Abstract
A total of 324 Japanese rice accessions, including landrace, improved, and weedy types were used to 1) investigate genetic variations in blast resistance to standard differential isolates, and 2) across the genome using polymorphism data on 64 SSR markers. From the polymorphism data, the accessions were classified into two clusters. Accessions from irrigated lowland areas were included mainly in cluster I, and upland and Indica types were mainly in cluster II. The accessions were classified into three resistance subgroups, A2, B1 and B2, based on the reaction patterns to blast isolates. The accessions in A2 were postulated to have at least two resistance genes Pish and Pik-s, whereas those in B1 had various combinations of the resistance genes Pish, Pia, Pii, Pi3, Pi5(t), and Pik alleles. The B2 accessions were resistant to almost all isolates, and many accessions of cluster II were included, and had Pish, Pia, Pii, Pi3, Pi5(t), certain Pik, Piz and Pita alleles, and unknown genes. The frequencies of accessions of B1 originating in Hokkaido, and those of B2 originating in the Kanto and Tohoku regions were remarkably higher than in the other regions.
Keywords: blast (Pyricularia oryzae Cavara), differential system, genetic variation, resistance, rice (Oryza sativa L.)
Introduction
Rice blast, caused by the fungus Pyricularia oryzae Cavara, is the most serious rice disease in Japan and worldwide. The interaction between host resistance and fungus virulence in the rice blast pathosystem can be explained by the gene-for-gene theory: for every resistance gene in the host, there is a corresponding avirulence gene in the pathogen (Flor 1956, Silue et al. 1992). On the basis of the gene-for-gene theory, Kiyosawa (1984) and Yamada et al. (1976) selected nine and 12 differential varieties (DVs), respectively targeting nine and 12 blast resistance genes. Kobayashi et al. (2007), Telebanco-Yanoria et al. (2010) and Tsunematsu et al. (2000) developed monogenic lines and Lijiangxintuanheigu (LTH) near-isogenic lines as new DV sets. These new DV sets targeted 23 resistance genes and made it possible to efficiently characterize the pathogenicity of P. oryzae isolates. Telebanco-Yanoria et al. (2008a) clarified the pathogenicities of P. oryzae isolates from the Philippines and selected standard differential blast isolates (SDBIs) by using the new DVs. In Japan, SDBIs were selected by Hayashi (2005) and have been used in pathological and genetic studies. These sets of DVs and SDBIs, and data on their reactions, have been used as a differential system for determining the blast resistance of rice varieties and for assessing the virulence of the pathogen.
Yaegashi et al. (1983) tried to postulate the blast resistance genes in recommended Japanese rice varieties by using several Japanese P. oryzae isolates. They reported that 185 accessions (33.1%) did not have any resistance genes that were effective against the test isolates. A total of 256 (45.8%) and 59 (10.6%) accessions were estimated to have Pia and Pii, respectively, but a few had other genes, such as Pik, Pik-m, Piz, Pib, Pita, and Pita-2. Kiyosawa et al. (1986) found that varieties in Japan showed different reactions to P. oryzae isolates from Indica type rice from Brazil, the Philippines (International Rice Research Institute, IRRI), and China. Telebanco-Yanoria et al. (2008b) assessed the genetic diversity of blast resistance using a worldwide collection of 922 varieties including accessions from Japan. They found that susceptible varieties were dominant in Japan and the variation in resistance was narrow compared with that in rice from tropical Asia and Africa. It is therefore likely that genetic variation in the blast resistance of rice varieties in Japan is limited, and susceptible varieties are common.
Yokoo et al. (2005) reviewed the annual changes in leading rice varieties grown in Japan over a 45-year period from 1956 to 2000. The 10 most popular varieties accounted for 70–78.9% of the total rice area, and since the 1970s only a small number of varieties have been used in Japan’s irrigated lowlands. The top six varieties cultivated in Japan from 2000 to 2010 were Koshihikari, Hitomebore, Hinohikari, Akitakomachi, Kirara397 and Kinuhikari. In Japan’s irrigation lowlands, the area grown to Koshihikari was 35% to 37%, and the area of all six varieties together was 67% to 70% of the total rice area (http://ineweb.narcc.affrc.go.jp/, http://www.maff.go.jp/j/tokei/kouhyou/sakumotu/index.html). Hitomebore, Kinuhikari, Akitakomachi, Hinohikari, and Kirara397 all have Koshihikari in their pedigrees. It is therefore likely that little genetic variation is present among Japan’s leading rice varieties.
Since 1981, trials to develop super-high-yielding rice varieties were started by using Indica-type varieties bred by, and introduced from, the IRRI and Korea (Sunohara 1990). These approaches have succeeded in breeding animal-feed varieties (Kato 2008). Indica-type rice varieties began to be used in Japanese rice breeding in the 1980s, but their cultivation is still limited in Japan. Ushiki et al. (2005) found weedy rices in Okayama Prefecture that included Japonica, Indica, and intermediate types. Kawasaki et al. (2009) suggested that these weedy rices were of multiple origin and may have been derived from the animal-feed rice variety Moretsu or from other Indica-type modern varieties. Hosoi et al. (2008) reported the occurrence of weedy rice in Nagano Prefecture. These Indica-type varieties and weedy-type accessions may have genetic backgrounds different from those of conventional Japanese rice varieties, but their genetic variation and genotype in terms of blast resistance have not yet been elucidated fully.
Kono et al. (2000) investigated DNA polymorphism among 15 Japonica varieties (10 landraces and five improved varieties) by using restriction fragment length polymorphism (RFLP), random amplified polymorphic DNAs, amplified fragment-length polymorphism, and simple sequence repeat (SSR) markers. Although there was little polymorphism with all marker types,the results indicated that the use of RFLP and SSR markers could facilitate the genetic analysis of temperate Japonica varieties. Okoshi et al. (2004) used polymorphism analysis and SSR markers to investigate 73 landraces originating from Japan. They classified the landraces into two major groups, Indica and Japonica, including upland rice and paddy rice subgroups. There were thus two rice variety types in Japan, Indica and Japonica, and these could be classified into upland and irrigated lowland varieties using SSR markers. Yamasaki and Ideta (2013) analyzed 114 accessions composed of 94 improved varieties and 20 landraces, which were representative and important for Japanese rice breeding, and a total of 706 alleles from 134 SSR markers. They showed that the landraces exhibited greater genetic diversity than improved lines. Using genome-wide single-nucleotide polymorphisms (SNPs), Yonemaru et al. (2012) investigated the change in haplotype structure and composition of 177 Japanese rice accessions, including landraces and improved varieties and found differences between landraces and improved varieties. Yamasaki and Ideta (2013) and Yonemaru et al. (2012) indicated that the genetic diversity of landraces was higher compared to improved varieties in Japan. To understand genetic variation in rice varieties, polymorphism data obtained by using DNA markers, such as SSRs, will be useful for differentiating between upland and irrigated lowland varieties, between Japonica and Indica types, and between landrace and improved varieties.
To study genetic variation in Japanese rice varieties, we used SSR markers and rice accessions collected from throughout the country. We then evaluated the genetic variation for blast resistance in Japanese rice by comparing their reaction patterns to several SDBIs from Japan (Hayashi 2005) and the Philippines (Telebanco-Yanoria et al. 2008a) with the reactions of DVs according to Kobayashi et al. (2007), Telebanco-Yanoria et al. (2010) and Tsunematsu et al. (2000). Based on genomic differences clarified by polymorphism data of SSR markers, we also discuss the genetic variation in blast resistance in rice accessions and the present situation of rice varieties in Japan in terms of their relationships between upland and irrigated lowland varieties, between Japonica and Indica types, and among landrace, improved and weedy types.
