Abstract
Background
Chronic kidney disease (CKD) is increasing being recognized as a global public health problem. However, there is dearth of information on the prevalence, determinants, and management of CKD from low- and middle-income countries. The objectives of the study were to determine the 1) prevalence of CKD; 2) socio-demographic and clinical factors associated with CKD; and 3) the existing management of these patients with regards to blood pressure control, and use of antihypertensive medications.
Methods
We conducted a cross-sectional study on 2873 participants aged ≥40 years in 12 representative communities in Karachi, Pakistan. The primary outcome was clinically significant CKD defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 estimated by CKD-EPI (CKD Epidemiology Collaboration) Pakistan equation (0.686 × CKD-EPI1.059) or urinary albumin to creatinine ratio ≥3 mg/mmol (i.e. KDOQI CKD stage G3, A2 or worse).
Results
The overall prevalence (95% CI) of CKD was 12.5% (11.4 – 13.8%). The factors independently associated with CKD were older age, hypertension, diabetes, elevated systolic blood pressure, raised fasting plasma glucose, raised triglycerides, and history of stroke (p < 0.05 for each). About 267 (74.4%, 69.5 – 78.8%) adults with CKD had concomitant hypertension. Of these, 130 (48.7%, 42.6 – 54.9%) were on antihypertensive medications, and less than 20% had their BP controlled to conventional target of ≤140/90 mm Hg, and only 16.9% (12.6 – 21.9%) were on blockers of renin-angiotensin system alone or in combination with other drugs.
Conclusions
Clinically significant CKD is common among Pakistani adults. The conventional risk factors for CKD and poor control of blood pressure among patients with CKD highlight the need to integrate CKD prevention and management in the primary care infrastructure in Pakistan, and possibly neighbouring countries.
Keywords: Albuminuria, Chronic kidney disease, CKD-EPI Pakistan, Glomerular filtration rate, South Asians
Background
Chronic kidney disease (CKD) is increasing being recognized as a major public health problem globally [1]. The adverse outcomes associated with CKD including kidney failure, accelerated cardiovascular disease (CVD), and premature mortality have greater societal and economical impact in low- and middle-income countries [2]. A glomerular filtration rate (GFR) level of less than 60 ml/min/1.73 m2 (GFR stages G3a – G5), indicating CKD represents loss of half or more of the adult level of normal kidney function, the level below which the risk of adverse outcomes has been shown to increase. As demonstrated in a large meta-analysis of a large general-population cohort of 105,872 participants, albuminuria is an independent marker of increase CVD mortality [3].
The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines 2012 for the Evaluation and Management of Chronic Kidney Disease classify CKD based on eGFR stages (G1 through G5 using eGFR thresholds (G3 split G3a and G3b using eGFR threshold of 45 ml/min/1.73 m2), and albuminuria stages (A1 (<3 mg/mmol), A2 (3 to 30 mg/mmol) and A3 (>30 mg/mmol)). The guidelines also recommend using locally validated CKD-EPI equation as preferred methods for estimating GFR where available [4].
The burden of CKD may be further exaggerated in rapidly urbanizing South Asian country like Pakistan where a substantial proportion of 180 million are predisposed to chronic diseases including diabetes and hypertension by virtue of low birth weight possibly associated with reduced renal reserve [5]. Moreover, South Asian countries are undergoing an epidemiological transition with an increase in risk factors of CKD, and consequently posing a burden on health systems [6]. Furthermore, CKD is also known to progress fast in Asians compared to Western counterparts underscoring the need for prevention through early detection and management of risk factors [7]. However, there is dearth of representative data on the prevalence and determinants of CKD from South Asian countries including Pakistan. Furthermore, despite publications of clear guidelines regarding the importance of blood pressure (BP), control and trials demonstrating effectiveness of blockers of renin-angiotensin system in patients with CKD, it is not known how these patients are managed in low- and middle-income countries that have traditionally diverted resources for treating acute infectious diseases [8,9].
The objectives of this study were to determine the 1) prevalence of CKD stage G3, A2 or worse; 2) socio-demographic and clinical factors associated with CKD; and 3) the existing management of patients with CKD with regards to BP control, and use of antihypertensive medications among adults in Karachi, Pakistan.
Methods
Study setting
This was a cross-sectional study representative of urban city of Karachi, Pakistan, conducted as part of baseline within a factorial design cluster randomized controlled trial [10]. In brief, the Federal Bureau of Statistics has divided the city of Karachi, the most populous city in Pakistan with 18 million inhabitants, into 5000 clusters each of about 250 households each. A multistage random sampling was employed to identify 12 of 4200 low to middle income (mean household monthly income $70) clusters (Additional file 1: Figure S1). All adults aged ≥ 40 years in these households were invited and the written informed consent was obtained from all study participants. Ethical approval was obtained from the Aga Khan University Ethics Review Committee.
Screening
Trained research staff visited all households in each of the 12 clusters, and informed consent was obtained for screening from all eligible adults; who then underwent for measurement of BP three times. The study was conducted over one year during 2004 – 2005.
