Abstract
Introduction
The objective of this study was to evaluate the efficacy and safety of vildagliptin, a potent dipeptidyl peptidase-4 inhibitor, as an add-on to metformin in Japanese patients with type 2 diabetes mellitus (T2DM).
Methods
This multicenter, 12-week, randomized, double-blind, placebo-controlled, parallel-arm study compared vildagliptin 50 mg bid with placebo in T2DM patients who were inadequately controlled [glycosylated hemoglobin (HbA1c) 7.0–10.0%] on a stable daily dose of metformin monotherapy (250 mg bid or 500 mg bid).
Results
A total of 139 patients were randomized to receive either vildagliptin (n = 69) or placebo (n = 70). Patient demographics were comparable between the groups at baseline. After 12 weeks of treatment, adjusted mean change in HbA1c was −1.1% in the vildagliptin group (baseline 8.0%) and −0.1% in the placebo group (baseline 8.0%), with a between-treatment difference of −1.0% (P < 0.001). Vildagliptin showed a similar reduction in HbA1c of −1.1% for both the subpopulations of patients receiving metformin 250 mg bid or 500 mg bid (P < 0.001 vs. baseline). Significantly more patients in the vildagliptin group achieved an HbA1c target of ≤6.5% (30.9%) and <7.0% (64.1%) compared with the placebo group (P < 0.001). The between-treatment difference in adjusted mean change in fasting plasma glucose was −1.6 mmol/L (P < 0.001) in favor of vildagliptin. Patients in the vildagliptin and placebo groups reported comparable incidences of adverse events (44.1% vs. 41.4%). No deaths or hypoglycemic events were reported in the study.
Conclusions
Vildagliptin 50 mg bid added to metformin improved glycemic control without any tolerability issues and hypoglycemia in Japanese patients with T2DM inadequately controlled on metformin monotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-014-0059-x) contains supplementary material, which is available to authorized users.
Keywords: Antidiabetic drug, Dipeptidyl peptidase-4 inhibitor, Glycemic control, Metformin, Randomized trial, Type 2 diabetes mellitus, Vildagliptin
Introduction
In Japan, the estimated number of individuals with type 2 diabetes mellitus (T2DM) is approximately 7.1 million, which is the ninth largest prevalence in the world [1]. In recent years, the prevalence of T2DM in Japan has increased due to lifestyle changes, genetic predisposition, and an aging population [2, 3]. Most of the Japanese T2DM patients are non-obese with an average body mass index (BMI) of 23–25 kg/m2, impaired insulin secretion plays a key role in the development of T2DM in these patients [4].
Despite major advances in the management of T2DM and availability of a range of antidiabetic agents, evidence suggests that up to ~60% of patients in Japan [5] fail to achieve the recommended target of glycosylated hemoglobin (HbA1c) levels <7.0% [6].
Metformin is one of the commonly used oral antidiabetic agents (OADs) in Japan. Metformin improves blood glucose levels primarily by inhibiting hepatic glucose production and also improving insulin sensitivity in the liver and skeletal muscles [7]. However, due to the progressive nature of T2DM, long-term glycemic control is difficult to achieve with a single agent, thus often requiring addition of further agents. Addition of a dipeptidyl peptidase-4 (DPP-4) enzyme inhibitor with metformin is beneficial due to their complementary mechanisms of action [8].
Vildagliptin, a potent and selective DPP-4 inhibitor, increases the active levels of incretin hormones, glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide (GIP), thereby improving pancreatic α- and β-cell sensitivity to glucose [9]. In large-scale clinical trials, vildagliptin improved glycemic control when given as monotherapy [10] or in combination with metformin [11], sulfonylurea [12], thiazolidinedione [13] or insulin [14], with low risk of hypoglycemia and weight gain. Vildagliptin 50 mg bid showed notable improvement in blood glucose levels and better tolerability compared with placebo [15] or voglibose [16] in Japanese patients with T2DM inadequately controlled on diet and exercise. Combination therapy of vildagliptin with low-dose (500 mg bid) and high-dose (1,000 mg bid) metformin showed improved glycemic control compared with individual monotherapies in a large global study [17]. The high dose of metformin (>750 mg/day) was approved in Japan in 2010. However, there are limited clinical data on the use of DPP-4 inhibitors in combination with metformin (>750 mg/day) in Japanese patients with T2DM. The aim of the present study was to evaluate the efficacy and safety of vildagliptin as add-on therapy in Japanese patients with T2DM inadequately controlled with metformin 500 or 1,000 mg/day. The study was conducted to support registration of the fixed-dose combination of vildagliptin and metformin for the treatment of T2DM in Japan.
