Abstract
Introduction/aim
Insulin degludec/insulin aspart (IDegAsp) is a soluble co-formulation of long-acting and short-acting insulin analogs. The primary objective of this study was to investigate the pharmacodynamic response of once-daily IDegAsp dosing in patients with type 1 diabetes. Pharmacokinetic response, as well as safety and tolerability, were assessed as secondary objectives.
Methodology
This was a single-center, open-label, single-arm study. Twenty-two subjects received once-daily insulin degludec (IDeg) (0.42 U/kg) for five consecutive days [with separate bolus insulin aspart (IAsp) as needed for safety and glycemic control], to achieve clinical steady state of the basal component. On Day 6, they received a single injection of IDegAsp (0.6 U/kg, comprising 0.42 U/kg IDeg and 0.18 U/kg IAsp). Pharmacodynamic response was assessed using a 30-h euglycemic glucose clamp, with blood glucose stabilized at a target of 5.5 mmol/L.
Results
The glucose infusion rate profile showed a rapid onset of action and a distinct peak due to IAsp, followed by a separate, flat and stable basal glucose-lowering effect due to the IDeg component. Modeling data suggested that the pharmacodynamic profile of IDegAsp was retained with twice-daily dosing (allowing for coverage of two main meals daily). IDegAsp was well tolerated and no safety issues were identified in this trial.
Conclusions
In conclusion, the IAsp component of IDegAsp has a fast onset of appearance and a peak covering the prandial phase, while the IDeg component has a flat and an evenly distributed pharmacokinetic profile over 24 h. IDegAsp is the first co-formulation of a basal insulin analog with an ultra-long duration of action and a mealtime insulin analog in a single soluble injection. These properties translate into clinically relevant benefits, including improved glycemic control and reduction in hypoglycemia.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-014-0070-2) contains supplementary material, which is available to authorized users.
Keywords: Insulin degludec/insulin aspart, Pharmacodynamics, Pharmacokinetics, Steady state, Type 1 diabetes mellitus
Introduction
In managing the progressive nature of type 2 diabetes mellitus (T2DM) through insulin therapy, treatment intensification is often required. When a basal insulin analog is no longer enough to maintain glycemic control, patients mainly follow one of two treatment options. One of these is the addition of separate bolus insulin injections (a basal–bolus approach); however, patients can find this difficult due to the complexity arising from the separate titration of two different insulin formulations [1]. The alternative approach of switching to premixed insulins [2] shows superior glycemic control to basal insulin but is associated with reduced fasting plasma glucose (FPG) control and, in some studies, less overall reduction in glycated hemoglobin (HbA1c), compared to basal–bolus insulin treatment [3–5].
One reason for these findings may be the non-optimal pharmacokinetic and pharmacodynamic characteristics of premixed formulations, which consistently use protaminated insulins for the basal insulin component, resulting in greater variability, a prolonged glucose-lowering effect beyond the time required for prandial control, and a shorter duration of action compared to basal insulin analogs [6–10]. Until now, it has not been possible to co-formulate a basal and a short-acting insulin analog due to interactions between the two insulins that blunt absorption, particularly that of the short-acting insulin [11]. Insulin glargine is soluble at an acidic pH of 4, which has prevented a co-formulation with bolus insulins that are soluble at a neutral pH of 7.4. Similarly, co-formulation of insulin detemir with rapid-acting insulin analogs has been shown to result in the formation of mixed hexamers displaying a pharmacokinetic/pharmacodynamic profile unsuitable for optimum glycemic control [11].
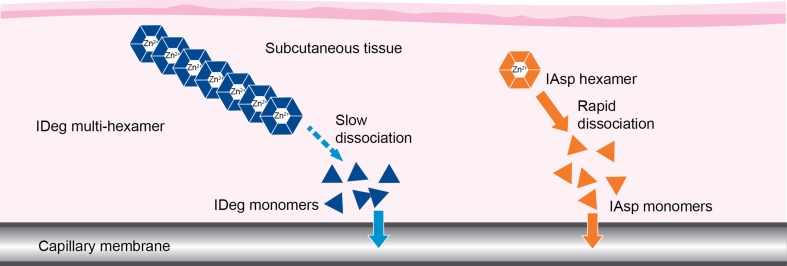
Insulin degludec/insulin aspart (IDegAsp) is a soluble co-formulation of two distinct insulin analogs in the ratio 70% insulin degludec (IDeg) to 30% insulin aspart (IAsp) [12]. Figure 1 [13–15] shows the mechanism of action of IDegAsp whereby IAsp is rapidly absorbed into the circulation [16], while IDeg provides stable coverage of basal insulin needs due to its flatter and more consistent pharmacodynamic profile with a duration of action exceeding 42 h and four times less within-subject variability compared to insulin glargine [13, 17, 18].
