Summary
Abnormal hypothalamic-pituitary-adrenal (HPA) axis function, as indexed by elevated diurnal cortisol levels and/or a blunted cortisol awakening response (CAR), has been observed among patients with first episode psychosis and associated with neurocognitive deficits in this population. However, the extent to which these features precede illness onset is unclear. The current study aimed to determine whether children who are at putatively elevated risk for psychosis because they present multiple antecedents of schizophrenia (ASz), and high-risk children with a family history of illness (FHx), are characterized by abnormal cortisol levels when compared with their typically developing (TD) peers. A further aim was to investigate the extent to which cortisol levels are associated with psychosocial stress and neurocognitive function. Thirty-three ASz children, 22 FHx children, and 40 TD children were identified at age 9–12 years using a novel community-based screening procedure or as relatives of individuals with schizophrenia. All participants were antipsychotic-naive and not currently seeking treatment for their symptoms. At age 11–14 years, participants provided salivary cortisol samples and completed psychosocial stress measures and tests of memory and executive function. Results indicated that FHx children, but not ASz children, were characterized by a blunted CAR relative to their TD peers (effect size = −0.73, p = 0.01) that was not explained by psychosocial stress exposure or by distress relating to these experiences. Neither FHx nor ASz children were characterized by elevated diurnal cortisol. Among both FHx and ASz children, more pronounced HPA axis function abnormalities (i.e., higher diurnal cortisol levels and greater blunting of the CAR) were associated with poorer performance on tests of verbal memory and executive function. These findings support the notion that at least some HPA axis abnormalities described in psychosis precede illness onset, rather than being a subsequent epiphenomenon. We speculate that the blunted CAR may constitute an early (potentially genetically mediated) marker of psychosis vulnerability, whilst elevated diurnal cortisol levels may emerge only proximally to disease onset.
Keywords: Psychosis, High-risk, HPA axis, Negative life events, Hassles, Memory, Executive function, Development
1. Introduction
The neural diathesis-stress model of schizophrenia (Walker and Diforio, 1997; Walker et al., 2008) posits that psychosocial stress may act via the hypothalamic-pituitary-adrenal (HPA) axis (the primary system involved in coordinating the physiological response to stress) to trigger psychosis onset among individuals with an underlying vulnerability for the disorder. Abnormal HPA axis function, as indexed by elevated diurnal cortisol levels and/or a blunted cortisol awakening response (CAR), has been observed in individuals who have recently experienced their first psychotic episode (Borges et al., 2013). Additionally, elevated cortisol levels among first episode patients have been associated with reduced hippocampal volume (Mondelli et al., 2010b), and greater blunting of the CAR with more pronounced deficits in verbal memory and processing speed (Aas et al., 2011). However, the extent to which abnormal HPA axis function precedes the onset of psychosis is currently unclear. Whilst these studies suggest that the first psychotic episode is characterized by HPA axis dysfunction and that this may be associated with some of the neuroanatomical abnormalities and cognitive deficits observed in psychosis patients, it is possible that these HPA axis abnormalities may simply be a consequence of the stress associated with illness onset.