Materials and Methods
Study Design
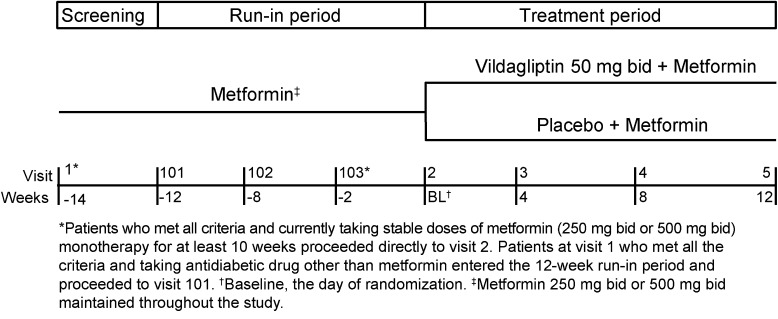
This was a 12-week, multicenter, randomized, double-blind, parallel-group, placebo-controlled study conducted across 20 centers in Japan in patients with T2DM inadequately controlled on metformin and diet/exercise. Following a screening period (visit 1), eligible patients who were on a stable daily dose of metformin (250 mg bid or 500 mg bid) for at least 10 weeks proceeded directly to randomization (baseline, visit 2) to receive either vildagliptin 50 mg bid or placebo as add-on to metformin in a 1:1 ratio. Patients taking OADs other than metformin were switched to either metformin 250 mg bid or 500 mg bid at the investigator’s discretion and were randomized after completing a 12-week run-in period (Fig. 1). This was followed by three scheduled visits from baseline (weeks 4, 8, and 12) during which efficacy and tolerability were assessed. Randomization was stratified to adjust for metformin dose in 1:1 ratio in both the treatment groups. The dose of metformin remained unchanged throughout the study and no rescue medication (additional OADs or insulin) was allowed. Patients with unsatisfactory therapeutic effect [fasting plasma glucose (FPG) ≥15.0 mmol/L] were to be discontinued from the study.
Fig. 1.
Study design
Study Population
The study enrolled men and women with T2DM, aged ≥20 to <75 years, BMI ≥20 to ≤35 kg/m2, baseline HbA1c values ≥7.0% to ≤10.0%, who were inadequately controlled on diet, exercise and metformin monotherapy. The patients were required to be on a stable daily dose of metformin 250 mg bid or 500 mg bid for at least 10 weeks prior to randomization.
The key exclusion criteria included history of type 1 diabetes, diabetes due to pancreatic injury or secondary forms, acute metabolic complications such as ketoacidosis or lactic acidosis, liver diseases such as cirrhosis or hepatitis, impaired renal function, congestive heart failure (New York Heart Association Class III or IV), myocardial infarction, stroke or transient ischemic attacks in the past 6 months. Patients with any of the following laboratory abnormalities at baseline were excluded: FPG ≥15 mmol/L; alanine transaminase, aspartate transaminase, or total bilirubin >2 times the upper limit of normal; and fasting triglycerides >5.7 mmol/L.
Study Endpoints and Assessments
The primary efficacy endpoint was the change in HbA1c from baseline to week 12 or the study endpoint. The key secondary efficacy endpoint was change in HbA1c from baseline to study endpoint within subpopulations of patients treated with vildagliptin and metformin (250 mg bid or 500 mg bid). Other secondary efficacy endpoints included percentage of patients (responder rate) achieving predefined HbA1c targets (≤6.5%, <7.0%, and reductions of ≥0.5% and ≥1.0%) and change in FPG levels after 12 weeks of treatment. Changes in HbA1c (reported in National Glycohemoglobin Standardization Program units) and FPG were assessed at each scheduled visit (weeks 0, 4, 8, and 12).
Adverse events (AEs) and serious AEs (SAEs) were recorded at each visit, and were assessed for severity, duration, and suspected relationship to the study drug. Standard hematology, biochemistry, liver function tests, urinalysis, vital signs, and body weight were measured at the screening visit and at weeks 0, 4, 8, and 12. Electrocardiograms were recorded at screening and at the last study visit (week 12). Fasting lipid profile was assessed at baseline and at the last study visit. All the patients were provided with a calibrated home glucose monitor and were instructed regarding its use. The patients were educated regarding hypoglycemic symptoms, possible triggers and were asked to record hypoglycemic event in a study diary. Hypoglycemia was defined as symptoms suggestive of hypoglycemia that was further confirmed by a self-monitored blood glucose measurement of <3.1 mmol/L. The event was considered grade 1 if the patient was able to initiate self-treatment, and grade 2 if the patient required assistance of another person or hospitalization. All the laboratory assessments were performed at a central laboratory (Mitsubishi Chemical Medience Corporation, Japan).
Statistical Analysis
A total of 136 patients (68 patients per group) were to be randomized (1:1) to achieve a target sample size of 128 patients (64 per group), assuming a dropout rate of 5%. This sample size would ensure at least 92% power to detect a clinically relevant between-group difference of 0.6% absolute units in HbA1c change from baseline, assuming a one-sided significance level of 0.025, to demonstrate the superiority of vildagliptin 50 mg bid over placebo as add-on to metformin in reducing HbA1c after 12 weeks of treatment. Moreover, randomization was stratified by metformin dose to ensure that patients on metformin 250 mg bid and 500 mg bid each constituted ~50% of the randomized population. The planned sample size of 136 patients (34 patients in each metformin subpopulation in the vildagliptin group) would provide at least 90% power to detect a statistically significant reduction in HbA1c of 0.6% from baseline in each metformin subgroup (250 mg bid or 500 mg bid), assuming a one-sided significance level of 0.025.