A routine physical examination was performed and the following information collected: (i) smoking status, food frequency and physical activity (IPAQ, international physical activity questionnaire), co-morbidities (history of stroke, cardiovascular disease, known diabetes, known hypertension); (ii) anthropometry (height, weight and waist circumference); (iii) BP was measured thrice with a calibrated automated device (Omron HEM-737 IntelliSense; Omron Healthcare Inc., Vernon Hills, IL) in the sitting position after 5 minutes of rest. If BP was elevated (systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg) based on mean of last 2 of 3 readings, subjects were visited again after 1 – 4 weeks for re-measurement of BP to confirm hypertension status if BP were persistently elevated, and (iv) Blood specimens for glucose, creatinine and lipids profile were collected in the morning after an overnight fast of about 10 – 12 hours. For urine microalbumin and creatinine, first morning sample was collected. All samples were transported in about one hour to the clinical laboratory at the Aga Khan University Hospital where samples were processed and stored at 2 – 8°C for appropriate tests performed within 24 hours. Serum creatinine and fasting blood glucose were measured on Synchron Cx-7/Delta; Beckman Coulter, Fullerton, CA; the lipid profile was measured on Hitachi-912; Roche, Basel, Switzerland. Urine microalbumin and creatinine were measured using nephelometry by the Array Systems method on a Beckman Coulter and Synchron Cx-7/Delta. All tests were performed to a standard protocol that confirmed to the international standards for definitions and measurements.
Serum creatinine measurements were calibrated at the Cleveland Clinic laboratory-reference laboratory, where serum creatinine levels were measured again using the Roche enzymatic creatinine assay (in duplicate) which is traceable to the National Institute of Standards and Technology creatinine reference measurement [11].
Variable definitions
Glomerular Filtration Rate (GFR) was estimated using the CKD-EPI (CKD Epidemiology Collaboration) Pakistan (CKD-EPIPK) equation, a modified version of CKD-EPI creatinine equation with a correction factor (0.686 × CKD-EPI1.059) for South Asians (estimated GFR (eGFR) based on this equation denoted as eGFRCKD-EPI(PK)) [11].
Hypertension was defined as persistent elevation of SBP ≥140 mm Hg or DBP ≥90 mm Hg on the basis of average of last two of three readings measured 5 minutes apart at each visit, on two separate occasions, or taking antihypertensive medications.
Diabetes was defined as fasting blood glucose ≥7.0 mmol/L, or taking anti-diabetic medications.
CKD (stage G3, A2 or worse) was defined as eGFRCKD-EPI(PK) < 60 mL/min/1.73 m2 (reduced eGFR) or urinary albumin to creatinine ratio (UACR) ≥3 mg/mmol (albuminuria) based on a single spot urine sample.
Statistical analysis
The analyses were performed in IBM SPSS v20 and Stata release 12.1 statistical software. The descriptive statistics were performed on 2873 participants by CKD status. Differences in means and proportions were assessed by t-test and chi-square test respectively. The distribution of eGFR in men and women was assessed graphically by plotting median, 25th and 75th percentiles respectively using quantile regression. The crude and age-standardized prevalence (95% CI) of CKD was computed. The reference population for the latter was WHO (World Health Organization) World Standard Population [12].
The final sample size for multivariable models was 2823 because of missing information on hypertension status on 50 subjects (Additional file 2: Figure S2). Two multivariable models were built for primary outcome of CKD by stepwise logistic regression analysis using forward selection procedure with an entry criteria of p = 0.05 and removal criteria p = 0.20. The first model was based on socio-demographic determinants (age, sex, education, tobacco use, and physical activity), and the second model was based on socio-demographic plus clinical predictors, i.e. hypertension, diabetes, self-reported history of coronary heart disease, self-reported history of stroke, body mass index (BMI), fasting plasma glucose, serum cholesterol, high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), and serum triglycerides. Both the multivariable models developed using stepwise logistic regression were adjusted for clustering; and furthermore, second model was also adjusted for co-morbidities, i.e. history of coronary heart disease and history of stroke.
Moreover, all CKD patients with hypertension were also assessed for 1) control of BP to levels ≤ 130/80 mm Hg, and ≤140 /90 mm Hg, and 2) use of anti-hypertensive medications by UACR status, i.e. UACR <3 mg/mmol and ≥3 mg/mmol, respectively. For these analyses, KDIGO 2012 Clinical Practice Guideline based disease-specific definition of hypertension in CKD (BP >140/90 or BP >130/80 mm Hg for those with UACR <3 mg/mmol and ≥3 mg/mmol, respectively) was used [8]. The use of antihypertensive medications by drug classes was assessed. Single drugs used alone represented mono-therapy and counted as individual drug; whereas two or more single drugs taken together either separately or as fixed dose combination drugs were counted under combination category (and not in the individual drug categories). The p-value <0.05 was considered statistically significant.
Results
A total of 3143 adults were aged 40 years or older in the 12 communities, and were invited for blood and urine tests; these measurements were available on 2873 (91.4%) individuals. The baseline characteristics of the population with and without CKD are shown in Table 1. Moreover, the baseline characteristics according to eGFR and UACR categories are shown in the Additional file 3: Tables S1 and S2.