Materials and Methods
Plant materials
A total of 324 Japanese rice (Oryza sativa L.) accessions, consisting of 230 irrigated lowland (9 landraces and 221 improved varieties), 69 upland (52 landraces and 17 improved varieties), 24 weedy types collected and characterized by Hosoi et al. (2008), Kawasaki et al. (2009), and Ushiki et al. (2005), the Indica-type check variety Kasalath, and the Japonica-type check variety Taichung 65, were used to investigate genetic variation in resistance to blast disease (Table 1).
Table 1.
Rice accessions used in this study
| Ecosystem and type | No. of accessions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Regiona | Total | ||||||||||
|
| |||||||||||
| Hokkaido | Tohoku | Kanto | Hokuriku | Tokai | Kinki | Chugoku/Shikoku | Kyushu | Other | |||
| Lowland | Landrace | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 2 | 2 | 10 |
| Improved | 24 | 33 | 20 | 34 | 41 | 19 | 16 | 33 | 1 | 221 | |
|
| |||||||||||
| Total | 24 | 33 | 21 | 35 | 42 | 19 | 19 | 35 | 3 | 231 | |
|
| |||||||||||
| Upland | Landrace | 0 | 7 | 19 | 0 | 5 | 2 | 3 | 7 | 9 | 52 |
| Improved | 1 | 3 | 7 | 0 | 1 | 4 | 0 | 1 | 0 | 17 | |
|
| |||||||||||
| Total | 1 | 10 | 26 | 0 | 6 | 6 | 3 | 8 | 9 | 69 | |
|
| |||||||||||
| Weedy | 0 | 0 | 0 | 0 | 13 | 0 | 11 | 0 | 0 | 24 | |
|
| |||||||||||
| Grand total | 25 | 43 | 47 | 35 | 61 | 25 | 33 | 43 | 12 | 324 | |
Location where variety was released (improved) or originally grown (landrace or weedy).
Weedy-type accessions were collected from Nagano Prefecture in the Tokai region and from Okayama Prefecture in the Chugoku region (Ushiki et al. 2005).
Other: Including Japonica variety Taichung 65 (Taiwan) and the Indica variety Kasalath (India) as controls and several accessions for which origin is unknown.
Rice accessions developed by cross-breeding after 1921 were categorized as improved types.
The accessions were collected from eight regions of Japan including Hokkaido, Tohoku, Kanoto, Hokuriku, Tokai, Chugoku/Shikoku, Kyushu, and Okinawa. Accessions developed by cross-breeding after 1921 were categorized as improved types, and the other accessions were classified as landraces or weedy types.
To postulate the presence of specific resistance genes in the test accessions, their reaction patterns to the SDBIs were compared to those of a set of 26 control varieties. The controls included 23 monogenic lines (Kobayashi et al. 2007, Tsunematsu et al. 2000) with resistance genes as follows; IRBLsh-B for Pish, IRBLt-K59 for Pit, IRBLb-B for Pib, IRBLa-A for Pia, IRBLi-F5 for Pii, IRBL3-CP4 for Pi3, IRBL5-M for Pi5(t), IRBLks-F5 for Pik-s, IRBLkm-Ts for Pik-m, IRBL1-CL for Pi1, IRBLkp-K60 for Pik-p, IRBL7-M for Pi7(t), IRBL9-W for Pi9(t), IRBLz-Fu for Piz, IRBLz5-CA for Piz-5, IRBLzt-T for Piz-t, IRBLta2-Re and IRBLta2-Pi for Pita-2, IRBL12-M for Pi12(t), IRBLta-K1 and IRBLta-CP1 for Pita, IRBl19-A for Pi19(t) and IRBL20-IR24 for Pi20(t), two near isogenic lines, IRBLkh-K3[LT] for Pik-h and IRBLk-K[LT] for Pik (Telebanco-Yanoria et al. 2010), and a susceptible variety Lijiangxintuanheigu (LTH).
Extraction of DNA and genotyping by using SSR markers
To assess the relationship among Japanese rice accessions based on SSR marker profile, a total of 64 SSR markers distributed over the 12 rice chromosomes were used (Table 2). All SSR markers were selected from a public database (http://www.gramene.org).
Table 2.
Classification of rice accessions on the basis of polymorphism of DNA markers
| Cultivated ecosystems | Variety type | Regions of varieties bred | No. of accessions (%) | ||
|---|---|---|---|---|---|
|
| |||||
| Cluster group | Sum | ||||
|
| |||||
| I | II | ||||
| Irrigated lowland | Landrace | Kanto | 1 | 0 | 1 |
| Hokuriku | 1 | 0 | 1 | ||
| Tokai | 1 | 0 | 1 | ||
| Chugoku/Shikoku | 2 | 1 | 3 | ||
| Kyushu | 0 | 2 | 2 | ||
| Others | 0 | 2 | 2 | ||
|
| |||||
| Sum | 5 (1.5) | 5 (1.5) | 10 (3.1) | ||
|
| |||||
| Improved | Hokkaido | 24 | 0 | 24 | |
| Tohoku | 33 | 0 | 33 | ||
| Kanto | 17 | 3 | 20 | ||
| Hokuriku | 32 | 2 | 34 | ||
| Tokai | 40 | 1 | 41 | ||
| Kinki | 19 | 0 | 19 | ||
| Chugoku/Shikoku | 16 | 0 | 16 | ||
| Kyushu | 32 | 1 | 33 | ||
| Others | 1 | 0 | 1 | ||
|
| |||||
| Sum | 214 (66.0) | 7 (2.2) | 221 (68.2) | ||
|
| |||||
| Total | 219 (67.6) | 12 (3.7) | 231 (71.3) | ||
|
| |||||
| Upland | Landrace | Tohoku | 4 | 3 | 7 |
| Kanto | 2 | 17 | 19 | ||
| Tokai | 1 | 4 | 5 | ||
| Kinki | 1 | 1 | 2 | ||
| Chugoku/Shikoku | 2 | 1 | 3 | ||
| Kyushu | 2 | 5 | 7 | ||
| Others | 2 | 7 | 9 | ||
|
| |||||
| Sum | 14 (4.3) | 38 (11..7) | 52 (16.0) | ||
|
| |||||
| Improved | Hokkaido | 0 | 1 | 1 | |
| Tohoku | 0 | 3 | 3 | ||
| Kanto | 0 | 7 | 7 | ||
| Tokai | 1 | 0 | 1 | ||
| Kinki | 1 | 3 | 4 | ||
| Kyushu | 0 | 1 | 1 | ||
|
| |||||
| Sum | 2 (0.6) | 15 (4.6) | 17 (5.2) | ||
|
| |||||
| Total | 16 (4.9) | 53 (16.4) | 69 (21.3) | ||
|
| |||||
| Weedy | Tokai | 12 | 1 | 13 | |
| Chugoku/Shikoku | 4 | 7 | 11 | ||
|
| |||||
| Total | 16 (4.9) | 8 (2.5) | 24 (7.4) | ||
|
| |||||
| Grand total | 251 (77.5) | 73 (22.5) | 324 (100.0) | ||
Rice accessions were classified by using Ward’s hierarchical clustering method on the basis of polymorphism data on 64 SSR markers distributed over the 12 rice chromosomes.
