooijmans R., Groenen M. A. M., et al. , 2000. Mapping the Naked Neck (NA) and Polydactyly (PO) mutants of the chicken with microsatellite molecular markers. Genet. Sel. Evol. 32: 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. L., Patterson N. J., Plenge R. M., Weinblatt M. E., Shadick N. A., et al. , 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38: 904–909 [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., et al. , 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. E., Haaning A., Ware S. M., 2012. Preaxial polydactyly caused by Gli3 haploinsufficiency is rescued by Zic3 loss of function in mice. Hum. Mol. Genet. 21: 1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb E., Delany M., 2012. The expression of preaxial polydactyly is influenced by modifying genetic elements and is not maintained by chromosomal inversion in an avian biomedical model. Cytogenet. Genome Res. 136: 50–68 [DOI] [PubMed] [Google Scholar]
- Sagai T., Masuya H., Tamura M., Shimizu K., Yada Y., et al. , 2004. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog (Shh). Mamm. Genome 15: 23–34 [DOI] [PubMed] [Google Scholar]
- Sagai T., Hosoya M., Mizushina Y., Tamura M., Shiroishi T., 2005. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development 132: 797–803 [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhao G., Liu R., Zheng M., Hu Y., et al. , 2013. The identification of 14 new genes for meat quality traits in chicken using a genome-wide association study. BMC Genomics 14: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Syu A., Ishiguro H., Inada T., Horiuchi Y., et al. , 2011. DPP6 as a candidate gene for neuroleptic-induced tardive dyskinesia. Pharmacogenomics J. 13: 27–34 [DOI] [PubMed] [Google Scholar]
- Visscher P. M., Thompson R., Haley C. S., 1996. Confidence intervals in QTL mapping by bootstrapping. Genetics 143: 1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D., 1944. Inheritance of polydactylism in the fowl. Genetics 29: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Luo C., Zhang C., Zhang R., Tang J., et al. , 2012. Genome-wide association study identified a narrow chromosome 1 region associated with chicken growth traits. PLoS ONE 7: e30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Chen Y., Gu C., Zhou Z., He C., et al. , 2013. Polydactyly mutation is linked with chromosome 2p region in Beijing fatty chicken. Anim. Genet. 44: 118–119 [DOI] [PubMed] [Google Scholar]
- Zhang J., Tam W. L., Tong G. Q., Wu Q., Chan H. Y., et al. , 2006. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol. 8: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Ersoz E., Lai C. Q., Todhunter R. J., Tiwari H. K., et al. , 2010. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42: 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. H., 2007. gap: Genetic analysis package. J. Stat. Softw. 23: 1–18 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.