Table 1.
Socio-demographic and clinical characteristics of individuals with and without Chronic Kidney Disease
| Characteristics | Total (n = 2873) | No CKD 2514 (87.4) | CKD* 359 (12.5) | P-value |
|---|---|---|---|---|
| Age in years, mean ± SD |
51.5 ± 10.7 |
50.5 ± 10.0 |
58.8 ± 12.3 |
<0.001 |
| Women, n (%) |
1499 (52.2) |
1299 (51.7) |
200 (55.7) |
0.152 |
| Education, n (%) |
|
|
|
|
| No education |
992 (34.5) |
832 (33.1) |
160 (44.6) |
<0.001 |
| Primary & middle |
929 (32.3) |
813 (32.3) |
116 (32.3) |
|
| Secondary & higher secondary |
622 (21.6) |
565 (22.5) |
57 (15.9) |
|
| Graduate and above |
330 (11.5) |
304 (12.1) |
26 (7.2) |
|
| Tobacco use, n (%) |
|
|
|
|
| Current users |
1113 (38.7) |
980 (39.0) |
133 (37.0) |
0.003 |
| Past users |
280 (9.7) |
227 (9.0) |
53 (14.8) |
|
| Never users |
1480 (51.5) |
1307 (52.0) |
173 (48.2) |
|
| Employed in any occupation, n (%) |
1209 (42.1) |
1115 (44.4) |
94 (26.2) |
<0.001 |
| Physical Activity, METs < 840, n (%) |
1725 (60.0) |
1473 (58.6) |
252 (70.2) |
<0.001 |
| Hypertension, n (%)║§ |
1267 (44.9) |
1013 (41.0) |
254 (72.2) |
<0.001 |
| Diabetes Mellitus, n (%)† |
615 (21.4) |
465 (18.5) |
150 (41.8) |
<0.001 |
| History of CHD, n (%)†† |
246 (8.6) |
203 (8.1) |
43 (12.0) |
0.013 |
| History of stroke, n (%) |
88 (3.1) |
59 (2.3) |
29 (8.1) |
<0.001 |
| Weight in Kg, mean ± SD |
64.8 ± 14.4 |
65.0 ± 14.3 |
63.4 ± 14.6 |
0.063 |
| Body mass index, mean ± SD║ |
25.8 ± 5.5 |
25.8 ± 5.5 |
25.7 ± 5.1 |
0.767 |
| Systolic BP, mean ± SD |
137 ± 24 |
135 ± 22 |
153 ± 27 |
<0.001 |
| Diastolic BP, mean ± SD |
86 ± 13 |
85 ± 12 |
91 ± 15 |
<0.001 |
| Fasting plasma glucose, mean ± SD‡ |
6.4 ± 2.8 |
6.2 ± 2.6 |
7.7 ± 3.8 |
<0.001 |
| Serum Cholesterol, mean ± SD‡ |
4.9 ± 1.0 |
4.8 ± 1.0 |
5.0 ± 1.2 |
0.003 |
| LDL, mean ± SD‡ |
3.0 ± 0.8 |
3.0 ± 0.8 |
3.1 ± 1.0 |
0.087 |
| HDL, mean ± SD‡ |
1.0 ± 0.3 |
1.0 ± 0.3 |
1.1 ± 0.3 |
0.196 |
| Triglycerides, mean ± SD‡ |
1.8 ± 1.1 |
1.8 ± 1.1 |
2.0 ± 1.2 |
<0.001 |
| eGFR, median (25th –75th percentiles) |
92.8 (82.7 – 101.5) |
94.6 (85.9 – 102.1) |
68.2 (52.1 – 92.5) |
0.001 |
| UACR, median (25th –75th percentiles) | 0.58 (0.38 – 1.07) | 0.53 (0.36 – 0.83) | 6.24 (3.46 – 19.60) | <0.001 |
CKD = Chronic Kidney Disease; METs = Metabolic Equivalents; CHD = Coronary Heart Disease; BP = Blood Pressure; LDL = Low Density Lipoprotein cholesterol; HDL = High Density Lipoprotein cholesterol; UACR = Urinary Albumin to Creatinine Ratio.
*CKD is defined as the eGFR < 60 mL/min/1.73 m2 estimated by CKD-EPIPK (0.686 × CKD-EPI1.059) or urinary albumin to creatinine ratio (UACR) ≥3 mg/mmol.
║Missing observations: for hypertension status 50 were missing and for BMI 2 were missing.
§Hypertension was defined as persistent elevation of SBP ≥140 mm Hg or DBP ≥90 mm Hg on the basis of average of last two of three readings measured 5 minutes apart at each visit, on two separate occasions, or taking antihypertensive medications.
†Diabetes defined as fasting blood glucose ≥ 7.0 mmol/L or on anti-diabetic medications.
††CHD was defined as self-reported history of coronary heart disease.
‡Reported in SI units (mmol/L).