Genomic DNA was extracted from a young leaf from each accession. Leaf tissue was ground in 100 μl of 0.25 N NaOH with zirconium beads in 2.0-ml tubes. A volume of 400 μl of 100 mM Tris-HCl (pH 7.5) was added to each tube. The sample was then mixed and centrifuged for 10 min at 10,000 rpm. The supernatant was poured into a fresh 1.5-ml tube. PCR was performed in a 10-μl PCR mixture containing 1 μl sterile H2O, a total of 1.5 μl forward primer (2 μM) and reverse primer (2 μM), 7.5 μl of 2× Quick Taq HS DyeMix (Toyobo Co., Ltd.), and 5 μl DNA concentrated to about 5 to 10 ng/μl. PCR amplification was performed with the following profile: 94°C for 2 min, 40 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 68°C. To detect polymorphisms, the amplified products were separated by electrophoresis on 2% agarose gels in 1× TAE buffer at 150 V for 90 to 120 min, and the DNA fragment was detected with ethidium bromide.
Inoculation of Pyricularia oryzae isolates and evaluation of resistance
Rice accessions were inoculated with 11 standard P. oryzae isolates from Japan (Hayashi 2005, Yamazaki and Kiyosawa 1966) and five isolates from the Philippines (Telebanco-Yanoria et al. 2008a), and the degree of infection was rated.
Six seeds of each accession were sown in a plastic cell tray (ϕ16 × 25 mm, 5 holes × 7 holes) and grown to the 4th- to 5th-leaf stage in a greenhouse at 27°C. Each cell tray also contained LTH as a susceptibile check. The spore concentration was standardized to 1 × 105/ml and 40-ml of the suspension was sprayed onto each tray with a fine atomizer 21 days after sowing. The degree of infection of each seedling was evaluated 7 days after inoculation by using the scale of 0 to 5 of Hayashi et al. (2009).
The resistance genes present in each rice accession were postulated based on the reaction patterns of 16 standard differential blast isolates from Japan and the Philippines in comparison to those of the 23 DVs and the susceptible check, LTH. It was assumed that only one resistance gene contributed the reaction of each rice accession for chromosome regions corresponding to Piz, Piz-t, Piz-5 and Pi9(t) on chromosome 6, Pii, Pi3 and Pi5(t) on chromosome 9, Pik-s, Pik-m, Pik, Pik-h, Pi1, Pik-p and Pi7(t) onchromosome 11, and Pita, Pita-2, Pi12(t), Pi19(t) and Pi20(t) on chromosome 12.
Classification of varieties
Rice accessions were classified based on marker data and reactions to the SDBIs using Ward’s hierarchical clustering method with the computer program JMP7.0.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Variation in SSR marker profile
Sixty-one markers of the 64 markers tested showed polymorphism among the 324 accessions (Supplemental Table 1). The number of alleles detected per marker ranged from two to five (average 2.9), and a total of 176 alleles were found (data not shown).
The rice accessions were classified into two clusters, I and II (Table 2). Most of the irrigated lowland accessions (219, 67.6%) were in cluster I, and most of the upland accessions (69, 21.3%) were in cluster II. Among the 24 weedy types, 16 and 8 accessions were classified into clusters I and II, respectively. Nipponbare and the control Taichung 65 (Japonica) fell into cluster I, and Kasalath (Indica) fell into cluster II. In addition, many accessions with Indica genetic backgrounds, such as animal-feed varieties, high-yielding varieties, and Tetep (animal-feed variety parent), were also in cluster II.
These results indicated the tendency for accessions from irrigated lowland and Japonica types to be classified into cluster I, and for upland and Indica types to be classified into cluster II. Weedy types were included in both clusters.
Genetic variation in blast resistance
The DVs for resistance genes, Pia, Pish, Pii, Pi3, Pi5(t), Pik-s, Pik-m, Pi1, Pik, Pik-h, Pik-p, Pi7, Pita, Piz, Piz-t, Piz-5, Pi9(t), Pita-2, Pi20(t), Pi12(t), Pib and Pit, were resistant to at least one of the three SDBIs, but the DV for Pi19(t) was susceptible to all SDBIs. Thus, the presence of Pi19(t) could not be detected in the genetic backgrounds of rice accessions. Accessions were classified into two major groups, A and B, including two respective subgroups, A1 and A2 and B1 and B2 (Supplemental Table 2).
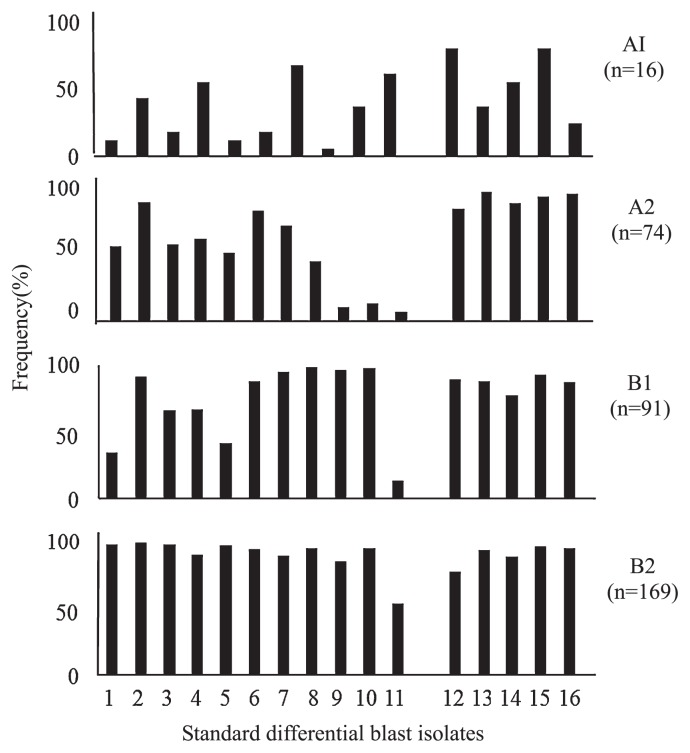
Infection ratings of 0, 1 and 2, were classified as resistant, and ratings of 3, 4 and 5 were classified as susceptible. The resistantce frequencies of accessions to each SDBI were compared among the four subgroups (Fig. 2). A total of 16 accessions were classified into subgroup A1. This subgroup consisted of 15 DVs, namely IRBLsh-B for Pish, IRBLa-A for Pia, IRBLi-F5 for Pii, IRBL3-CP4 for Pi3, IRBL5-M for Pi5(t), IRBLks-F5 for Pik-s, IRBLkm-Ts for Pik-m, IRBLk-Ka for Pik, IRBLkh-K3 for Pik-h, IRBL1-CL for Pi1, IRBLkp-K60 for Pik-p, IRBL7-M for Pi7(t), IRBLta-K1 and IRBLta-CP1 for Pita, IRBL19-A for Pi19(t) and the susceptible check, LTH. These DVs showed gene-specific reactions against SDBIs from both Japan and the Philippines.
Fig. 2.