The primary and secondary efficacy analyses were based on the full analysis set, which included all randomized patients who received at least one dose of the study drug and had at least one post-randomization efficacy parameter assessment. Changes in HbA1c and FPG from baseline to study endpoint were analyzed using the analysis of covariance model (ANCOVA), with treatment groups and metformin dose as classification variables and baseline HbA1c as covariate. The study endpoint is the final available post-randomization assessment value at any visit (scheduled or unscheduled) up to final visit (week 12). The between-treatment difference in HbA1c and FPG was also analyzed using ANCOVA. Change in HbA1c from baseline to study endpoint within the metformin subpopulations was analyzed using a paired t test. Missing data because of early discontinuation were handled using the last observation carried forward method. The impact of various baseline characteristics (age, gender, BMI, HbA1c, and FPG) on absolute change in HbA1c from baseline to endpoint was analyzed using descriptive statistics. The proportion of responders (HbA1c ≤6.5% at endpoint, HbA1c <7% at endpoint, and reductions in HbA1c ≥0.5% and ≥1%) in each treatment group was computed and compared using the Chi-square test. The data analysis for this study was carried out using SAS software (version 9.2, SAS Institute Inc., Cary, NC, USA). The safety set consisted of all patients who received at least one dose of the study drug. Safety data were summarized descriptively by treatment. The incidences of treatment-emergent AEs were summarized by system organ class (SOC), preferred term (PT), severity, and relationship to the study drug. AEs were coded by primary SOC and PT according to Medical Dictionary for Drug Regulatory Activities (MedDRA version 15.1).
Ethics and Good Clinical Practice
The study protocol was reviewed and approved by the Independent Ethics Committee/Institutional Review Board at each center. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), the Helsinki Declaration of 1975, as revised in 2000 and 2008 and Good Clinical Practice guidelines. Informed consent was obtained from all patients for being included in the study. The study is registered with ClinicalTrials.gov, identifier: NCT01497522.
Results
Patient Disposition and Baseline Characteristics
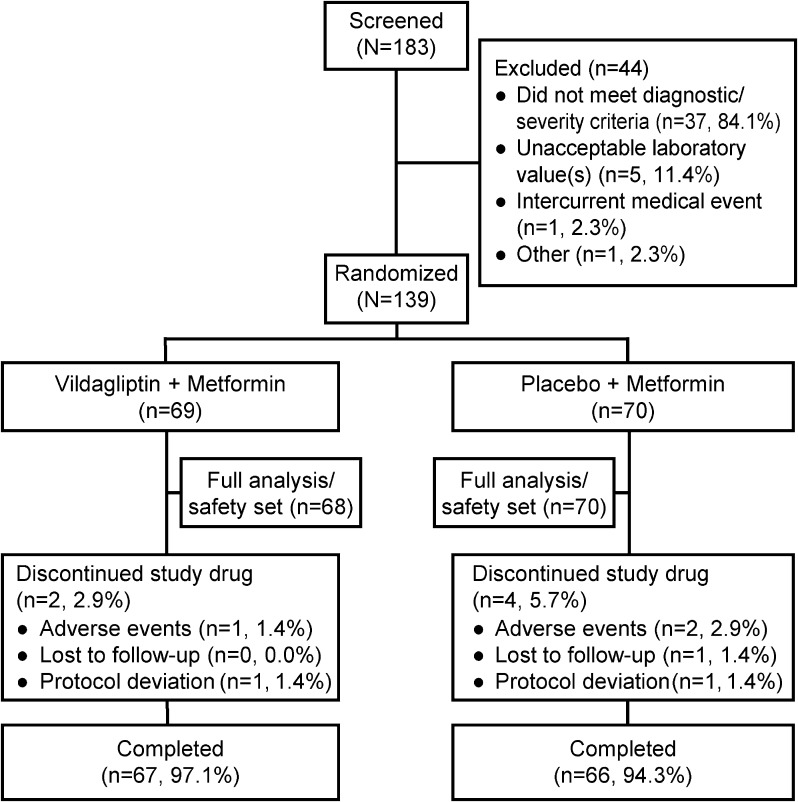
Of the 139 randomized patients (vildagliptin, n = 69; placebo, n = 70), 133 patients (95.7%) completed the study (Fig. 2). The primary reasons for discontinuation in the study were AEs (3 patients) and protocol deviations (2 patients) (Fig. 2). Patient demographics and baseline characteristics were comparable between the treatment groups (Table 1). Overall mean age, BMI, baseline HbA1c, baseline FPG, and duration of T2DM were 58.1 years, 25.6 kg/m2, 8.0%, 9.2 mmol/L, and 7.1 years, respectively. The patients were predominantly men (66.2%), and more patients were aged ≥65 years in the vildagliptin group (31.9%) than in the placebo group (22.9%).
Fig. 2.
Patient disposition
Table 1.