Fig. 1.
Schematic illustration showing the mechanism of action of IDegAsp combination insulin. In solution, the IDeg component forms soluble dihexamers at neutral pH, whereas IAsp remains as distinct hexamers. Upon subcutaneous injection, as illustrated, IDeg dihexamers immediately self-associate into stable multi-hexamers in the subcutaneous tissue from which IDeg monomers dissociate slowly and continuously. By contrast, IAsp hexamers promptly dissociate to monomers that are rapidly absorbed into the circulation [13–15]. IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart
As a result of the >24 h half-life of IDeg, the pharmacodynamic properties of IDegAsp should be evaluated at steady state. As patients with T2DM may retain some beta-cell function, and may be characterized by high insulin resistance, patients with type 1 diabetes mellitus (T1DM) are the preferred population in which to investigate insulin pharmacodynamic endpoints. T1DM is characterized by absolute insulin deficiency as a result of an autoimmune destruction of pancreatic β cells [19]. As a consequence, patients with T1DM are dependent on insulin replacement therapy. In pharmacological studies, the insulins used for therapy are washed off, so that the effect of the study insulin can be evaluated without influence of exogenous or endogenous insulin. The main objective of the present study was to investigate the steady-state pharmacodynamic and pharmacokinetic response of once-daily IDegAsp in subjects with T1DM who had been receiving insulin treatment for at least the past 12 months.
Materials and Methods
Study Design
This single-center, single-arm, open-label trial was registered with clinicaltrials.gov (NCT01590836), and the protocol was reviewed and approved by the local health authority (Bundesinstitut für Arzneimittel und Medizinprodukte) in accordance with regulations, and by the appropriate ethics committee (Ärztekammer Nordrhein). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. The study was performed in accordance with Good Clinical Practice as defined by the International Conference on Harmonisation. Subjects were fully informed of the risks of the trial and were made aware that they could withdraw from the trial at any time for any reason. Patients were informed verbally and in writing, and written consent was obtained before any trial-related procedures were initiated.
Subjects
Trial participants included 22 men and women aged 18–65 years, with T1DM, treated with insulin for at least 12 months with a current daily basal insulin requirement of ≥0.2 U/kg/day and a total insulin dose of <1.2 U/kg/day. Eligible subjects were also required to have a body mass index (BMI) in the range of 18.0–28.0 kg/m2, an HbA1c level of ≤9.5%, and a fasting C-peptide level of ≤0.3 nmol/L.
Subjects were excluded from participation if they had a history or presence of cancer or cardiovascular disease, supine blood pressure beyond the normal range (90–140 mmHg systolic; 50–90 mmHg diastolic), proliferative retinopathy or maculopathy and/or severe neuropathy, recurrent severe hypoglycemia (more than one episode requiring assistance in the past 12 months) or hypoglycemia unawareness, or smoked >5 cigarettes or the equivalent, per day.
Interventions
The trial consisted of a screening visit (Visit 1), followed by a treatment period (Visits 2–8), and a follow-up visit (Visit 9). After screening, all subjects received IDeg (0.42 U/kg) once daily for 5 days to reach steady state [20]. IAsp was given as required, which enabled individualization of bolus doses on Days 1–5 (IAsp has a short exposure and therefore does not accumulate over time). On Day 6, subjects received a single dose of IDegAsp (0.6 U/kg, comprising 0.42 U/kg IDeg and 0.18 U/kg IAsp). At each dosing visit, trial product was administered at approximately 8 p.m. This time was chosen for convenience in performing the clamp procedure and does not reflect requirements in normal clinical practice.
IDeg and IDegAsp were dosed as subcutaneous injections into a lifted skinfold of the lower abdominal wall above the inguinal area. Both were provided in 3 mL Penfill® cartridges (Novo Nordisk, Bagsvaerd, Denmark) (100 U/mL) and administered using a syringe and needle.