Frequency of resistant in rice accessions and differential varieties to blast isolates from Japan and the Philippines. Classification by Ward’s hierarchical clustering method of 350 rice accessions, including 25 differential varieties and a susceptible control, LTH, was performed on the basis of the reaction patterns against 16 blast isolates from Japan and the Philippines. A total of 11 blast isolates from Japan (Hayashi 2005), namely 1 (Ken53-33), 2 (Sasamori121), 3 (P-2b), 4 (Kyu92-22), 5 (GFOS8-1-1), 6 (Mu183), 7 (TH68-140), 8 (Mu95), 9 (H05-72-1), 10 (93-406(B)), and 11 (H07-76-1), and five isolates from the Philippines (Telebanco-Yanoria et al. 2008a), namely 12 (M64-1-4-9-1), 13 (V86010), 14 (BN111), 15 (V850196), and 16 (IK81-25) were used. Degrees of infection by blast isolates on rice accessions were evaluated on a scale of 0 to 5, where 0 is resistant and 5 is susceptible, by using the method of Hayashi and Fukuta (2009).
A total of 74 accessions were classified into subgroup A2. The accessions in this subgroup had specific reactions to SDBIs from Japan, but were highly resistant to all the SDBIs from the Philippines. Almost all accessions were commonly susceptible to the three Japanese SDBIs, 9 (H05-72-1), 10 (93-406(B)) and 11 (H07-76-1), which were all virulent to DVs for Pish, Pik-s, and Pi19(t). Thus almost all accessions in A2 probably have only Pish and Pik-s in their genetic backgrounds. Since unexpected reactions to the other 14 SDBIs were also found, unknown gene(s) were likely present in all accessions of this group. The Japonica check Taichung 65, Nipponbare, and the leading variety Koshihikari, which has excellent eating quality, were included in this group.
A total of 91 accessions were classified into subgroup B1, including five DVs, IRBLz-Fu for Piz, IRBLz5-CA for Piz-5, IRBLta2-Pi and IRBLta2-Re for Pita-2 and IRBL20-IR24 for Pi20(t). The resistance of this subgroup was higher than that of subgroup A2. Clear susceptible reactions were found to three SDBIs from Japan: 1 (Ken53-33, which was virulent to DVs for Pish, Pia, Pii, Pi3, Pi5(t), Pik-s, Pik-m, Pi1, Pik-h, Pik, Pik-p, Pi7, Pita, Pi19(t), Piz-5 and Pita-2), 5 (GFOS8-1-1, which was virulent to DVs for Pish, Pia, Pik-s, Pita, Pi19(t), Pita-2 and Pi20(t)), and 11 (H07-76-1, which was virulent to DVs for Pish, Pia, Pii, Pi3, Pi5(t), Pik-s, Pik-m, Pik, Pik-h, Pik-p, Pi7(t) and Pi19(t)). Almost all accessions were susceptible to H07-76-1. Some of these 9 resistance genes (Pia, Pii, Pi3, Pi5(t), Pik-m, Pik, Pik-h, Pik-p and Pi7(t)) may be present in these accessions, in addition to Pish or Pik-s. Kirara 397 and Kinuhikari were included in this group. Kirara 397 was postulated to have some Pia, one of three (Pii, Pi3 or Pi5(t)), or one of six (Pik-s, Pik-m, Pik, Pik-h, Pik-p or Pi7(t)); Kinuhikari was posutulated to have Pish, and either one of three (Pii, Pi3 or Pi5(t)) or one of five (Pik-s, Pik-m, Pik, Pik-p or Pi7(t)). This group also showed higher resistance to SDBIs from the Philippines than to those from Japan, and probably carry additional unknown gene(s) in all accessions based on unexpected reactions to the SDBLs.
A total of 164 rice accessions and five DVs (IRBLb-B for Pib, IRBLt-K59 for Pit, IRBL9-W for Pi9, IRBLzt-T for Piz-t, and IRBL12-M for Pi12) were classified into subgroup B2. Almost all of these accessions were resistant to all SDBIs from Japan and the Philippines, and B2 was the most resistant among the four groups. The genotypes of these resistant accessions could not be estimated. However, the rice accessions which showed susceptible reactions probably had Pib, Pit, Piz alleles (Piz, Piz-t, Piz-5 or Pi9(t)) and Pita alleles (Pi12(t), Pita or Pi20(t)), in addition to Pish, Pia, Pii, Pi3, Pi5(t) or Pik alleles or Pi7(t). The Japanese leading varieties Hitomebore, Hinohikari, and Akitakomachi were included in this group. The genotypes of Hinohikari and Akitakomachi were postulated as Pish, Pia, one of three genes (either Pii, Pi3 or Pi5(t)), and one of five (either Pik-s, Pik-m, Pik, Pik-p or Pi7(t)). Hitomebore was postulated to have Pia, one of three genes (Pii, Pi3 or Pi5(t)), and one of four genes (either Pik-m, Pik, Pik-p or Pi7(t)). In addition to the irrigated lowland varieties, this subgroup included many blast resistant upland varieties and high-yielding varieties with Indica-type genetic backgrounds.
Thus, Japanese rice accessions were classified into three cluster subgroups, A2, B1 and B2, among which the degrees of resistance varied. Among these three groups, the reactions to three SDBIs, H05-72-1, 93-406(B) and H07-76-19, which were all virulent to DVs for Pish, Pik-s and Pi19(t), varied dramatically. This variation was likely due to the presence of other resistance gene(s) in the genetic background of the rice accessions. Therefore, Pish, Pik-s or Pi19(t) are probably commonly present in Japanese varieties, and the other resistance genes, Pia, Pii, Pi3, Pi5(t), Piz alleles, Pik alleles and Pi7(t), and Pita alleles, played a basic role in the classification. There were no avirulence for Pi19(t) among the isolates used, and thus the present of Pi19(t) could not be detected in this study.
Rice accessions showed specific reactions to SDBLs from Japan, but almost all accession were resistance to isolates from the Phlippines. The difference in the reactions to blast isolates between Japan and the Philippines indicate that some gene(s) in the genetic backgrounds of the rice accessions gave different reactions between SDBIs from Japan and the Philippines.
Relationships between variety groups, as classified by resistance and by polymorphism patterns of SSR markers
We found some unique relationships between the three resistance subgroups (A2, B1 and B2) and the two clusters (I and II), as classified by the polymorphism patterns of SSR markers, in terms of the frequencies of rice accessions (Table 3).
Table 3.
Relationships between variety groups classified on the basis of resistance to Pyricularia oryzae isolates and polymorphism patterns of SSR markers
| Group classified on the basis of reactions to blast isolates | Clusters classified on the basis of data on polymorphism of SSR markers (%) | ||
|---|---|---|---|
|
| |||
| I | II | Total | |
| A2 | 71 (21.9) | 3 (0.9) | 74 (22.8) |
| B1 | 83 (25.6) | 3 (0.9) | 86 (26.5) |
| B2 | 97 (29.9) | 67 (20.7) | 164 (50.6) |
|
| |||
| Total | 251 (77.5) | 73 (22.5) | 324 (100.0) |
The propotion of the resistance subgroups varied greatly between clusters of accessions based on SSR polymorphism data. The accessions in cluster I included mainly cultivated varieties or weedy-type rice from irrigated lowland ecosystems; the frequencies of the subgroups were A2, 28.3%; B1, 33.1%; and B2, 38.6%. The frequencies of the three subgroups in cluster II were A2, 4.1%, B1, 4.1% and B2, 91.8%, and thus almost all of these accessions were upland varieties and those with Indica-type genetic backgrounds (i.e. B2).
These results indicated that the accessions in cluster I including the lowland accessions had a wide variation of blast resistance from susceptible to highly resistant. Many of the accessions in cluster II, including the upland and Indica-type varieties, tended to be highly resistant.