Patient demographics and baseline characteristics (randomized set)
| Parameter | Vildagliptin + metformin n = 69 | Placebo + metformin n = 70 | Total N = 139 |
|---|---|---|---|
| Age, years | 58.7 (9.81) | 57.5 (9.15) | 58.1 (9.47) |
| ≥65 years, n (%) | 22 (31.9) | 16 (22.9) | 38 (27.3) |
| Men, n (%) | 44 (63.8) | 48 (68.6) | 92 (66.2) |
| Body weight, kg | 67.9 (12.70) | 70.0 (13.02) | 68.9 (12.85) |
| BMI, kg/m2 | 25.3 (3.56) | 25.9 (4.01) | 25.6 (3.79) |
| HbA1c, % | 8.0 (0.83) | 8.0 (0.96) | 8.0 (0.90) |
| ≤8%, n (%) | 40 (58.0) | 40 (57.1) | 80 (57.6) |
| >8 to ≤9%, n (%) | 17 (24.6) | 14 (20.0) | 31 (22.3) |
| >9%, n (%) | 12 (17.4) | 16 (22.9) | 28 (20.1) |
| FPG, mmol/L | 9.1 (1.80) | 9.3 (2.40) | 9.2 (2.12) |
| ≥8.9 mmol/L, n (%) | 28 (40.6) | 36 (51.4) | 64 (46.0) |
| Duration of T2DM, years | 7.2 (6.18) | 7.0 (5.92) | 7.1 (6.03) |
| Metformin total daily dose, mg | 753.6 (251.81) | 750.0 (251.81) | 751.8 (250.90) |
| Metformin ≤500 mg/day, n (%) | 34 (49.3) | 35 (50.0) | 69 (49.6) |
| Metformin >500 mg/day, n (%) | 35 (50.7) | 35 (50.0) | 70 (50.4) |
| eGFR (MDRD), mL/min/1.73 m2, n (%) | |||
| Normal, >80 | 66 (95.7) | 64 (91.4) | 130 (93.5) |
| Mild, ≥50 to ≤80 | 3 (4.3) | 6 (8.6) | 9 (6.5) |
| Moderate, ≥30 to <50 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are expressed as mean (standard deviation) unless specified otherwise
BMI body mass index, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, HbA 1c glycosylated hemoglobin, MDRD modification of diet in renal disease, OADs oral antidiabetic drugs, T2DM type 2 diabetes mellitus
Efficacy
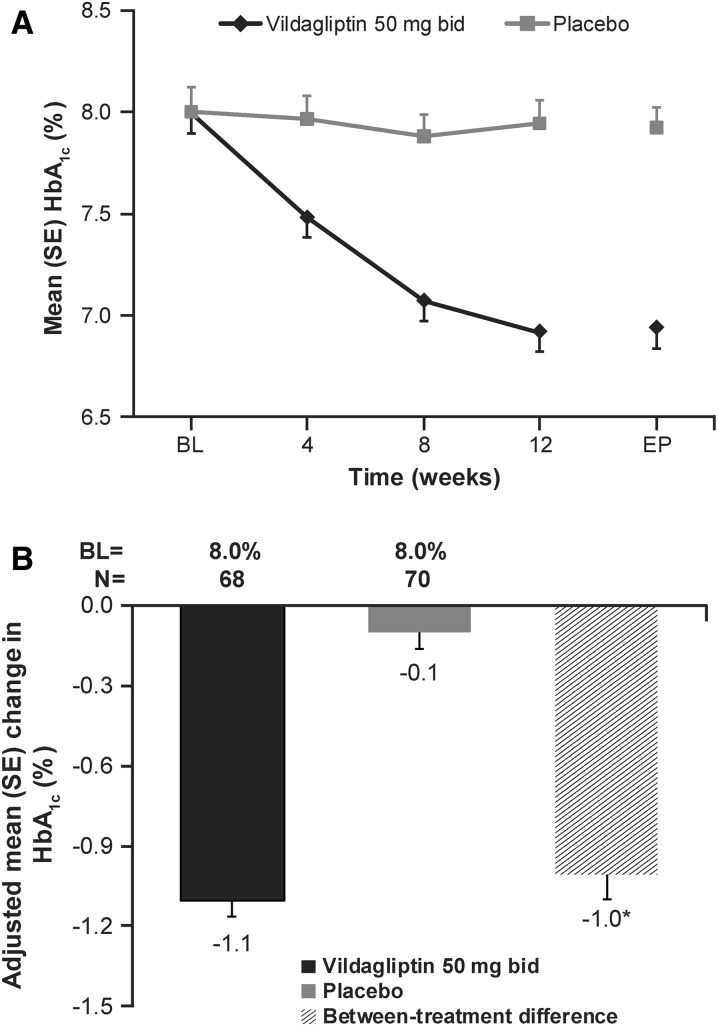
The mean change in HbA1c during the 12 weeks of treatment was consistently lower with vildagliptin than with placebo (Fig. 3a). The overall adjusted mean change (AM∆) ± SE in HbA1c was −1.1 ± 0.06% in the vildagliptin group (baseline 8.0%) and −0.1 ± 0.06% in the placebo group (baseline 8.0%), with a statistically significant between-treatment difference of −1.0 ± 0.09% (P < 0.001) in favor of vildagliptin (Fig. 3b). Vildagliptin also showed statistically significant reductions from baseline in HbA1c for subpopulations of patients receiving metformin 250 mg bid and 500 mg bid (Table 2). Significantly more patients with vildagliptin achieved HbA1c targets of ≤6.5% (30.9%) and <7.0% (64.1%) compared with placebo (P < 0.001). A higher proportion of patients in the vildagliptin group achieved HbA1c reductions of ≥1% and ≥0.5% than in the placebo group (P < 0.001) (Table 3).