After administration of IDegAsp on Day 6, the steady-state pharmacodynamic response was evaluated using a 30-h euglycemic glucose clamp performed with a Biostator® device (MTB Medizintechnik, Amstetten, Germany), as described previously [18]. In brief, 5–6 h before dosing of the trial product, subjects received a variable intravenous infusion of human insulin (15 IU Actrapid®, 100 IU/mL in 49 mL saline and 1 mL of the subject’s blood) or glucose to obtain a blood glucose clamp target level of 5.5 mmol/L (100 mg/dL). After trial product administration, when only insulin was infused to maintain the glucose clamp target, the rate of insulin infusion was decreased gradually and stopped completely when blood glucose had decreased by 0.3 mmol/L (5 mg/dL); glucose infusion was then initiated to keep the glucose concentration constant at the glucose clamp target of 5.5 mmol/L (100 mg/dL). The clamp continued for 30 h post-dosing of trial product, but was terminated earlier if the blood glucose consistently exceeded 13.9 mmol/L (250 mg/dL) without any glucose having been administered for at least 30 min. During the entire clamp procedure, subjects remained fasting (with no oral intake other than water) and stayed in a supine or semi-supine position.
The glucose infusion rate (GIR) required to keep the blood glucose concentration at the target level was recorded every minute throughout the euglycemic clamp. Approximately every 30 min throughout the glucose clamp, blood glucose measurements from the Biostator were checked against blood glucose measurements performed by a glucose analyzer (Super GL Glucose Analyzer, Hitado, Möhnesee, Germany). Blood samples for determination of serum IAsp concentration were taken on Day 6 at the following times: 5 min predose, 0 min, at 10 min intervals until 2 h post-dose, then every 15 min to 3 h, then at 3½, 4, 5, 6, 8, 10, 11 and 12 h post-dose. Serum IDeg concentrations were measured in blood samples taken 5 min predose and at 0 min on Days 1–5 then on Day 6, at 5 min predose, 0 min, 30 min, 1 h and thereafter every hour until 16 h, then at 18, 20, 22, 24, 30, 36 and 48 h.
Each subject remained in the clinic for 48 h after IDegAsp dosing. Subjects returned to the clinic at time points 72, 96 and 120 h after dosing to have blood samples taken for pharmacokinetic assessment of IDeg and for monitoring of blood glucose.
Serum IAsp concentrations were quantified using a validated IAsp-specific enzyme-linked immunosorbent assay (ELISA). Serum IDeg concentrations were measured using a validated IDeg-specific sandwich ELISA.
Assessments
The primary endpoint was the total glucose-lowering effect estimated by the area under the GIR curve during one 24-h dosing interval at steady state (AUCGIR,τ,SS) after administration of IDegAsp.
Secondary endpoints included time to GIRmax,SS (t GIRmax,SS), duration of action of IDegAsp, total exposure of IDeg (AUCIDeg,τ,SS) and distribution of IDeg exposure over the 24-h dosing interval at steady state (AUCIDeg,0–12h,SS/AUCIDeg,τ,SS), total exposure of IAsp (AUCIAsp,0–12h), and time to maximum observed serum concentration of IAsp (t max,IAsp). In addition, using the data obtained from once-daily dosing in this trial, a pharmacokinetic/pharmacodynamic model was used to simulate steady-state GIR response following twice-daily dosing of IDegAsp.
Safety was monitored for both IDeg and IDegAsp administration. Safety endpoints included treatment-emergent adverse events, confirmed hypoglycemic episodes, physical examination, vital signs, electrocardiogram (ECG), and clinical laboratory values. Confirmed hypoglycemia was defined as any episode of severe hypoglycemia (as per the American Diabetes Association definition [21]) or minor hypoglycemia [plasma glucose <3.1 mmol/L (56 mg/dL) or blood glucose <2.8 mmol/L (50 mg/dL)].
Statistical Methods
Pharmacokinetic and pharmacodynamic analyses were based on the full analysis set, which included 22 subjects. The primary endpoint, AUCGIR,τ,SS, was derived from individual GIR profiles and calculated as the area under the smoothed GIR profile using the linear trapezoidal technique on interpolated data points.