Geographic distribution of variety groups classified according to blast resistance
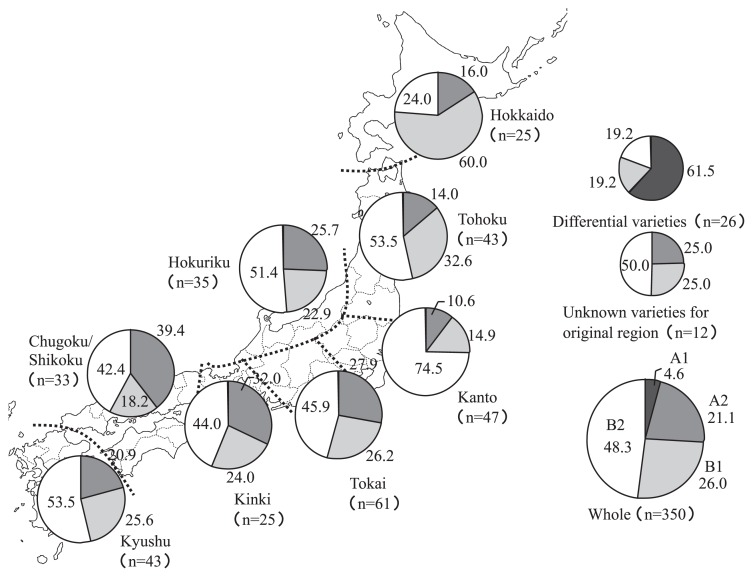
We investigated the geographic distributions of rice variety groups classified according to blast resistance among the eight Japanese regions, namely Hokkaido, Tohoku, Hokuriku, Kanto, Tokai, Kinki, Chugoku/Shikoku, and Kyushu (Fig. 1).
Fig. 1.
Geographic distribution of rice resistance groups in eight Japanese regions. Cluster analysis was carried out based on data on patterns of reaction to blast isolates from Japan and the Philippines, and classified into 4 groups, A1, A2, B1 and B2. Frequency of varieties in each group (no. of varieties) is shown for each region.
The frequencies of the 350 rice accessions in subgroups A1, A2, B1 and B2 were 4.6%, 21.1%, 26.0%, and 48.3%, respectively, and some differences in frequency were found among the eight regions. In the Hokkaido region, the frequencies of subgroups A2, B1 and B2 were 16.0%, 60.0% and 24.0%, respectively, the frequency of B1 was higher than in the other regions and in all regions as a whole. The frequency of B1 in Tohoku was 32.6%—a little higher than in the other seven regions. In the Kanto region, the frequencies of the three subgroups were 10.6%, 14.9% and 74.5%, respectively; with the frequency of B2 being higher than in all other regions. The frequencies of A2 in the four regions of Hokuriku, Tokai, Kinki and Chugoku/Shikoku were a little higher than in the other regions. In the Kyushu region the frequencies of the three subgroups were similar to the frequencies across all regions.
These results indicated that rice types with different patterns of resistance were distributed among the eight regions. The rice accessions from the Hokkaido and Kanto regions were markedly different from those from the other regions.
Discussion
The present study clarified the genetic variation of all Japanese rice accessions including landraces, improved types (including high-yielding or animal-feed rice with Indica-type genetic backgrounds), and weedy rice using polymorphism data of SSR markers and reaction patterns of resistant to SDBLs. In addition, this study used a wider range of rice materials compared with the previous studies.
On the basis of the resistance to 16 SDBIs from Japan and the Philippines, we classified Japanese rice accessions into three subgroups, A2, B1 and B2. The reactions to three Japanese isolates, 9 (H05-72-1), 10 (93-406(B)) and 11 (H07-76-1), which were all virulent to DVs for Pish, Pik-s and Pi19(t), played the main role in classification of the accessions (Fig. 2). Because there were no avirulent isolates for DVs containing Pi19(t), it was not possible to identify accessions with Pi19(t) in this study. Almost all of the A2 accessions were susceptible to all three isolates. By using a differential system based on DVs targeting 23 resistance genes (Kobayashi et al. 2007, Telebanco-Yanoria et al. 2008a, Tsunematsu et al. 2000), we estimated that these accessions could have the resistance genes, Pish and Pik-s, but none of the other 20 resistance genes. In subgroup B1, almost all of the accessions were susceptible to isolate 11 (H07-76-1), and we estimated that various combinations from among 11 resistance genes (Pish, Pia, Pii, Pi3, Pi5(t), Pik-s, Pik-m, Pik, Pik-h, Pik-p or Pi7(t)) were present. The accessions in subgroup B2 were resistant to almost all isolates and estimating the genotypes of the resistance genes was difficult. However, several rice accessions showing susceptible reactions were postulated to have these additional resistance genes, Pia, Pii, Pi3, Pi5(t), Pik alleles (Pik-s, Pik-m, Pik, Pik-h or Pik-p), Pi7(t), Pib, Pit, Piz alleles (Piz, Piz-t, Piz-5 or Pi9(t)) and Pita alleles (Pi12(t) Pita or Pi20(t)). Several upland varieties and high-yielding and weedy-type accessions with Indica genetic backgrounds were included mainly in this subgroup. Our results indicated that two resistance genes (Pish and Pik-s) were distributed widely and that some of the other 20 resistance genes were also present in Japanese accessions. Unexpected reactions to SDBLs were also found in almost all accessions and unknown resistance gene(s) were present probably. Yaegashi et al. (1983) postulated the blast resistance genes in recommended Japanese rice varieties by using several Japanese P. oryzae isolates. They estimated that 33.1% of varieties did not have any resistance genes, 45.8% had only Pia, 10.6% had only Pii, and a few had the other genes such as Pik, Pik-m, Piz, Pib, Pita, and Pita-2. Kiyosawa et al. (1986) found that varieties in Japan showed different reactions from Indica types from Brazil and the Philippines (International Rice Research Institute, IRRI), but did not postulate the genotype(s) in these varieties in detail. Our study demonstrated clearly the genetic variation of resistance genes in Japanese rice accessions by using SDBIs and their reactions on DVs for targeting 23 resistance genes, and showed more a detail estimation of the blast resistance genes present compared to previous studies, (Kiyosawa et al. 1986, Yaegashi et al. 1983). This information in rice accessions will be useful, to understand the differentiation and components of blast resistance gene(s) in current rice varieties, and as a foundation for new strategies for the genetic improvement of varieties in Japan. In other words, this study demonstrated that the limited resistance genes and genetic backgrounds have been used in Japanese rice breeding and that new sources of genetic diversity are needed.