The study of individuals at elevated risk for schizophrenia provides the opportunity to directly test the assumptions of the neural diathesis-stress model (Walker and Diforio, 1997; Walker et al., 2008) and thus determine whether vulnerability for schizophrenia is associated with HPA axis dysfunction. Traditionally, high-risk approaches have focused on individuals with a family history of illness. Studies comparing young adult relatives of patients with psychosis to healthy controls have yielded inconsistent findings, perhaps due to methodological differences. A study examining salivary cortisol at random time-points throughout the day (values averaged) reported elevated cortisol among relatives (Collip et al., 2011), whilst two studies measuring fasting plasma cortisol at a single time-point did not (Brunelin et al., 2008; Yang et al., 2012). A more recent strategy focuses on youth considered at ultra high-risk (UHR) for psychosis whose clinical features (typically, attenuated psychotic symptoms) indicate that they may be in the prodromal phase of illness. Higher salivary cortisol levels (obtained at a single time-point in the morning) have been reported among UHR youth relative to healthy controls, with more pronounced effects among those who were medication-free (Sugranyes et al., 2012). Elevated cortisol levels in UHR youth were similarly observed in a study which examined salivary cortisol obtained at three time-points throughout the morning (values averaged) (Walker et al., 2013). Using a similar sampling protocol, work by Walker and colleagues also indicates that adolescents with schizotypal personality disorder (SPD), who are at greater risk for psychosis on account of their diagnosis, exhibit higher daytime salivary cortisol levels (Walker et al., 2001; Mittal et al., 2007). Thus, elevated salivary cortisol during the day has been consistently observed among youth whose clinical features designate them as being at elevated risk for psychosis. However, none of these studies obtained the multiple samples distributed throughout the day that are required for the examination of diurnal patterns of cortisol secretion (which may be a more reliable marker of HPA axis function given that cortisol levels vary substantially through the day). Furthermore, as no study of high-risk youth has yet examined the CAR, it remains unclear whether the blunted CAR observed among individuals with first episode psychosis also characterizes these youth.
Whilst studies of UHR youth suggest that HPA axis dysfunction precedes the onset of psychosis, these youth are, by definition, already sufficiently distressed as to seek treatment for their symptoms. Thus, elevations in cortisol might feasibly be due to distress relating to emerging illness, as opposed to external psychosocial stressors. Indeed, only one study of UHR youth has examined the association between psychosocial stress exposure and HPA axis dysfunction (Thompson et al., 2007). In this study, higher plasma cortisol levels obtained at a single time-point in the morning were correlated with the number of minor hassles but not with the number of major life events experienced during the past month. Studies of youth with SPD, although not typically confounded by help-seeking behaviour, are limited by the fact that these individuals already present with symptoms of sufficient severity as to meet diagnostic criteria for a schizophrenia spectrum disorder.1 Establishing whether abnormal HPA axis function is present in younger samples of high-risk individuals, who are not yet sufficiently distressed as to seek help for their symptoms, will help to determine the extent to which HPA axis dysfunction increases the risk for psychosis. Furthermore, given that neurocognitive deficits have been consistently identified among individuals at elevated risk for psychosis (Fusar-Poli et al., 2012; Agnew-Blais and Seidman, 2013), it is important to establish whether these deficits are associated with HPA axis dysfunction.
We have developed a novel community screening method for identifying children who may be at elevated risk for psychosis because they present with a triad of antecedents of schizophrenia (ASz), defined as (i) a speech and/or motor delay or abnormality, (ii) a social, emotional, and/or behavioural problem, and (iii) a psychotic-like experience (Laurens et al., 2007). We have previously demonstrated that, relative to their typically developing (TD) peers, ASz children, as well as children with a family history of schizophrenia (FHx), are more frequently exposed to psychosocial stressors and more distressed by these experiences (Cullen et al., 2014), and that both groups show poorer neurocognitive function (Cullen et al., 2010; Dickson et al., 2014). The current study aimed to determine whether ASz and FHx children are also characterized by abnormal HPA axis function (elevated diurnal cortisol and/or a blunted CAR) relative to TD children. A further aim was to investigate whether among ASz and FHx children, HPA axis function is associated with experiences of psychosocial stress and performance on tasks of memory and executive function.