Fig. 3.
a Mean glycosylated hemoglobin (HbA1c) by treatment and visit (full analysis set). b Adjusted mean change in HbA1c from baseline to endpoint (full analysis set). BL baseline, EP endpoint, SE standard error. *P < 0.001
Table 2.
Change in HbA1c (%) in subpopulations of patients taking metformin 250 mg bid or 500 mg bid (full analysis set)
| Treatment | n | Baseline mean (SE) | Mean change (SE) | 95% CI (P value) |
|---|---|---|---|---|
| Vildagliptin + metformin 250 mg bid | 34 | 7.9 (0.13) | −1.1 (0.09) | −1.24, −0.88 (P < 0.001) |
| Vildagliptin + metformin 500 mg bid | 34 | 8.1 (0.15) | −1.1 (0.09) | −1.24, −0.88 (P < 0.001) |
CI confidence interval, HbA 1c glycosylated hemoglobin, SE standard error
Table 3.
HbA1c responder rates (full analysis set)
| Responder criteria | Vildagliptin + metformin n = 68 | Placebo + metformin n = 70 |
|---|---|---|
| HbA1c ≤6.5%, n/N a (%) | 21/68 (30.9)* | 2/70 (2.9) |
| HbA1c <7.0%, n/N b (%) | 41/64 (64.1)* | 9/59 (15.3) |
| Reduction of HbA1c ≥1%, n/N c (%) | 39/68 (57.4)* | 3/70 (4.3) |
| Reduction of HbA1c ≥0.5%, n/N c (%) | 59/68 (86.8)* | 13/70 (18.6) |
HbA 1c glycosylated hemoglobin
* P < 0.001
aDenominator includes patients with a baseline of HbA1c >6.5% and endpoint HbA1c measurement
bDenominator includes patients with a baseline of HbA1c ≥7% and endpoint HbA1c measurement
cDenominator includes patients with both baseline and endpoint HbA1c measurements
The mean changes in HbA1c from baseline to endpoint in the subgroups of patients by age, gender, baseline BMI, baseline HbA1c and baseline FPG are presented in Table 4. The mean changes in HbA1c were greater for vildagliptin compared with placebo across all the subgroups. Mean reductions in HbA1c in the vildagliptin group were higher in the subgroups of patients with higher baseline HbA1c (HbA1c >8% to ≤9% or >9%) or FPG (≥8.9 mmol/L) and in those with lower baseline BMI (<25 kg/m2).
Table 4.
Mean changes in HbA1c (%) from baseline to endpoint by subgroups (full analysis set)
| Subgroups | Vildagliptin + metformin n = 68 | Placebo + metformin n = 70 | ||||
|---|---|---|---|---|---|---|
| n | BL mean | Change (SE) | N | BL mean | Change (SE) | |
| Age (years) | ||||||
| <65 | 47 | 7.9 | −1.1 (0.08) | 54 | 8.0 | −0.1 (0.08) |
| ≥65 | 21 | 8.3 | −1.1 (0.15) | 16 | 7.9 | −0.2 (0.08) |
| Gender | ||||||
| Male | 44 | 7.9 | −1.0 (0.10) | 48 | 8.1 | −0.2 (0.07) |
| Female | 24 | 8.2 | −1.2 (0.09) | 22 | 7.9 | −0.1 (0.13) |
| BMI (kg/m2) | ||||||
| <25 | 32 | 8.0 | −1.2 (0.11) | 35 | 7.8 | −0.2 (0.08) |
| ≥25 | 36 | 8.0 | −0.9 (0.08) | 35 | 8.2 | 0.0 (0.10) |
| HbA1c (%) | ||||||
| ≤8 | 40 | 7.4 | −0.9 (0.07) | 40 | 7.3 | 0.0 (0.08) |