The accessions in subgroup B2 were highly resistant to the blast fungus and many rice accessions showed resistance to all of the SDBLs. Many upland accessions were classified into cluster II on the basis of the SSR markers’ polymorphisms (Table 2) and were also categorized into subgroup B2 (Supplemental Table 2). Thus, many highly resistant accessions were found in the uplands. Several partial (field) resistance genes with a wide spectra of resistance to many blast isolates have been found among the upland varieties Owarihatamochi (pi21; Fukuoka and Okuno 2001), Kahei (Pi36: qBRF4-1; Miyamoto and Hirasawa 2003, Miyamoto et al. 2001), and Sensho (some QTLs on chromosomes 4, 11 and 12; Kato et al. 2002). These three accessions were also included in subgroup B2. The lowland variety Asanohikari, with the partial resistance gene PB1 (Fujii et al. 1999), was also included in this subgroup. The presence of these known or unknown partial resistance genes in upland and lowland accessions might be one of the reasons for the high levels of resistance in subgroup B2. Several high-yielding varieties, such as Ohchikara (Horisue et al. 1991), Takanari (Imbe et al. 2004), Habataki (Miura et al. 1991), Hokuriku193 (Goto et al. 2009), Kusahonami (Sakai et al. 2003), and Momiroman (Hirabayashi et al. 2010), as well as Indica varieties, Kasalath and Tetep, were also categorized into this subgroup (Supplemental Table 2). These highly resistant accessions among the upland accessions and high-yielding varieties with Indica genetic backgrounds may be useful either as new sources of resistance or for increasing the genetic diversity of rice varieties cultivated in Japan.
Rice accessions in Japan were classified into two clusters on the basis of data on the polymorphism of SSR markers. Many accessions from irrigated lowlands were categorized into cluster I and many from the uplands were classified into II (Table 2). Accessions with Indica genetic background tended to be classified into cluster II (Supplemental Table 2). Thus, the genetic background from irrigated lowlands, differs from those of upland and Indica types. Almost all highly resistant rice accessions of group B2 were included in cluster II, which mainly consisted of upland and Indica accessions. Kiyosawa et al. (1986) have already indicated that the reaction patterns of Japanese rice varieties to the blast fungus were different from those of Indica types in tropical areas such as Brazil, the Philippines (IRRI), and China. Ebron et al. (2004, 2005) and Fukuta et al. (2007) tried to clarify the resistance gene constitution of 42 Indica varieties bred by the IRRI. They found that two to six resistance genes were present in these varieties, and thus the genetic basis of their resistance was complex. Kato (2008) pointed out that several animal-feed varieties with Indica genetic backgrounds have been developed and grown in Japan by using these IRRI-bred varieties or Korean Indica varieties. The accessions with Indica genetic backgrounds no doubt inherited their different and complex genetic mechanisms of blast resistance from these IRRI and Korean varieties. These upland and Indica accessions in subgroup B2 showed resistance to all SDBIs and were postulated to have various combinations of known or unknown resistance genes. These highly resistant accessions are interesting as new resistance gene sources, such as partial resistance from upland accessions or novel resistance originated from Indica varieties. The genetic evaluation of highly resistant accessions using many SDBIs has not yet been done. Such studies will elucidate genetic basis of their high resistance. These varieties with high resistance might harbor novel partial resistance gene(s) or unique genetic mechanism(s), and be useful for breeding for durable resistant to blast disease.
We found several differences in frequency in subgroups A2, B1 and B2 among the eight regions of Japan (Fig. 1). Markedly high frequencies of B1 and B2 were apparent in the Hokkaido and Kanto regions, respectively, and the frequency of B1 in Tohoku was also somewhat higher than in the other regions, except for Hokkaido. The frequencies of B2 in Tokai, Kinki and Chugoku/Shikoku were lower than those of the other regions, except for Hokkaido. Slightly higher frequencies of A2—the most susceptible group—were found in four regions, Hokuriku, Tokai, Kinki, and Chugoku/Shikoku, located over a wide area of Central and Western Japan. These results indicate that Hokkaido, Tohoku, and Kanto had different frequencies of subgroups compared with Hokuriku, Tokai, Kinki and Chugoku/Shikoku. In the other words, the accessions in these four regions—Hokuriku, Tokai, Kinki and Chugoku/Shikoku—have high diversity for blast resistance, and that the blast resistance deployed in Hokkaido, Tohoku, and Kanto was less diverse. Nearly all Japanese varieties carry blast resistance genes Pish and Pik-s. Varieties with various additional resistance gene combinations of Pia, Pii, Pi3, Pi5(t), Pik-m, Pik, Pik-h, Pik-p, and Pi7(t) are distributed at high frequencies from Tohoku to Hokkaido. Upland varieties with complex genetic mechanisms have been cultivated mainly in the Kanto region and very few are grown elsewhere. The resistance of rice accessions in subgroups A2 and B1 to SDBIs from the Philippines was higher than that of SDBIs from Japan (Fig. 2). The DVs for Pish showed the same reaction pattern to the two sets of P. oryzae isolates. Kozaki et al. (1970) reported the same results, namely that Japonica-type varieties in Japan are susceptible to blast isolates from Japan, but resistant to those from South and Southeast Asian countries. Yaegashi et al. (1983) reported that 41% of Japanese varieties had an unknown dominant gene, Pi-x, which could be detected only by the blast isolate Kyu7707A, isolated from the rice variety Reiho. Imbe and Matsumoto (1985) designated it as a new dominant resistance gene, Pish, and localized it on chromosome 1. The number of accessions in subgroups A2 and B1 was 160, which was 49% of the total; similar to that of found byYaegashi et al. (1983). The differences in reaction patterns between SDBIs from Japan and the Philippines and the subgroup A2 and B1 accessions likely occurred because of the presence of Pish, and we confirmed here that it was present in many Japanese varieties. Because almost all blast isolates in Japan are virulent to Pish (Unpublished data), such isolates cannot be used to identify Pish. Thus, blast isolates form Tropical countries, such as the Philippines, were useful for clarifying the resistance gene(s) present in Japanese rice.
The top five varieties cultivated in Japan from 2000 to 2010 were Koshihikari, Hitomebore, Hinohikari, Akitakomachi, and Kirara 398/Kinuhikari, and Koshihikari accounted for 35% of Japanese rice average, and all five varieties together accounted for 67% to 70% to the area grown of rice (http://ineweb.narcc.affrc.go.jp/, http://www.maff.go.jp/j/tokei/kouhyou/sakumotu/index.html). On the basis of the reaction patterns of the accessions and DVs against the SDBIs, we estimated the cluster groups and resistance genes of the leading Japanese varieties to be as follows: Koshihikari (A2, Pish and Pik-s); Hitomebore (B2, Pia, Pii or Pi3, and one of five, either Pik-m, Pik-h, Pik, Pik-p or Pi7(t)); Hinohikari and Akitakomachi (B2, Pish, Pia, various combinations of three (Pii, Pi3 and Pi5(t)), and one of either Pi1, Pik-m, Pik-h, Pik, Pik-p or Pi7(t)); Kirara 394 (B1, Pish, Pia, and one of either Pik-s, Pik-m, Pik-h, Pik, Pik-p or Pi7(t)); and Kinuhikari (B1, Pish, various combinations of three (Pii, Pi3 or Pi5(t)), and one of either Pik-s, Pik-m, Pik-h, Pik, Pik-p or Pi7(t)). Therefore, either the resistance genes Pish and Pia plus various combinations of either Pii, Pi3 or Pi5(t)) on chromosome 9, or one of the Pik alleles (Pik-s, Pi1, Pik-m, Pik-h, Pik, Pik-p or Pi7(t)) on chromosome 11, are present in the genetic background of leading Japanese varieties. A limited numbers of varieties have been cultivated over wide areas of Japan, and our results show that only a few genes for blast resistance have been deployed in these varieties.