Secondary pharmacodynamic endpoints were derived from individual GIR profiles (smoothed) and blood glucose profiles at steady state. Duration of action was calculated as the time from administration of IDegAsp until blood glucose concentration was consistently >8.3 mmol/L (end of action) during the euglycemic glucose clamp at steady state [22]. Statistical analyses were performed using SAS® 9.1.3 software (SAS Institute Inc., Cary, NC, USA).
The pharmacokinetic/pharmacodynamic model used to simulate steady-state GIR response consisted of separate pharmacokinetic and pharmacodynamic components for IDeg and for IAsp. For IDeg, the pharmacokinetic component was based on absorption (with a depot compartment and a transit compartment, an absorption-rate parameter and a transit-rate parameter), and disposition (with a single distribution compartment, a clearance parameter, and a volume-of-distribution parameter), and the pharmacodynamic component linked the concentration in the distribution compartment to GIR by means of an insulin-action compartment, a turnover parameter, and an insulin-sensitivity parameter. For IAsp, the pharmacokinetic component was based on absorption (with a depot compartment and four transit compartments, an absorption-rate parameter, and a transit-rate parameter), and disposition (with a single distribution compartment, a clearance parameter, and a volume-of-distribution parameter), and the pharmacodynamic component linked the concentration in the distribution compartment to GIR by means of an insulin-action compartment, a turnover parameter, and an insulin-sensitivity parameter. The GIR contributions for IDeg and IAsp were subsequently added to simulate total GIR effect. The parameters of the model were estimated in a population pharmacokinetic/pharmacodynamic setting, using a nonlinear mixed-effects approach, which allowed individual sets of parameters to be obtained for each of the subjects included in the trial. Using the estimated individual parameters, a simulation of twice-daily multiple dosing was conducted to obtain a mean steady-state profile. Twice-daily multiple dosing for 6 days at a dose level of 0.3 U/kg was simulated for each of the subjects, and the mean of the profiles on Day 6 was subsequently calculated. A dose of 0.3 U/kg was used for the simulation based on the assumption that the once-daily dose of 0.6 U/kg would be divided into two for the twice-daily dose. The modeling was performed using NONMEM® version 7.1.2 (ICON Development Solutions, Ellicott City, MD, USA) and the corresponding figure was produced using S-Plus version 8.2 (TIBCO, Palo Alto, CA, USA).
Safety analyses were based on the safety analysis set (all subjects who received ≥1 dose of either IDeg or IDegAsp). Safety endpoints were summarized using descriptive statistics.
Results
Subject Characteristics
Of the 25 subjects screened, a total of 22 subjects (18 men, 4 women) with T1DM were exposed to IDeg and IDegAsp during the trial. No subjects withdrew from the trial. The mean age of the subjects was 40 years (range 20–56 years), mean HbA1c was 7.9% (range 5.8–9.0%), mean duration of diabetes was 23.1 years (range 8.9–42.9 years), and mean BMI was 24.6 kg/m2 (range 20.2–27.9 kg/m2) (Table 1).
Table 1.
Subject characteristics
| Characteristic | Mean (min, max) |
|---|---|
| Mean age, years | 40.0 (20.0, 56.0) |
| Mean BMI, kg/m2 | 24.6 (20.2, 27.9) |
| Sex, n | |
| Female | 4 |
| Male | 18 |
| Race, n | |
| White | 22 |
| Duration of diabetes, years | 23.1 (8.9, 42.9) |
| HbA1c, % | 7.9 (5.8, 9.0) |
| Fasting C-peptide, nmol/L | 0.02 (0.02, 0.08) |
BMI, body mass index; HbA1c, glycated hemoglobin
Pharmacodynamics
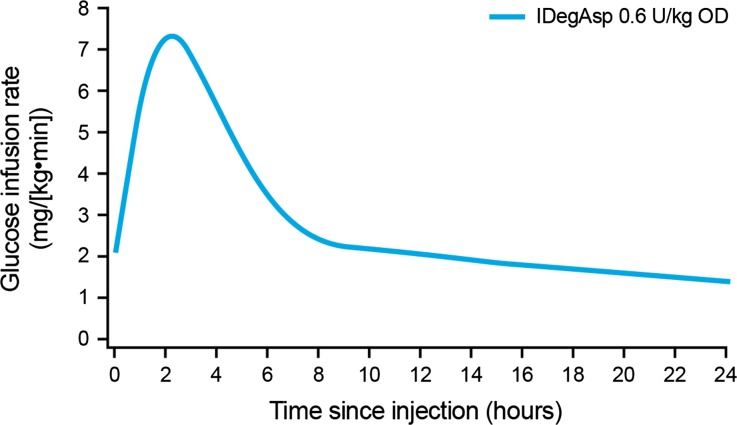
The mean GIR profile at steady state is shown in Fig. 2, reflecting the primary endpoint, AUCGIR,τ,SS [geometric mean (coefficient of variation): 3,859 mg/kg (33%)]. The profile showed a rapid onset of action and a distinct peak due to the IAsp component, and a separate, stable basal glucose-lowering effect attributable to the IDeg component. The median t GIRmax,SS for IDegAsp was 2.5 h. The clamp was performed when steady state of the basal component had been established. The GIR was already elevated at the time of injection of IDegAsp, owing to the duration of action of IDeg being >42 h [13], so that the effect of the previous IDeg injections on Days 1–5 was expected to elicit a GIR response already at time-point 0 on Day 6.