Missing information
Information on serum creatinine and UACR measurements was missing in 261 (8.3%), and 253 (8.0%) participants respectively. The characteristics of subjects with (n = 270) and without missing information on CKD (n = 2873) (i.e. serum creatinine and/or UACR) did not differ by age (51.5 vs. 52.6 years, p = 0.14), gender (men, 48 vs. 48%, p = 0.99), SBP (137 vs. 137 mm Hg, p = 0.576) or history of stroke (3.1 vs. 3.7%, p = 0.563). However, mean BMI (25.8 vs. 24.1 Kg/m2, p < 0.001), mean DBP (86 vs. 84 mm Hg, p = 0.024) or proportion of hypertension (44.9 vs. 32.9%, p < 0.001) was higher among subjects with available CKD information compared to those with missing information. An additional 50 individuals did not have hypertension status confirmed. A comparison of the above-mentioned characteristics with the individuals with complete information (n = 2,823) did not show any significant difference with respect to age, gender, SBP, DBP, or stroke.
CKD prevalence
The distribution of eGFR among adult men and women is illustrated in Figure 1. The crude prevalence (95% CI) of reduced eGFR (eGFRCKD-EPI(PK) <60.0 mL/min/1.73 m2), albuminuria (UACR ≥3 mg/mmol), and CKD was 5.3% (4.5 – 6.2%), 9.4% (8.4 – 10.5%), and 12.5% (11.4 – 13.8%) respectively.
Figure 1.
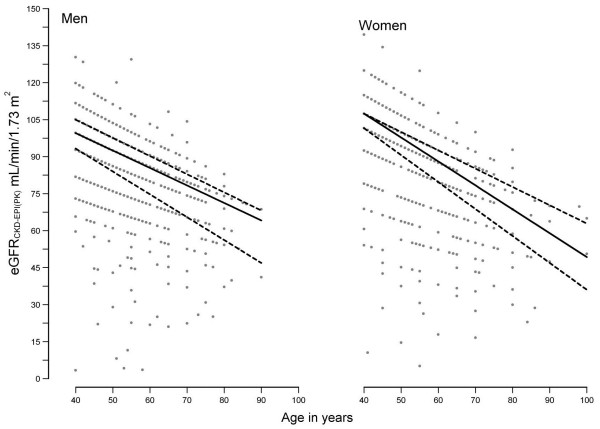
eGFRCKD-EPI(PK) distribution by age in men and women. The lines have been plotted using quantile regression in Stata. The solid line represent median eGFR and dashed lines represents 25th and 75th percentiles respectively. Abbreviation: eGFRCKD-EPI(PK), eGFR calculated by CKD-EPI Pakistan (0.686 × CKD-EPI1.059).
The prevalence of CKD and reduced eGFR was higher in women compared to men, and increased with age (Figure 2). The age standardized prevalence (95% CI) of reduced eGFR (eGFRCKD-EPI(PK) <60.0 mL/min/1.73 m2), albuminuria (UACR ≥3 mg/mmol), and CKD was 7.4% (6.2 – 8.6%), 11.1% (9.8 –12.4%), and 15.3% (13.7 – 16.9%) respectively.
Figure 2.
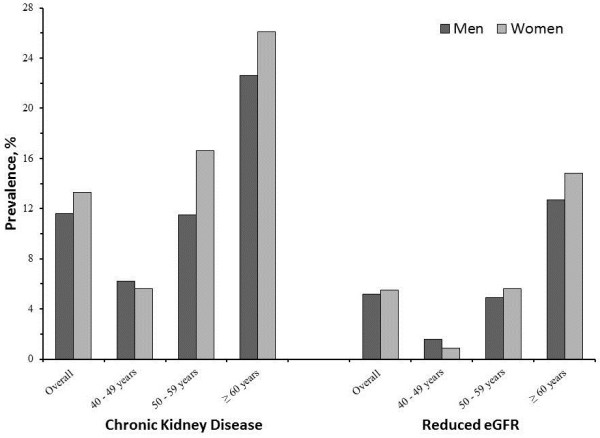
Age and sex specific crude prevalence of chronic kidney disease and reduced eGFR. The dark and light grey bars represent men and women respectively. The prevalence percentages are plotted on y-axis and the age groups are plotted on the x-axis.
CKD determinants
Among the 2873 adults, a total of 359 (12.5%) had CKD. These patients were significantly older, more likely to be less physically active, have concomitant hypertension, diabetes, coronary heart disease and stroke compared to those without CKD (p < 0.05 for each).
Table 2 shows adjusted odds ratios and 95% CI of factors associated with CKD. The socio-demographic and clinical factors independently associated with presence of CKD were older age, hypertension, diabetes, elevated SBP, raised fasting plasma glucose, raised triglycerides, and history of stroke, (p < 0.05 for each)
Table 2.