| >8 to ≤9 | 17 | 8.3 | −1.1 (0.14) | 14 | 8.5 | 0.0 (0.10) |
| >9 | 11 | 9.5 | −1.6 (0.26) | 16 | 9.4 | −0.3 (0.17) |
| FPG (mmol/L) | ||||||
| <8.9 | 41 | 7.6 | −1.0 (0.08) | 34 | 7.3 | −0.1 (0.06) |
| ≥8.9 | 27 | 8.6 | −1.2 (0.14) | 36 | 8.7 | −0.1 (0.11) |
BL baseline, BMI body mass index, FPG fasting plasma glucose, HbA 1c glycosylated hemoglobin, SE standard error
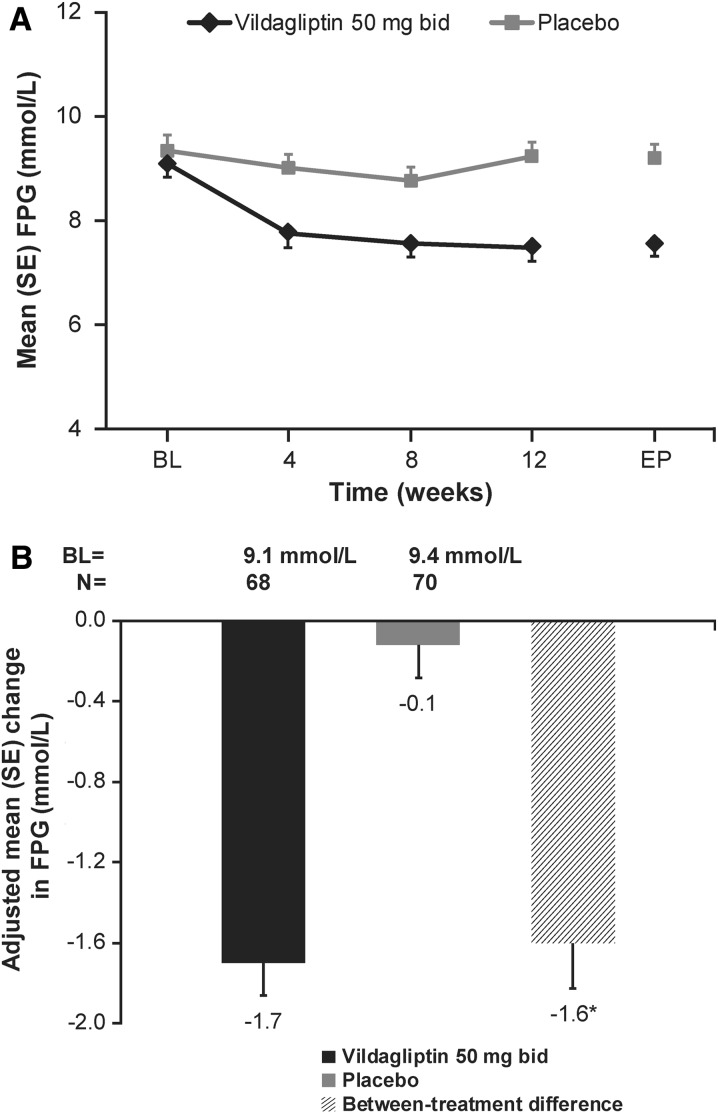
Vildagliptin showed sustained reduction in FPG over placebo during the 12 weeks of treatment (Fig. 4a). The AM∆ ± SE in FPG from baseline to endpoint was greater in patients receiving vildagliptin (−1.7 ± 0.16 mmol/L) compared with those receiving placebo (−0.1 ± 0.16 mmol/L), with a between-treatment difference of −1.6 ± 0.22 mmol/L (P < 0.001) (Fig. 4b).
Fig. 4.
a Fasting plasma glucose (FPG) by treatment and visit (full analysis set). b Adjusted mean change in FPG from baseline to endpoint (full analysis set). BL baseline, EP endpoint, SE standard error. *P < 0.001
Safety
The overall proportion of patients experiencing AEs was comparable between the vildagliptin (44.1%) and placebo (41.4%) groups. The most commonly reported AE by primary SOC was “infections and infestations” (13.2% for vildagliptin and 14.3% for placebo). The most frequently reported AE (≥2% in any group) by PT was “nasopharyngitis” (7.4% for vildagliptin and 5.7% for placebo) (Table 5). While incidence of AEs was low across PTs in both the treatment groups, “amylase increased” was reported in more patients with vildagliptin (4 patients; 5.9%) than with placebo (1 patient; 1.4%) and anemia was more frequent with placebo (3 patients; 4.3%) than with vildagliptin (0 patient). All the events of increased amylase levels were classified as mild and clinically asymptomatic. All the reported AEs were mild or moderate in severity. The incidence of AEs suspected to be related to the study drug was slightly higher in the vildagliptin group than in the placebo group (16.2% vs. 10.0%). One patient in the vildagliptin group and two patients in the placebo group discontinued the study. No SAEs were reported in the vildagliptin group, whereas one SAE of myocardial infarction was reported in the placebo group. There were no deaths during the study. No hypoglycemic events were reported in the study. There was no change in body weight from baseline to endpoint for both treatment groups (+0.3 kg for vildagliptin and −0.2 kg for placebo). There were no clinically relevant changes or trends in the hematological, biochemical (including lipid parameters), hepatic enzyme, urinalysis parameters, and vital signs in either treatment group.
Table 5.