We found limited numbers of resistance genes, such as Pish, Pia, and various combinations of Pii, Pi3, Pi5(t) or some of the Pik allele genes, among recent leading Japanese rice varieties indicating as narrow genetic base of blast resistance. Telebanco-Yanoria et al. (2008b) also found that susceptible varieties are dominant in Japan and that the genetic variation is not as high as in Tropical regions of Asian and African. Environmental and physiological conditions predispose plants to plant pathogens (Yarwood 1976). Environmental conditions, including low temperatures and high nitrogenous fertilizer, significantly increase the susceptibility of rice plants to the rice blast fungus (Hashioka 1985, Matsuyama and Dimond 1973, Ogta et al. 1966, Ou 1985). In the northern regions of Japan, cold summers increase the frequency and severity of the rice blast disease and bring heavy yield losses. These findings suggest that there is a high blast disease risk for leading Japanese varieties, and the outbreak of blast disease can occur under the abnormal environmental and physiological conditions.
Supplementary Material
Supplemental Table 1. SSR maerkers used in this study
Supplemental Table 2. Reaction data to SDBIs of rice accessions used in this study
Acknowledgements
We thank Genebank, National Institute of Agrobiological Sciences (NIAS), the Hokkaido Research Organization Agriculture Research Department’s Kamikawa Agricultural Experiment Station; the Hokkaido Research Organization Agriculture Research Department’s Central Agricultural Experiment Station; the Aomori Prefectural Industrial Technology Research Center; the Akita Prefectural Agriculture, Forestry, and Fisheries Research Center; the Yamagata Integrated Agricultural Research Center; Miyagi Prefectural Furukawa Agricultiral Experiment Station; National Institute of Crop Science, the National Agriculture and Food Research Organization (NARO); Ibaraki Agricultural Institute, Gunma Agricultural Technology Center, NIAS; Saitama Prefectural Agriculture and Forestry Research Center; Chiba Prefectural Agriculture and Forestry Research Center; Niigata Crop Research Center; Toyama Prefectural Agriculture, Forestry and Fisheries Research Center; Ishikawa Prefectural Agriculture and Forestry Research Center’s Agricultural Experiment Station; the Agriculture, Forestry and Fisheries Department; Fukui Prefecture Agricultural Experiment Station; Aichi Agricultural Research Center; Fukuoka Agricultural Research Center; the NARO Kyushu Okinawa Agricultural Research Center; Saga Prefectural Agricultural Research Center; Kumamoto Agricultural Research Center; and Miyazaki Agricultural Research Institute for providing the rice varieties. We thank Dr. J. Ushiki, National Agricultural Research Center, for providing the weedy rice and Dr. N. Hayashi, NIAS, for providing Japanese blast isolates. This study was performed under the Japan International Research Center for Agricultural Science Research Projects “Blast Research Network for Stable Rice Production” (2006 to 2010) and “Rice Innovation for Environmentally Sustainable Production Systems” (2011 onward).
Literature Cited
- Ebron, L.A., Fukuta, Y., Imbe, T., Kato, H., Yanoria, J.M.T., Tsunematsu, H., Khush, G.S. and Yokoo, M. (2004) Estimation of genes in blast resistance in elite Indica-type rice (Oryza sativa L.) varieties – bred at International Rice Research Institute. Breed. Sci. 54: 381–387 [Google Scholar]
- Ebron, L.A., Fukuta, Y., Imbe, T., Kato, H., Yanoria, M.J.T., Tsunematsu, H., Khush, G.S., Kobayashi, N. and Yokoo, M. (2005) Identification od blast resistance genes in elite Indica-type varieties of rice (Oryza sativa L.). SABRAO Journal of Breeding and Genetics 37: 1–11 [Google Scholar]
- Flor, H.H. (1956) The complementary genetic systems in flax and flax rust. Adv. Genet. 8: 29–54 [Google Scholar]
- Fujii, K., Hayano-Saito, Y., Sugiura, N., Hayashi, N., Saka, N., Tooyama, T., Izawa, T. and Shumiya, A. (1999) Gene analysis of panicle blast resistance in rice cultivars with rice stripe resistance. Breed. Res. 1: 203–210 [Google Scholar]
- Fukuoka, S. and Okuno, K. (2001) QTL analysis and mapping of pi21, a recessive gene for field resistance to rice blast in Japanese upland rice. Theor. Appl. Genet. 103: 185–190 [Google Scholar]
- Fukuta, Y., Ebron, L.A. and Kobayashi, N. (2007) Genetic and breeding analysis of blast resistance in elite Indica-type rice (Oryza sativa L.) bred in International Rice Research Institute. JARQ 41: 101–114 [Google Scholar]
- Goto, A., Sasahara, H., Shigemune, A. and Miura, K. (2009) Hokuriku 193: A new high-yielding Indica rice cultivar bred in Japan. JARQ 43: 13–18 [Google Scholar]
- Hashioka, Y. (1985) Effects of environmental factors on development of causal fungus, infection, disease development, and epidemiology in rice blast disease. In: The rice blast disease. Proceedings of a symposium at the International Rice Research Institute, 1963 Johns Hopkins Press, Baltimore, pp. 153–161 [Google Scholar]
- Hayashi, N. (2005) Rice blast fungus, MAFF Microorganism Genetic Resources Manual No. 18. National Institute of Agrobiological Sciences, Tsukuba, Inaraki, December 2005. [Google Scholar]
- Hayashi, N. and Fukuta, Y. (2009) Proposal for a new international system of differentiating races of blast (Pyricularia oryzae Cavara) by using LTH monogenic lines in rice (Oryza sativa L.). JIRCAS working report No. 63. Japan International Research Center for Agricultural Sciences, Tsukuba, Ibaraki, Japan, pp. 11–15 [Google Scholar]
- Hayashi, N., Kobayashi, N., Vera Cruz, M.C.M. and Fukuta, Y. (2009) Protocols for the sampling of diseased specimens and evaluation of blast disease in rice. JIRCAS Working Report No. 63. Japan International Research Center for Agricultural Sciences, Tsukuba, Ibaraki, pp. 17–33 [Google Scholar]
- Hirabayashi, H., Nemoto, H., Ando, I., Kato, H., Oota, H., Satou, H., Takeuchi, Y., Ishii, T., Maeda, H., Imbe, T.et al. (2010) “Momiroman”, a new rice cultivar for feed use. Bull. Natl. Inst. Crop Sci. 11: 31–47 [Google Scholar]
- Horisue, H., Miura, K., Kobayashi, A., Koga, Y., Okuno, K., Fujita, Y., Uchiyamada, H. and Samoto, S. (1991) Breeding process and characteristics of a new rice variety “Oochikara”. The Hokuriku Crop Sci. 26: 56–59 [Google Scholar]
- Hosoi, J., Ushiki, J., Sakai, N., Aoki, M. and Tezuka, M. (2008) Seed shattering habit of weedy rice (Oryza sativa) in Nagano prefecture, Japan and germination ability of shattered seeds. Jpn. J. Crop Sci. 77: 321–325 [Google Scholar]