Multivariable regression models for Chronic Kidney Disease
| Characteristics | Model 1* † Adjusted OR (95% CI) | Model 2* ‡ Adjusted OR (95% CI) |
|---|---|---|
| Age in years |
1.35 (1.28 – 1.41) For each 05 year increase |
1.31 (1.24 – 1.38) For each 05 year increase |
| Physical activity |
|
|
| < 840 METs |
1.35 (1.04 – 1.75) |
- |
| ≥ 840 METs |
1.00 |
|
| Hypertension |
|
|
| Hypertensive |
NA |
1.90 (1.40 – 2.57) |
| Non-hypertensive |
|
1.00 |
| Diabetes mellitus |
|
|
| Diabetic |
NA |
1.69 (1.18 – 2.43) |
| Non-diabetic |
|
1.00 |
| Systolic BP, mm Hg |
NA |
1.15 (1.09 – 1.22) For each 10 mm Hg increase |
| Fasting plasma glucose, mmol/L |
NA |
1.08 (1. – 1.13) For each 1 mmol/L increase |
| Triglycerides, mmol/L |
NA |
1.07 (1.01 – 1.13) For each 0.5 mmol/L increase |
| History of stroke |
|
|
| Positive |
NA |
1.73 (1.03 – 2.92) |
| Negative | 1.00 |
NA = Not applicable; METs = Metabolic Equivalents; BP = Blood Pressure.
*Final sample size for multivariable models was 2823 and models are based on stepwise forward selection method.
†The candidate variables for model 1 include socio-demographic determinants only, i.e. age, gender, educational status, tobacco use and physical activity. The final model was adjusted for clustering.
‡The candidate variables for model 2 include all socio-demographic and clinical variables, i.e. age, gender, educational status, tobacco use and physical activity, hypertension status, diabetes status, history of CHD, history of stroke, body mass index, systolic BP, diastolic BP, fasting plasma glucose, serum cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and total triglycerides. The final model was adjusted for clustering and history of coronary heart disease.
CKD and hypertension
Among 359 individuals with CKD, 267 (74.4%, 95% CI: 69.5 – 78.8%) had concomitant hypertension using CKD specific definition. About 48.7% (42.6 – 54.9%) of these patients were on antihypertensive medications, and less than 20% had their BP controlled to conventional target of ≤140/90 mm Hg (Table 3). Beta blockers were the single most commonly prescribed antihypertensive agent (16.1%, 95% CI: 11.9 – 21.1), followed by various combinations of antihypertensive medications (13.5%, 95% CI: 9.6 – 18.2). Only 9.7% (6.5 – 13.9%) were on blockers of renin-angiotensin system alone whereas 16.9% (12.6 – 21.9%) patients were using these medications either as mono-therapy or in combination with other antihypertensive drugs (Table 4).
Table 3.
Blood pressure control and use of antihypertensive medication among CKD patients with hypertension according to level of UACR
| Characteristics |
Overall, n (%, 95% CIs) |
UACR, mg/mmol, n (%, 95% CIs) |
||
|---|---|---|---|---|
|
<3.0 |
≥ 3.0 |
≥3.0 and diabetes |
||
| n = 267 | N = 56 | N = 211 | N = 107 † | |
| BP ≤ 140/90 mm Hg, n (%, 95% CI) |
51 (19.1, 14.6 – 24.3) |
13 (23.2, 13.0 – 36.4) |
38 (18, 13.1 – 23.9) |
20 (18.7, 11.8 – 27.4) |
| BP ≤ 130/80 mm Hg, n (%, 95% CI) |
17 (6.4, 3.8 – 10.0) |
6 (10.7, 4.0 – 21.9) |
11 (5.2, 2.6 – 9.1) |
6 (5.6, 2.1 – 11.8) |
| Use of antihypertensive medications, n (%, 95% CI) | 130 (48.7, 42.6 – 54.9) | 33 (58.9, 45.0 – 71.9) | 97 (46, 39.1 – 52.9) | 54 (50.5, 40.6 – 60.3) |
UACR = Urinary Albumin to Creatinine Ratio; BP = Blood Pressure.
CKD (G3, A2 or worse) is defined as the eGFR < 60 mL/min/1.73 m2 estimated by CKD-EPIPK equation (0.686 × CKD-EPI1.059) or UACR ≥3 mg/mmol.
†Patients with diabetes mellitus and UACR ≥3 mg/mmol along with CKD and hypertension.
Table 4.