Number (%) of patients reporting common adverse events (≥2% in any group) by preferred term (safety set)
| Preferred term, n (%) | Vildagliptin + metformin n = 68 | Placebo + metformin n = 70 |
|---|---|---|
| Any preferred term | 30 (44.1) | 29 (41.4) |
| Nasopharyngitis | 5 (7.4) | 4 (5.7) |
| Amylase increased | 4 (5.9) | 1 (1.4) |
| Dental caries | 2 (2.9) | 0 (0.0) |
| Gastritis erosive | 2 (2.9) | 0 (0.0) |
| Tinea infection | 2 (2.9) | 0 (0.0) |
| Lipase increased | 2 (2.9) | 1 (1.4) |
| Hypoesthesia | 2 (2.9) | 0 (0.0) |
| Anemia | 0 (0.0) | 3 (4.3) |
| Diarrhea | 0 (0.0) | 2 (2.9) |
| Gastroenteritis | 0 (0.0) | 2 (2.9) |
| Alanine aminotransferase increased | 0 (0.0) | 2 (2.9) |
| Aspartate aminotransferase increased | 0 (0.0) | 2 (2.9) |
| Back pain | 0 (0.0) | 2 (2.9) |
| Headache | 0 (0.0) | 2 (2.9) |
| Tension headache | 0 (0.0) | 2 (2.9) |
Discussion
This 12-week, randomized, double-blind study evaluated the efficacy and safety of vildagliptin 50 mg bid in Japanese patients with T2DM inadequately controlled on metformin monotherapy. Vildagliptin produced a statistically significant and clinically meaningful change in HbA1c compared with placebo (−1.1% vs. −0.1%; P < 0.001) as add-on to metformin (250 mg bid or 500 mg bid) after 12 weeks of treatment in Japanese patients with T2DM. Despite the lower baseline mean HbA1c and daily dose of metformin in this study, the between-treatment difference (−1.0%) seen was consistent with the findings previously reported in a predominantly Caucasian population, where vildagliptin-treated patients showed a decrease in HbA1c of 1.1% vs. placebo over 24 weeks of treatment [11]. Moreover, the reduction in HbA1c levels reported with vildagliptin therapy was consistent with other DPP-4 inhibitors with different study designs in Japanese population [18–20]. These findings indicate that vildagliptin is effective in Japanese patients with T2DM when added to metformin monotherapy.
Further, vildagliptin showed statistically significant and clinically meaningful reduction in HbA1c after 12 weeks of treatment in the subpopulation of patients receiving metformin 250 mg bid or 500 mg bid. Treatment with vildagliptin produced greater reduction in HbA1c compared with placebo regardless of age, gender, baseline BMI, HbA1c and FPG. Vildagliptin was efficacious irrespective of the baseline HbA1c. Greater reduction was seen in patients with higher baseline, which is consistent with the results observed in a predominantly Caucasian population [11].
Approximately one-third of patients treated with vildagliptin (30.9%) achieved the predefined HbA1c target of ≤6.5%. Furthermore, almost two-thirds of patients (64.1%) reached the HbA1c target of <7.0%, a goal recommended by the Japanese Diabetes Society [6]. The responder rate (<7.0%) was higher than that reported in a predominantly Caucasian population (55.4%) [11]. Over half of the population (57.4%) achieved an HbA1c reduction of ≥1.0%, and 86.8% of patients reported a reduction of ≥0.5% in the vildagliptin group.
Vildagliptin showed statistically significant reduction in FPG levels vs. placebo (P < 0.001) as add-on to metformin monotherapy after 12 weeks of treatment. The decrease in FPG could be attributed to increased active levels of GLP-1 upon twice-daily administration of vildagliptin 50 mg, which enhances insulin secretion and suppresses glucagon levels relative to glucose levels, in turn decreasing the endogenous glucose production overnight [21].
Overall, vildagliptin added to metformin was safe with no new safety findings observed in Japanese patients with T2DM. The observed safety profile was similar with previously reported 52-week safety study of vildagliptin add-on to metformin in Japanese patients with T2DM [22], long-term study of vildagliptin add-on to metformin in a predominantly Caucasian population [23], and safety pooled analysis of vildagliptin studies of ≥12 to ≥104 weeks duration [24]. Four patients in the vildagliptin group and one patient in the placebo group reported clinically asymptomatic mild elevations of amylase and/or lipase; however, none of these cases were considered as an AE of acute pancreatitis by the investigators. Similar to the previously reported studies [25], treatment with vildagliptin as add-on to metformin confirmed its weight neutrality in Japanese patients.
There were no incidences of hypoglycemia reported in the study. Absence of hypoglycemic events in the vildagliptin group, in spite of lower mean baseline FPG and HbA1c levels than the global study [11], confirms the glucose-dependent action of vildagliptin. This is consistent with the results from a previously reported large pooled analysis of global safety data, which showed that vildagliptin, as monotherapy or in combination with metformin, thiazolidinedione, or sulfonylurea, is associated with fewer hypoglycemic events compared with comparators [24].
The notable benefit observed in improving HbA1c levels confirms the complementary mechanism of action of vildagliptin and metformin in Japanese patients with T2DM. Metformin increases the plasma concentration of incretin hormones and enhances the effects of DPP-4 inhibition on the increase of intact GLP-1, which might explain the improved efficacy of vildagliptin in combination with metformin [26].
In conclusion, vildagliptin 50 mg bid as add-on to metformin is effective in reducing HbA1c and FPG levels without any tolerability issues and hypoglycemia in Japanese patients with T2DM inadequately controlled on metformin monotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the patients and staff who participated in this study. Sponsorship and article processing charges for this study were funded by Novartis Pharma K.K. The authors take full responsibility for the content of the manuscript and participated at all stages of manuscript development and approved the final manuscript for publication. Masato Odawara and Manabu Suzuki contributed to study design, data analysis and interpretation and contributed to drafting of the manuscript. Izumi Hamada provided support for statistical analysis. All authors meet the ICMJE criteria for authorship, had full access to the study data and take complete responsibility for the integrity of the data and accuracy of the data analysis. Medical writing assistance, editorial assistance, and collation and incorporation of comments from all authors for this study were provided by Anuja Shah (Novartis Healthcare Pvt. Ltd., Hyderabad, India) and Amit Garg (Novartis Healthcare Pvt. Ltd., Hyderabad, India).