Fig. 2.
Mean glucose infusion rate profile of once-daily insulin degludec/insulin aspart (IDegAsp) administered at steady state in subjects with type 1 diabetes. OD, once daily
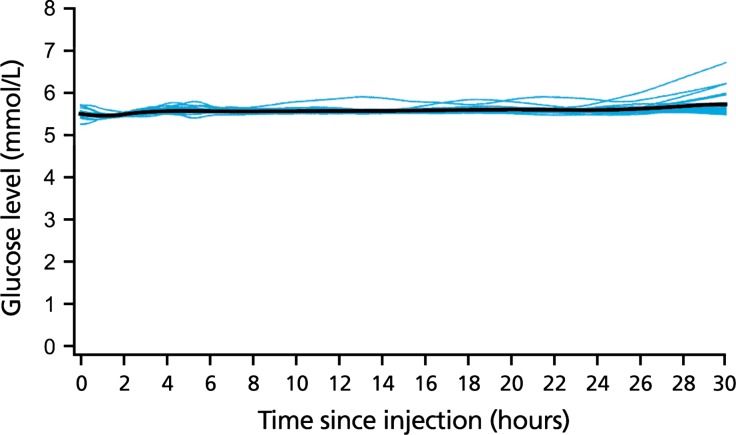
Mean blood glucose levels remained at the clamp target level throughout the euglycemic clamp procedure (Fig. 3). No subject experienced end of action (defined as a blood glucose level >8.3 mmol/L) or early termination during the 30-h clamp, hence the duration of action of IDegAsp extended beyond 30 h in all 22 subjects.
Fig. 3.
30-h individual and mean (black line) blood glucose profiles—insulin degludec/insulin aspart (IDegAsp) at steady state
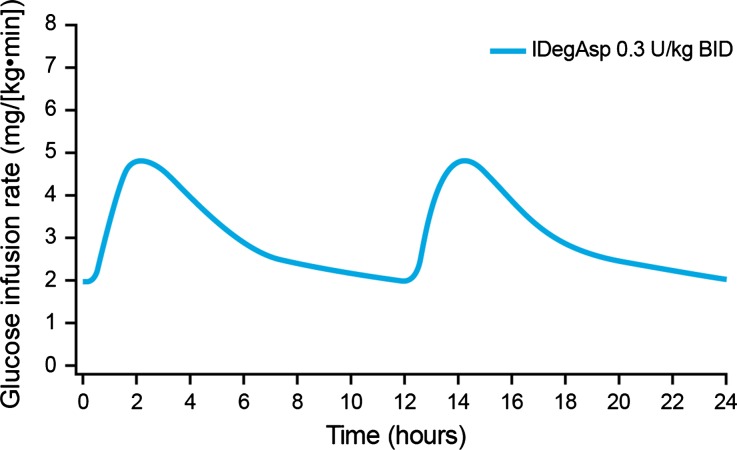
The model simulating the pharmacodynamic response to twice-daily dosing of IDegAsp at steady state indicated that the distinct peak (due to IAsp) and the separate, flat basal action (due to IDeg) were retained following each dose (Fig. 4).
Fig. 4.