Use of antihypertensive medication by class of drugs among CKD patients with hypertension according to level of UACR
| Anti-hypertensive medications |
Overall, n (%, 95% CIs) |
UACR, mg/mmol, n (%, 95% CIs) |
||
|---|---|---|---|---|
|
<3.0 |
≥3.0 |
≥3.0 and diabetes |
||
| n = 267 | N = 56 | N = 211 | N = 107 ‡ | |
| Antihypertensive medications, mean ± SD |
1.4 ± 0.8 |
1.4 ± 0.7 |
1.4 ± 0.8 |
1.5 ± 0.9 |
| ACEI/ARB only |
26 (9.7, 6.5 – 13.9) |
7 (12.5, 5.2 – 24.1) |
19 (9.0, 5.5 – 13.7) |
10 (9.3, 4.6 – 16.5) |
| ACEI/ARB only or in combination with others |
45 (16.9, 12.6 – 21.9) |
12 (21.4, 11.6 – 34.4) |
33 (15.6, 11–21.3) |
20 (18.7, 11.8 – 27.4) |
| Beta blockers only |
43 (16.1, 11.9 – 21.1) |
9 (16.1, 7.6 – 28.3) |
34 (16.1, 11.4 – 21.8) |
13 (12.1, 6.6 – 19.9) |
| Beta blockers only or in combination with others |
64 (24.0, 19.0 – 29.6) |
15 (26.8, 15.8 – 40.3) |
49 (23.2, 17.7 – 29.5) |
22 (20.6, 13.4 – 29.5) |
| Calcium channel blockers only |
18 (6.7, 4.0 – 10.4) |
4 (7.1, 2.0 – 17.3) |
14 (6.6, 3.7 – 10.9) |
10 (9.3, 4.6 – 16.5) |
| Calcium channel blockers only or in combination with others |
40 (15.0, 10.9 – 19.8) |
9 (16.1, 7.6 – 28.3) |
31 (14.7, 10.2 – 20.2) |
21 (19.6, 12.6 – 28.4) |
| Diuretics only |
3 (1.1, 0.2 – 3.2) |
2 (3.6, 0.4 – 12.3) |
1 (0.5, 0.0 – 2.6) |
1 (0.9, 0.0 – 5.1) |
| Diuretics only or in combination with others |
19 (7.1, 4.3 – 10.9) |
6 (10.7, 4–21.9) |
13 (6.2, 3.3 – 10.3) |
12 (11.2, 5.9 – 18.8) |
| Any combinations† | 36 (13.5, 9.6 – 18.2) | 10 (17.9, 8.9 – 30.4) | 26 (12.3, 8.2 – 17.5) | 18 (16.8, 10.3 – 25.3) |
UACR = Urinary Albumin to Creatinine Ratio; ACEI/ARB = Angiotensin converting enzyme inhibitor/Angiotensin receptor blocker.
†Combination antihypertensive medications are based on various combinations of 2 or more above-mentioned anti-hypertensive drugs. They are not included in the individual drug categories. Each individual drug refers to monotherapy.
‡Patients with diabetes mellitus and UACR ≥3 mg/mmol along with CKD and hypertension.
Discussion
Our population based study is the first to report prevalence and factors associated with CKD from a South Asian country using a locally validated eGFR equation. Our findings indicate a high (12.5%) prevalence of CKD, defined using validated eGFRCKD-EPI(PK) <60 mL/min/1.73 m2, and/or albuminuria ≥3 mg/mmol or higher, among adults aged 40 years or older in Karachi, Pakistan. The prevalence of reduced eGFRCKD-EPI(PK) alone was 5.3% (4.5 – 6.2%). We found that CKD was independently associated with older age, hypertension, diabetes, elevated SBP, raised fasting plasma glucose, raised triglyceride levels, and history of stroke (p < 0.05 for each). Despite a high proportion of associated co-morbidities, CKD remains under-treated with less than 10% and 20% of patients had their BP controlled to targets of ≤130/80 mm Hg, and ≤140/90 mm Hg, respectively per KDIGO 2012 Clinical Practice guidelines [8]. Our results of high prevalence of CKD underscore urgent need for efforts to prioritize CKD on the public health agenda of Pakistan.
The distribution of eGFR in this population illustrate an age related decline, (Figure 1) albeit with relatively well persevered median eGFR among men and women aged 60 years or older [13]. These findings underscore the significance of low eGFR values which should prompt further evaluation even in the elderly in this population.
The high prevalence of CKD is not surprising given the high burden of major CKD risk factors in South Asia. Hypertension and diabetes, both established risk factors for end stage kidney disease, were independently associated with CKD in this population [14,15]. Results of national surveys indicate that hypertension and diabetes affect about 1 in 3 and 1 in 5 adults, respectively, in Pakistan [16-18]. While, trends data on CKD are not available, the 2010 Global Burden of Disease for Pakistan reported a steep rise in the prevalence of major CKD risk factors during the past two decades [6,19,20]. Our finding of high prevalence of CKD is highly suggestive of a parallel increase in the neglected burden of CKD in Pakistan.
We observed that high triglyceride levels were independently associated with CKD. These findings are consistent with those in the Western population demonstrating raised triglycerides in individuals with CKD, [21] which in part confers increased cardiovascular risk. We also found patients with CKD were more likely to have concomitant stroke (adjusted OR, 95% CI: 1.73 (1.03 – 2.92). A number of studies have established that both reduced eGFR and urine albumin excretion, even in the high normal range, predict a graded increase in cardiovascular morbidity [3]. Furthermore, the adverse environmental exposures including high levels of ambient air pollutants and heavy metals are likely to further enhance the CVD risk associated with CKD in this population [22]. Although lead was phased out of gasoline in Pakistan in 2002, the chronic exposure would predispose this population to lead-related nephrotoxicity especially in the presence of CKD, hypertension or diabetes [23-25].
Recent trials data suggest benefit of lipid lowering on CVD morbidity and mortality among patients with CKD [26]. This practice needs to be integrated into CKD prevention efforts in Pakistan.