2. Methods
2.1. Recruitment of children presenting antecedents and typically developing children
ASz and TD children were recruited using a community-based screening procedure (Laurens et al., 2007, 2011). In brief, questionnaires assessing a triad of well-replicated antecedents of schizophrenia were completed independently by children aged 9–12 years at school and by their caregivers at home. The caregiver questionnaire included nine items (three quantitative and six qualitative) assessing delays or abnormalities in speech and/or motor development (Laurens et al., 2007). Social, emotional, and behavioural problems were defined as a score in the clinical range on at least one of the four Strengths and Difficulties Questionnaire [SDQ (Goodman, 2001)] psychopathology scales: emotional symptoms (child-reported), conduct problems, hyperactivity-inattention, or peer relationship problems (caregiver-reported). The child questionnaire also included a measure of psychotic-like experiences (PLEs) (Laurens et al., 2012), assessing nine delusion- and hallucination-like experiences, each rated on a three-point scale (0 = not true, 1 = somewhat true, 2 = certainly true; a score of 2 on any of the nine items indicated a positive rating). Children presenting each of the three antecedents were eligible for the ASz group. The TD group comprised children who presented none of the antecedents and who had no first-, second-, or third-degree relatives with a schizophrenia spectrum disorder, as confirmed subsequently with the child's caregiver via the Family Interview for Genetic Studies [FIGS (Maxwell, 1992)]. Screening questionnaires were completed by 1343 children and their caregivers; of these, 9.4% presented the ASz triad and 22.9% of children presented none of the antecedents. Of the 150 children eligible for the ASz or TD group who were subsequently invited to participate in a longitudinal study of child development, 41% of ASz and 42% of TD families declined participation, respectively; these rates are consistent with other studies recruiting children via schools in this region (Mackie et al., 2011; Stateva et al., 2012). Relative to the ASz and TD children who declined participation, ASz and TD children who participated in the study did not differ on age, gender, or ethnicity. Among ASz participants, the prevalence of the triad components did not differ significantly, with the exception that ASz participants were less likely to score in the ‘abnormal’ range on the SDQ emotional symptoms scale than ASz children who did not participate (p = 0.01).
2.2. Recruitment of children with a family history of schizophrenia
Children with a family history of schizophrenia or schizoaffective disorder (FHx) were identified via the caregiver screening questionnaire, which included items assessing child and family mental health difficulties. Additionally, medical records of mental health service users within the South London and Maudsley National Health Service (NHS) Foundation Trust were reviewed to identify patients with a diagnosis of schizophrenia or schizoaffective disorder who had a child relative aged between 9 and 12 years. Identified families were approached following liaison with the patient's care worker. All FHx children (identified either via medical records or via community screening) had at least one first- or second-degree relative with schizophrenia or schizoaffective disorder, as confirmed by the FIGS. Of the 1020 children for whom family history information on screening questionnaires was available, 3.4% were reported to have a family history of schizophrenia. A further 36 children who met criteria for the FHx group (i.e., had a family history of schizophrenia, fell within the appropriate age range, and for whom there were viable contact details) were identified via medical records. In total, 40% of FHx families declined to participate in the longitudinal study after initial contact.
2.3. Current sample and procedure
Caregivers and children provided written informed consent and assent, respectively, for participation. Ethical permission for the study was granted by the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee.
The current study examines cross-sectional data collected at a follow-up assessment completed when participants were aged between 11 and 14 years, on average 33 months after initial identification. Children completed a saliva collection protocol and assessments of psychosocial stress and neurocognitive function described in detail below. Caregivers provided information on ethnicity (determined during the FIGS interview) and parental occupation status [coded according to the UK National Statistics Socio-economic Classification (Office for National Statistics, 2010)]. Children self-reported substance use via questionnaire (McVie and Bradshaw, 2005), with regular tobacco use defined as having smoked at least once a week and regular cannabis use defined by use on at least four occasions. Pubertal status was assessed using the self-report Pubertal Developmental Scale (Carskadon and Acebo, 1993), with higher scores indicating more advanced pubertal development. Height and weight were collected by the researcher at the assessment session and used to compute Body Mass Index (BMI; kg/m2). Consistent with previous studies conducted in Northern Europe (Vreeburg et al., 2009), sampling month was categorized into light (March–September) and dark months (October–February).