Simulated mean glucose infusion rate profile of insulin degludec/insulin aspart (IDegAsp) administered twice daily at steady state in subjects with type 1 diabetes. BID, twice daily
Pharmacokinetics
Mean total serum exposure of IAsp in IDegAsp (AUCIAsp,0–12h) was 1,087 pmol h/L. The IAsp component of IDegAsp had a rapid onset of appearance; t max,IAsp was 80 min (median). The mean total serum exposure of IDeg in IDegAsp (AUCIDeg,τ,SS) at steady state, during one dosing interval, was 72,084 pmol h/L. Exposure to the IDeg component of IDegAsp was similar in the first and second 12 h periods. The mean ratio of AUCIDeg,0–12h,SS/AUCIDeg,τ,SS was 0.51, indicating that IDeg has an evenly distributed pharmacokinetic profile over 24 h at steady state. Clinical steady state was achieved after 2–3 days of once-daily IDeg dosing.
Safety
During 5 days of IDeg administration, one adverse event was reported that was considered possibly related to IDeg treatment—a case of mild headache. Furthermore, while IDeg steady state was being attained, there were 22 confirmed hypoglycemic episodes in 13 subjects.
After administration of IDegAsp on Day 6, there were no reported AEs that were considered to be related to IDegAsp. A total of six confirmed hypoglycemic episodes were recorded in five subjects after administration of IDegAsp on Day 6. Four occurred 30–48 h after IDegAsp administration, and the other two occurred 96–120 h after IDegAsp administration. These hypoglycemic episodes may therefore have been related to the administration of bolus insulin or the switch to other regimens following cessation of IDegAsp treatment.
There were no significant treatment-emergent changes in clinical laboratory parameters, vital signs, physical examination or ECG findings following subcutaneous IDeg and IDegAsp administration. No injection site reactions were reported during the trial.
Discussion
At steady state, the glucose-lowering effects of the prandial and basal components of IDegAsp were distinct and clearly separated. The IAsp component resulted in a rapid onset of action and a peak glucose-lowering effect covering the prandial phase, providing mealtime insulin control. This was followed by a stable, flat and ultra-long glucose-lowering effect due to IDeg that was maintained for >30 h in all trial subjects. These properties were consistent with those observed for the individual components [16, 23].
Historically, it has proven difficult to combine two different insulin analogs in one co-formulation, largely owing to interactions between them that blunt absorption, especially of the short-acting insulin [11]. In contrast, our findings show that IDegAsp has a distinct peak action (IAsp) followed by a separate stable and sustained basal effect (IDeg). This is reflective of the distinct mode of action of IDegAsp (see Fig. 1).
In the present study, the pharmacodynamic evaluation of IDegAsp was conducted at steady state due to the ultra-long duration of action of IDeg. As a result of this duration of action, metabolic action from the previous IDeg injection was still in effect when IDegAsp was injected (the mean GIR at time-point 0 was around 2 mg/kg/min as indicated in Fig. 2). In addition, no early termination of the 30-h clamp was required by any study subject and the duration of action exceeded 30 h in all patients.
IDeg is indicated for once-daily dosing, whereas IDegAsp may be administered once or twice daily with the main meal(s) according to patient needs and preferences [12, 24]. Based on simulated steady-state pharmacodynamic modeling, dividing the IDegAsp dose in two (0.3 U/kg twice-daily [BID]) gives the same basal glucose-lowering effect as once-daily dosing, due to the fact that the concentration of the basal part of IDegAsp, IDeg, remains unchanged (provided the total daily insulin dose is the same). Thus, at steady state, the basal glucose-lowering effect is only dependent on dose and not on dosing frequency. In contrast, the bolus peak associated with the IAsp component of IDegAsp will be half the size when switched from once-daily to twice-daily dosing. This dose schedule would provide insulin coverage for two main meals, which as the disease progresses is often required due to increasing hyperglycemia on basal-only therapy [25, 26].
Overall, the data from this study suggest that IDegAsp could provide flat and stable basal insulin coverage (provided by IDeg at steady-state conditions) and bolus mealtime insulin control with reduced injection burden compared to standard basal and bolus therapy. The preservation of these distinct glucose-lowering effects in the IDegAsp combination indicates potential improvements in FPG and reductions in hypoglycemia compared to premixed insulins. A meta-analysis of two recent phase 3 trials [27, 28] in patients with T2D comparing twice-daily IDegAsp to the premixed insulin preparation, BIAsp 30 (70% IAsp protamine suspension; 30% IAsp) indicates that these pharmacodynamic properties translate into meaningful clinical benefits. IDegAsp showed superior reductions in FPG, comparable HbA1c and reduced daily insulin dose at end of trial compared with BIAsp 30 [29]. Furthermore, rates of overall confirmed hypoglycemia and nocturnal confirmed hypoglycemia were both significantly lower with IDegAsp [29].