We found that BP control was grossly sub-optimal with less than 20% of patients having BP controlled to conventional target of ≤140/90 mm Hg. The recent 2012 KDIGO CKD Clinical Practice guidelines underscore BP target of 130/80 mm Hg or less if the patient has CKD with a higher degree of albuminuria (UACR ≥3 mg/mmol) [8]. Clearly the vast majority of patients with CKD failed to meet even the more relaxed target of ≤140/90 mm Hg in Pakistan (Table 3). These findings call for enhancing provider and patient education regarding importance of BP control among patients with CKD.
Evidence suggests benefit of antihypertensive therapy and blockers of renin-angiotensin system are especially protective in patients with CKD and albuminuria [27]. Strikingly, despite significant co-morbidities among patients with CKD, only 48.3% (95% CI: 42.6 – 54.9%) received antihypertensive medications. Moreover use of blockers of renin-angiotensin system was grossly inadequate with barely 17% of patients taking these nephro-protective agents [28] (Table 4). Our results highlight the sub-optimal delivery of CKD care in urban Pakistan where private physicians are the dominant source of service provision, and cost of medications is borne out of pocket [6]. Our previous survey of physicians in Pakistan identified serious deficiencies in knowledge and management of hypertension [29]. The situation is likely to be worse for CKD. Studies in the West have shown that screening for CKD can improve BP control among patients recognized to have CKD [30]. Thus, our findings underscore implementation of KDIGO guidelines for screening and management of CKD to be integrated along with management of hypertension and diabetes in the primary health infrastructure in Pakistan, coupled with provider training and public education on awareness of CKD. Appropriate referral mechanism need to be established for those with advanced stages of CKD. Such a model is likely to be cost-effective for prevention of CKD in Pakistan.
Our study has limitations. First, the cross-sectional design of the study does not permit conclusions regarding causation, and reverse causal association of CKD with BP, lipids, and other factors remains a possibility. However, evidence on the importance of controlling raised BP and glucose levels for slowing progression of CKD is well established. Second, since the study was conducted in Karachi, some variation in findings would be expected in other urban and rural areas of Pakistan. However, national survey indicates a high prevalence of hypertension and diabetes across urban and rural Pakistan [16]. Moreover, practices of providers in terms of management of CKD risk factors, and provision of health services in other areas of Pakistan are not better than in Karachi [29]. Thus, prevalence of CKD is also likely to be comparable and our findings generalizable. Third, we relied on single measurement of serum creatinine and UACR, whereas the clinical definition of CKD requires persistent decrease in eGFR or elevation in UACR for at least 3 months. However, single measurement is considered appropriate for epidemiological research, and has been used widely in other studies [31].
The study has several strengths. This is the first report of prevalence of CKD using a validated eGFR equation with a correction developed in the local South Asian population. Moreover, IDMS (Isotope Dilution Mass Spectrophotometry)-traceable serum creatinine, door to door survey and representative population based on census data of Karachi, and high response rate are the main strength of this study. Thus, our findings would be generalizable to the general population.
Conclusions
In summary, our findings are the first report of prevalence of CKD in Pakistan using a validated CKD-EPIPK estimating equation (eGFRCKD-EPI(PK) <60 mL/min/1.73 m2), or albuminuria (UACR ≥3 mg/mmol). We found that a high prevalence of CKD affecting 12.5% of adults aged 40 years or older. CKD was independently associated with older age, hypertension, diabetes, raised systolic BP, raised plasma fasting glucose, raised triglycerides, and history of stroke. Less than 20% of patients with CKD had BP controlled to conventional levels of ≤140/90 mm Hg. Our findings underscore the urgent need for integrating CKD prevention efforts in the primary care infrastructure in Pakistan, and possibly neighbouring countries with high burden of CKD.
Abbreviations
CKD: Chronic kidney disease; CVD: Cardiovascular disease; BP: Blood pressure; IPAQ: International physical activity questionnaire; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; GFR: Glomerular Filtration Rate; CKD-EPI: CKD Epidemiology Collaboration; CKD-EPIPK: CKD-EPI Pakistan; eGFR: Estimated GFR; eGFRCKD-EPI(PK): eGFR estimated by CKD-EPI Pakistan; UACR: Urinary albumin to creatinine ratio; BMI: Body mass index; HDL: High density lipoprotein cholesterol; LDL: Low density lipoprotein cholesterol; KDIGO: (Kidney Disease Improving Global Outcomes); IDMS: Isotope Dilution Mass Spectrophotometry.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
THJ conceptualized the analytic plan for the study. SJ analyzed the data along with RB and prepared the first draft with advice from THJ. All authors critically reviewed the manuscript and provided input to the interpretation of findings. All authors have read and approved the final manuscript. THJ is the guarantor.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Map of Karachi city with randomized study areas. This map showing the randomized study areas marked as (X) has been adapted from town maps published by the “Master Plan Group of Offices, City District Government, Karachi, Year 2002” publically available on URL: http://www.kmc.gos.pk/Contents.aspx?id=94.
Flow diagram of study participants aged ≥ 40 years.
Socio-demographic and clinical characteristics of individuals according to levels of estimated glomerular filtration rate. Table S2. Socio-demographic and clinical characteristics of individuals with and without albuminuria.