Conflict of interest
Manabu Suzuki is an employee of Novartis Pharma K.K. Izumi Hamada is an employee of Novartis Pharma K.K. Masato Odawara is the independent medical advisor for this study and has received consultancy fees from Novartis Pharma K.K.
Compliance with ethics guidelines
The study protocol was reviewed and approved by the Independent Ethics Committee/Institutional Review Board at each center. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), the Helsinki Declaration of 1975, as revised in 2000 and 2008 and Good Clinical Practice guidelines. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of investigators/institutions
Akira Numata (Ikebukuro Metropolitan Clinic), Hideki Hanashi (New Medical Research System Clinic), Otoya Miho (Miho Clinic), Munechika Noguchi (Shinagawa East One Medical Clinic), Hideki Kaizuka (Pedi Shiodome Clinic), Nobuki Wakao (KASAI Diabetes Clinic), Takashi Lizuka (Asahi Medical Clinic), Tatsuhiro Shimoyama (Shimamura Kinen Hospital), Hiroshi Shimomura (Musashino Polyclinic), Masafumi Sugawara (Dyna Medical Nezu Clinic), Shizuo Kajiyama (Medical Corporation Keisei-kai Kajiyama Clinic), Seiichi Tanaka (Kyushu Rosai Hospital), Yasuhiro Sako (Saiseikai Fukuoka General Hospital), Kiyohide Nunoi (St. Mary’s Hospital), Yasuhiro Ono (Takagi Hospital), Makoto Kunisaki (Kunisaki Makoto Clinic), Shizuka Kaneko (Takatsuki Red Cross Hospital), Ryuji Suzuki (Kawasaki Suzuki Medical Clinic), Tomio Inoue (Ageo Central General Hospital) and Kentaro Doi (Rakuwakai Otowa Hospital).
Footnotes
Trial registration: Clinical Trials.gov #NCT01497522.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. http://www.idf.org/diabetesatlas/5e/diabetes. Accessed Aug 5, 2013.
- 2.Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev. 2002;18:S9–S13. doi: 10.1002/dmrr.296. [DOI] [PubMed] [Google Scholar]
- 3.Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev. 2009;25:705–716. doi: 10.1002/dmrr.1012. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–835. doi: 10.1016/j.metabol.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Japan Diabetes Clinical Data Management Study Group. HbA1c (NGSP) in 2008. Ibaraki. http://jddm.jp/data/index.html. Accessed Aug 10, 2013.
- 6.Japan Diabetes Society, Treatment Guide for Diabetes edited by Japan Diabetes Society 2012–2013. Bunkodo Co. Ltd.; 2013.
- 7.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B, Foley JE, Bosi E. Clinical evidence and mechanistic basis for vildagliptin’s action when added to metformin. Diabetes Obes Metab. 2011;13:193–203. doi: 10.1111/j.1463-1326.2010.01321.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahrén B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011;13:775–783. doi: 10.1111/j.1463-1326.2011.01414.x. [DOI] [PubMed] [Google Scholar]
- 10.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 12.Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–1056. doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 13.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 14.Kothny W, Foley JE, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–257. doi: 10.1111/dom.12020. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233–240. doi: 10.1016/j.diabres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto Y, Kashiwagi A, Yamada N, et al. Efficacy and safety of vildagliptin and voglibose in Japanese patients with Type 2 diabetes: a 12-week, randomized, double-blind, active-controlled study. Diabetes Obes Metab. 2010;12:700–708. doi: 10.1111/j.1463-1326.2010.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506–515. doi: 10.1111/j.1463-1326.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Tajima N, Odawara M, Nishii M, Taniguchi T, Ferreira JCA. Addition of sitagliptin to ongoing metformin monotherapy improves glycemic control in Japanese patients with type 2 diabetes over 52 weeks. J Diabetes Investig. 2013;4:174–181. doi: 10.1111/jdi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab. 2012;14:927–936. doi: 10.1111/j.1463-1326.2012.01620.x. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki N, Watada H, Murai M, et al. Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:833–843. doi: 10.1111/dom.12110. [DOI] [PubMed] [Google Scholar]
- 21.Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–1255. doi: 10.1210/jc.2006-1882. [DOI] [PubMed] [Google Scholar]
- 22.Odawara M, Suzuki M, Hamada I, Iguchi A. Clinical evaluations of the vildagliptin combination therapy in type 2 diabetes patients – A long term safety study of 52 weeks treatment with vildagliptin as add-on therapy with metformin, TZD, α-GI or Glinides. J New Rem Clin. 2012;12:2593–611 (article in Japanese).
- 23.Matthews DR, Dejager S, Ahrén B, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12:780–789. doi: 10.1111/j.1463-1326.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer A, Dejager S, Foley JE, Kothny W. Assessing the general safety and tolerability of vildagliptin: value of pooled analyses from a large safety database versus evaluation of individual studies. Vasc Health Risk Manag. 2011;7:49–57. doi: 10.2147/VHRM.S16925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541–548. doi: 10.2147/vhrm.s10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther. 2007;88:801–808. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.