The current study was not powered to assess safety and tolerability. However, among the 22 patients enrolled, IDegAsp was well tolerated, there were no injection site reactions and no unexpected safety issues were identified.
The main limitation of this study was its uncontrolled design. Additional potential limitations include that some study subjects had a BMI in the overweight range (>25 kg/m2), and hence may have been characterized as having impaired insulin sensitivity. Finally, women were relatively under-represented among the study participants. A controlled study has been performed under single-dose conditions, showing that the 24-h AUCGIR and GIRmax increased significantly and proportionally as IDegAsp dose increased [30]. The aim of the present study was to examine the IDegAsp profile under steady-state conditions. The GIR profiles from the single-dose study [30] and the current study at steady state (Fig. 2) displayed the same shape, although, as expected, the baseline level was higher at steady state compared to the single-dose profile.
In conclusion, IDegAsp is the first soluble insulin combination to offer ultra-long basal insulin analog coverage in combination with a well-established mealtime insulin analog in a single injection. The clinical pharmacokinetic and pharmacodynamic findings from the present work have translated into comparable glycemic control, improved FPG, and significantly lower levels of hypoglycemia vs BIAsp 30 in phase 3 clinical trials [27–29]. IDegAsp may therefore represent a clinical advance in the management of diabetes by combining a convenient treatment regimen with reduced hypoglycemia compared to existing treatment options.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Sponsorship and article processing charges for this study were funded by Novo Nordisk. Medical writing assistance for this study was provided by Dr Nason Ma’ani Hessari of ApotheCom ScopeMedical, UK, and funded by Novo Nordisk (Bagsvaerd, Denmark). All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Tim Heise is a shareholder of Profil. Within the past year, this institution received research funds from Adocia, Becton–Dickinson, Biocon, Boehringer Ingelheim, Bristol-Myers Squibb, Dance Pharmaceuticals, Evolva, Hoffmann La-Roche, Johnson&Johnson, Eli Lilly, Marvel, Novartis, Novo Nordisk, Sanofi and Servier. In addition, Tim Heise received speaker honoraria from Eli Lilly and Novo Nordisk, received travel grants from Novo Nordisk and is a member of advisory panels for Novo Nordisk.
Hanne Haahr, Carsten Roepstorff and Suresh Chenji are employees of Novo Nordisk.
Leszek Nosek and Oliver Klein declare no conflict of interest.
Compliance with ethics guidelines
The study protocol was reviewed and approved by the local health authority (Bundesinstitut für Arzneimittel und Medizinprodukte) in accordance with regulations, and by the appropriate ethics committee (Ärztekammer Nordrhein). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. The study was performed in accordance with Good Clinical Practice as defined by the International Conference on Harmonisation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Trial registration: ClinicalTrials.gov #NCT01590836.
References
- 1.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 3.Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16:396–402. doi: 10.1111/dom.12225. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Cerriello A, Esposito K. Efficacy of insulin analogs in achieving the haemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomised controlled trials. Diabetes Care. 2011;34:510–517. doi: 10.2337/dc10-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER study: a randomized controlled trial. Diabetes Obes Metab. 2009;11:45–52. doi: 10.1111/j.1463-1326.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 6.Heise T, Eckers U, Kanc K, Nielsen JN, Nosek L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: a randomized, glucose clamp, crossover study. Diabetes Technol Ther. 2008;10:479–485. doi: 10.1089/dia.2008.0019. [DOI] [PubMed] [Google Scholar]
- 7.Hirao K, Maeda H, Urata S, et al. Comparison of the pharmacokinetic and pharmacodynamic profiles of biphasic insulin aspart 50 and 30 in patients with type 2 diabetes mellitus: a single-center, randomized, double-blind, two-period, crossover trial in Japan. Clin Ther. 2007;29:927–934. doi: 10.1016/j.clinthera.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Rave K, Heinemann L, Puhl L, et al. Premixed formulations of insulin lispro. Activity profiles in type 1 diabetic patients. Diabetes Care. 1999;22:865–866. doi: 10.2337/diacare.22.5.865. [DOI] [PubMed] [Google Scholar]
- 9.Heise T, Weyer C, Serwas A, et al. Time-action profiles of novel premixed preparations of insulin lispro and NPL insulin. Diabetes Care. 1998;21:800–803. doi: 10.2337/diacare.21.5.800. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Heise T, Heinemann L. Insulin aspart in a 30/70 premixed formulation. Pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care. 1997;20:1612–1614. doi: 10.2337/diacare.20.10.1612. [DOI] [PubMed] [Google Scholar]
- 11.Cengiz E, Swan KL, Tamborlane WV, Sherr JL, Martin M, Weinzimer SA. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes Care. 2012;35:690–692. doi: 10.2337/dc11-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryzodeg Summary of Product Characteristics. Novo Nordisk A/S. At: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002499/WC500139011.pdf. Accessed on April 25, 2014.