- Imbe, T. and Matsumoto, S. (1985) Inheritance of resistance of rice varieties to the blast fungus strains virulent to the variety “Reiho”. Jpn. J. Breed. 35: 332–339 [Google Scholar]
- Imbe, T., Akama, Y., Nakane, A., Hata, T., Ise, K., Ando, I., Uchiyamada, H., Nakagawa, N., Furutachi, H., Horisue, N.et al. (2004) Development of a multipurpose high-yielding rice variety “Takanari”. Bull. Natl. Inst. Crop Sci. 5: 35–51 [Google Scholar]
- Kato, H. (2008) Development of rice varieties for whole crop silage (WCS) in Japan. JARQ 42: 231–236 [Google Scholar]
- Kato, T., Endo, I., Yano, M., Sasaki, T., Inoue, M. and Kudo, S. (2002) Mapping of quantitative trait loci for field resistance to rice blast in upland rice, “Sensho”. Breed. Res. 4: 119–124 [Google Scholar]
- Kawasaki, A., Imai, K., Ushiki, J., Ishii, T. and Ishikawa, R. (2009) Molecular constitution of weedy rice (Oryza sativa L.) found in Okayama prefecture, Japan. Breed. Sci. 59: 229–236 [Google Scholar]
- Kiyosawa, S. (1984) Establishment of differential varieties for pathogenicity test of rice blast fungus. Rice Genet. Newsl. 1: 95–97 [Google Scholar]
- Kiyosawa, S., Mackill, D.J., Bonman, J.M., Tanaka, Y. and Ling, Z. (1986) An attempt of classification of world’s rice varieties based on reaction pattern to blast fungus strains. Bull. Natl. Inst. Agrobiol. Resour. 2: 13–39 [Google Scholar]
- Kobayashi, N., Telebanco-Yanoria, M.J., Tsunematsu, H., Kato, H., Imbe, T. and Fukuta, Y. (2007) Development of new sets of international standard differential varieties for blast resistance in rice (Oryza sativa L.) JARQ 41: 31–37 [Google Scholar]
- Kono, I., Takeuchi, Y., Shimano, T., Sasaki, T. and Yano, M. (2000) Comparison of efficiency of detecting polymorphism among japonica varieties in rice using RFLP, RAPD, AFLP and SSR makers. Breed. Res. 2: 197–203 [Google Scholar]
- Kozaki, T., Matsumoto, S. and Yamada, M. (1970) Reactions of foreign rice varieties to major race of Pyricularia oryzae in Asia. Bull. Nat. Inst. Agr. Sci. Ser. C, No. 24: 113–152 [Google Scholar]
- Matsuyama, N. and Dimond, A.E. (1973) Effect of nitrogenous fertilizer on biochemical processes that could affect lesion size of rice blast. Phytopathology 63: 1202–1203 [Google Scholar]
- Miura, K. (1991) Breeding process and characteristics of a new rice variety “Habataki”. The Hokuriku Crop Scinece 26: 52–55 [Google Scholar]
- Miyamoto, M., Yano, M. and Hirasawa, H. (2001) Mapping of quantitative trait loci conferring blast field resistance in the Japanese upland rice variety Kahei. Breed. Sci. 51: 257–261 [Google Scholar]
- Miyamoto, M. and Hirasawa, H. (2003) Relationship between RFLP loci with different alleles on chromosome 4 and the levels of blast field resistance in Japanese upland rice varieties. Breed. Sci. 53: 1–5 [Google Scholar]
- Okoshi, M., Hu, J., Ishikawa, R. and Fujimura, T. (2004) Polymorphic analysis of landraces of Japanese rice using microsatellite markers. Breed. Res. 6: 125–133 [Google Scholar]
- Ou, S.H. (1985) Blast-Pyricularia oryzae. In: Commonwealth mycological institute. Rice diseases 2nd ed. Kew, UK, pp. 109–201 [Google Scholar]
- Sakai, M., Imbe, T., Nemoto, H., Horisue, N., Nakagawara, N., Sato, H., Hirasawa, H., Takasate, M., Tamura, K., Ando, I.et al. (2003) A new rice variety for whole-crop silage “Kusahomare”. Bull. Natl. Inst. Crop Sci. 4: 1–15 [Google Scholar]
- Silue, D., Nottegherm, J.L. and Tharreau, D. (1992) Evidence of a gene-for-gene relationship in the Oryza sativa–Magnaporthe grisea pathosystem. Phytopathology 82: 577–580 [Google Scholar]
- Sunohara, Y. (1990) Development and utilization of rice cultivars with various grain properties for processing. Tohoku Agric. Res. Extra Issue 3: 5–13 [Google Scholar]
- Telebanco-Yanoria, M.J., Imbe, T., Kato, H., Tsunematsu, H., Ebron, L.A., Vera Cruz, C.M., Kobayashi, N. and Fukuta, Y. (2008a) A set of standard differential blast isolates (Magnaporthe grisea (Hebert) Barr.) from the Philippines for rice (Oryza sativa L.) resistance. JARQ 42: 23–34 [Google Scholar]
- Telebanco-Yanoria, M.J., Ohsawa, R., Senon, S., Kobayashi, N. and Fukuta, Y. (2008b) Diversity analysis for resistance of rice (Oryza sativa L.) to blast disease (Magnaporthe grisea (Hebert) Barr.) using differential isolates from the Philippines. Plant Breed. 127: 355–363 [Google Scholar]
- Telebanco-Yanoria, M.J., Koide, Y., Fukuta, Y., Imbe, T., Kato, H., Tsunematsu, H. and Kobayashi, N. (2010) Development of near-isogenic lines of Japonica-type rice variety Lijiangxintuanheigu as differentials for blast resistance. Breed. Sci. 60: 629–638 [Google Scholar]
- Tsunematsu, H., Telebanco-Yanoria, M.J., Ebron, L.A., Hayashi, N., Ando, I., Kato, H., Imbe, T. and Khush, G.S. (2000) Development of monogenic lines of rice for blast resistance. Breed. Sci. 50: 229–234 [Google Scholar]
- Ushiki, J., Ishii, T. and Ishikawa, R. (2005) Morpho-physiological characters and geographical distribution of japonica and indica weedy rice (Oryza sativa) in Okayama prefecture, Japan. Breed. Res. 7: 179–187 [Google Scholar]
- Yaegashi, H., Asaga, K. and Yamada, M. (1983) Presumed genotypes for true resistance of recommended rice varieties to rice blast. Bull. Tohoku Nat. Agric. Exp. Stat. 68: 1–19 [Google Scholar]
- Yamada, M., Kiyosawa, S., Yamaguchi, T., Hirano, T., Kobayashi, T., Kushibuchi, K. and Watanabe, S. (1976) Proposal of a new method of differentiating races of Pyricularia oryzae Cavara in Japan. Ann. Phytopathol. Soc. Japan. 42: 216–219 [Google Scholar]
- Yamasaki, M. and Ideta, O. (2013) Population structure in Japanese rice population. Breed. Sci. 63: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, Y. and Kiyosawa, S. (1966) Studies on inheritance of resistance of rice varieties to blast I. Inheritance of resistance of Japanese varieties to several strains of the fungus. Bull. Natl. Inst. Agr. Sci. D14: 39–69 [Google Scholar]
- Yarwood, C.E. (1976) Modification of the host response-predisposition. In: Heitefuss, R. and Williams, P.H. (eds.) Physiological plant pathology, Springer, Berlin, pp. 703–718 [Google Scholar]
- Yokoo, M., Hirao, M. and Imai, T. (2005) Annual change in leading rice varieties for the 45 years between 1956 and 2000 in Japan. Bull. Natl. Inst. Crop Sci. 5: 19–125 [Google Scholar]
- Yonemaru, J., Yamamoto, T., Ebana, K., Yamamoto, E., Nagasaki, H., Shibaya, T. and Yano, M. (2012) Genome-wide haplotype changes produced by artificial selection during modern rice breeding in Japan. PLoS One 7: e32982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. SSR maerkers used in this study
Supplemental Table 2. Reaction data to SDBIs of rice accessions used in this study