Contributor Information
Saleem Jessani, Email: saleem.jessani@aku.edu.
Rasool Bux, Email: rasool.bux@aku.edu.
Tazeen H Jafar, Email: tazeen.jafar@duke-nus.edu.sg.
Acknowledgements
The study was supported by a research award (070854/Z/03/Z) from Wellcome Trust, UK. The design, conduct, analysis, interpretation, and presentation of the data were the responsibility of the authors with no involvement from the funder.
We thank all research, and support staff for their hard work to the success of this study. We express our appreciation to all participants for their cooperation during the study.
Information about presentations of this work
The abstracts based on this work have been presented at the World Congress of Nephrology on June 02, 2013 in Hong Kong, and at the annual meeting of American Society of Nephrology on November 07, 2013, in Atlanta, GA.
References
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter, Suppl. 2013;3:1–150. [Google Scholar]
- Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;68:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Haaland BA, Rahman A, Razzak JA, Bilger M, Naghavi M, Mokdad AH, Hyder AA. Non-communicable diseases and injuries in Pakistan: strategic priorities. Lancet. 2013;381:2281–2290. doi: 10.1016/S0140-6736(13)60646-7. [DOI] [PubMed] [Google Scholar]
- Fischbacher CM, Bhopal R, Rutter MK, Unwin NC, Marshall SM, White M, Alberti KG. Microalbuminuria is more frequent in South Asian than in European origin populations: a comparative study in Newcastle, UK. Diabet Med. 2003;20:31–36. doi: 10.1046/j.1464-5491.2003.00822.x. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Inter Suppl. 2012;2:337–414. [Google Scholar]
- Baltatzi M, Savopoulos C, Hatzitolios A. Role of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in hypertension of chronic kidney disease and renoprotection. study results. Hippokratia. 2011;15:27–32. [PMC free article] [PubMed] [Google Scholar]
- Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, Bux R, Khan A, Jafary FH, Hameed A, Badruddin SH, Chaturvedi N. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med. 2009;151:593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- Jessani S, Levey AS, Bux R, Inker LA, Islam M, Chaturvedi N, Mariat C, Schmid CH, Jafar TH. Estimation of GFR in South Asians: A study from the general population in Pakistan. Am J Kidney Dis. 2014;63:49–58. doi: 10.1053/j.ajkd.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard. GPE Discussion Paper series no. 31. Geneva: WHO; 2001. [Google Scholar]
- Wang X, Vrtiska TJ, Avula RT, Walters LR, Chakkera HA, Kremers WK, Lerman LO, Rule AD. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85:677–685. doi: 10.1038/ki.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA. 1997;278:2069–2074. doi: 10.1001/jama.1997.03550230045035. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Levey AS, Jafary FH, White F, Gul A, Rahbar MH, Khan AQ, Hattersley A, Schmid CH, Chaturvedi N. Ethnic subgroup differences in hypertension in Pakistan. J Hypertens. 2003;21:905–912. doi: 10.1097/00004872-200305000-00014. [DOI] [PubMed] [Google Scholar]
- Shera AS, Rafique G, Khwaja IA, Ara J, Baqai S, King H. Pakistan national diabetes survey: prevalence of glucose intolerance and associated factors in Shikarpur, Sindh Province. Diabet Med. 1995;12:1116–1121. doi: 10.1111/j.1464-5491.1995.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Shera AS, Jawad F, Maqsood A. Prevalence of diabetes in Pakistan. Diabetes Res Clin Pract. 2007;76:219–222. doi: 10.1016/j.diabres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, Badawi A. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417–E423. doi: 10.1503/cmaj.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansha M, Ghauri B, Rahman S, Amman A. Characterization and source apportionment of ambient air particulate matter (PM2.5) in Karachi. Sci Total Environ. 2012;425:176–183. doi: 10.1016/j.scitotenv.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Kadir MM, Janjua NZ, Kristensen S, Fatmi Z, Sathiakumar N. Status of children's blood lead levels in Pakistan: implications for research and policy. Public Health. 2008;122:708–715. doi: 10.1016/j.puhe.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Jessani S, Jafary FH, Ishaq M, Orakzai R, Orakzai S, Levey AS, Chaturvedi N. General practitioners' approach to hypertension in urban Pakistan: disturbing trends in practice. Circulation. 2005;111:1278–1283. doi: 10.1161/01.CIR.0000157698.78949.D7. [DOI] [PubMed] [Google Scholar]
- Richards N, Harris K, Whitfield M, O'Donoghue D, Lewis R, Mansell M, Thomas S, Townend J, Eames M, Marcelli D. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23:549–555. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Map of Karachi city with randomized study areas. This map showing the randomized study areas marked as (X) has been adapted from town maps published by the “Master Plan Group of Offices, City District Government, Karachi, Year 2002” publically available on URL: http://www.kmc.gos.pk/Contents.aspx?id=94.
Flow diagram of study participants aged ≥ 40 years.
Socio-demographic and clinical characteristics of individuals according to levels of estimated glomerular filtration rate. Table S2. Socio-demographic and clinical characteristics of individuals with and without albuminuria.