- 13.Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetologia. 2011;54(suppl 1):S426. [Google Scholar]
- 14.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havelund S, Ribel U, Hubálek F, et al. Insulin degludec (IDeg) and insulin aspart (IAsp) can be co-formulated such that the formation of IDeg multi-hexamers and IAsp monomers is retained upon s.c. injection. Diabetes 2013;62(suppl 1):A241:945-P.
- 16.Heinemann L, Heise T, Jorgensen LN, Starke AA. Action profile of the rapid acting insulin analogue: human insulin B28Asp. Diabet Med. 1993;10:535–539. doi: 10.1111/j.1464-5491.1993.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 17.Korsatko S, Deller S, Mader JK, Glettler K, Koehler G, Treiber G, Urschitz M, Wolf M, Hastrup H, Søndergaard F, Haahr H, Pieber TR. Ultra-long pharmacokinetic properties of insulin degludec are comparable in elderly subjects and younger adults with type 1 diabetes mellitus. Drugs Aging. 2014;31:47–53. doi: 10.1007/s40266-013-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 19.Salsali A, Nathan M. A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am J Ther. 2006;13:349–361. doi: 10.1097/00045391-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Coester H-V, Heise T, Nosek L, Roepstorff C, Segel S, Lassota N, Haahr HL. Steady state is reached within two to three days of once-daily administration of ultra-long-acting insulin degludec. Diabetologia. 2012;55(suppl 1):909. [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 23.Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–950. doi: 10.1111/j.1463-1326.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 24.Tresiba® Summary of Product Characteristics. Novo Nordisk A/S. At: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002498/WC500138940.pdf. Accessed on April 25, 2014.
- 25.Vaag A, Lund S. Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. Eur J Endocrinol. 2012;166:159–170. doi: 10.1530/EJE-11-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garber AJ. Insulin intensification strategies in type 2 diabetes: when one injection is no longer sufficient. Diabetes Obes Metab. 2009;11:14–18. doi: 10.1111/j.1463-1326.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- 27.Fulcher GR, Christiansen JS, Bantwal G, Polaszewska-Muszynska M, Mersebach H, Andersen TH, Niskanen LK, on behalf of the BOOST: Intensify Premix I investigators. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes Care; 2014. doi:10.2337/dc13-2908. [DOI] [PubMed]
- 28.Christiansen JS, Chow FCC, Choi DS, Taneda S, Hirao K. Superior FPG control and less nocturnal hypoglycaemia with IDegAsp vs BIAsp 30 in Asian subjects poorly controlled on basal or pre/self-mixed insulin: randomised phase 3 trial. Diabetologia. 2013;56:S420. [Google Scholar]
- 29.Vaag A, Christiansen JS, Niskanen L, Rasmussen S, Johansen T, Fulcher G. Lower rates of overall, nocturnal and severe hypoglycaemia during maintenance treatment with IDegAsp vs biphasic insulin aspart 30 in patients with type 2 diabetes mellitus: a meta-analysis. Diabetologia. 2013;56:S83. [Google Scholar]
- 30.Heise T, Nosek L, Coester HV, et al. Insulin degludec/insulin aspart produces a dose-proportional glucose-lowering effect in subjects with type 1 diabetes. Poster presented at International Diabetes Federation, 2–6 December 2013, Melbourne, Australia. Poster P-1400.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.