Of the 111 children recruited via questionnaire screening and/or case record review to the longitudinal study, 91 (82%) provided salivary cortisol data at the follow-up assessment at age 11–14 years: 29 met ASz criteria only, 18 met FHx criteria only, 4 met both ASz and FHx criteria, and 40 children met TD criteria. In order to most accurately reflect the ASz and FHx populations from which the participants were sampled, the four ASz + FHx cases were retained in both groups; ASz and FHx groups were therefore analyzed relative to the TD group only and not relative to each other. FHx children, but not ASz children, were significantly less likely than TD children to provide salivary cortisol data at the follow-up assessment (p = 0.05). Within-group analyses indicated that children who provided salivary cortisol data did not differ from those who did not provide cortisol data on any of the four SDQ psychopathology scales or on total PLE scores at screening (p > 0.05). Sample characteristics are provided in Table 1; there were no significant between-group differences (i.e., FHx vs. TD, or ASz vs. TD) in age, pubertal status, lapse of time between identification and cortisol assessment, BMI, tobacco use, awakening time, or sampling month (p > 0.05). None of the participants reported regular cannabis use. ASz children were significantly more likely to be male compared to TD children (p = 0.02). Relative to the TD group, both the FHx and ASz group were found to differ on ethnicity (p ≤ 0.05) and socioeconomic status (p ≤ 0.01).
Table 1.
Sample demographics, psychosocial stress, and neurocognitive performance indices for each participant group.
| FHx (n = 22) | ASz (n = 33) | TD (n = 40) | Statistical results |
||||
|---|---|---|---|---|---|---|---|
| FHx vs. TD | ASz vs. TD | ||||||
| Age (years); mean ± SE | 13.3 ± 0.3 | 12.8 ± 0.2 | 13.1 ± 0.2 | t = −0.50 | p = 0.62 | t = 1.18 | p = 0.24 |
| Pubertal development scale scorea; mean ± SE | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 | t = −0.19 | p = 0.85 | t = −0.07 | p = 0.94 |
| Sex (male); n (%) | 11 (50) | 23 (70) | 17 (43) | χ2 = 0.32 | p = 0.57 | χ2 = 5.40 | p = 0.02 |
| Ethnicity; n (%) | FE = 17.46 | p < 0.001 | FE = 7.69 | p = 0.05 | |||
| White British | 3 (14) | 8 (24) | 20 (50) | ||||
| White other | 2 (9) | 8 (24) | 11 (27) | ||||
| Black Caribbean/African | 8 (36) | 4 (12) | 3 (8) | ||||
| Other | 9 (41) | 13 (40) | 6 (15) | ||||
| Socioeconomic status; n (%)a | FE = 9.74 | p = 0.006 | FE = 13.44 | p = 0.001 | |||
| Higher managerial, administrative, and professional | 11 (50) | 14 (43) | 33 (82) | ||||
| Intermediate | 5 (23) | 12 (36) | 6 (15) | ||||
| Routine and manual | 6 (27) | 7 (21) | 1 (3) | ||||
| Tobacco use; n (%) | 1 (5) | 2 (6) | 0 (0) | FE | p = 0.36 | FE | p = 0.20 |
| BMI (kg/m2); mean ± SE | 19.7 ± 0.7 | 20.4 ± 0.6 | 19.8 ± 0.5 | t = 0.09 | p = 0.93 | t = −0.76 | p = 0.45 |
| Waking time; mean ± SE | 8:36 ± 0:12 | 8:29 ± 0:13 | 8:34 ± 0:11 | t = −0.11 | p = 0.91 | t = 0.27 | p = 0.79 |
| Lighter sampling month (March–September); n (%) | 13 (59) | 17 (52) | 21 (53) | χ2 = 0.01 | p = 0.93 | χ2 = 0.25 | p = 0.62 |
| Psychosocial stress measure; mean ± SE (total) | |||||||
| Total number of negative life events | 1.8 ± 0.4 | 1.8 ± 0.2 | 1.1 ± 0.2 | t = −1.83 | p = 0.08 | t = −2.77 | p = 0.007 |
| Distress at the time of negative life event | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.3 ± 0.2 | U = 398.5 | p = 0.64 | U = 596.5 | p = 0.59 |
| Current distress related to negative life event | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.6 ± 0.1 | U = 365.5 | p = 0.32 | U = 536.0 | p = 0.20 |
| Frequency of daily hassles | 35.8 ± 2.9 | 43.3 ± 2.2 | 31.0 ± 1.8 | t = −1.48 | p = 0.14 | t = −4.33 | p < 0.001 |
| Distress related to daily hassles | 1.1 ± 0.1 | 1.3 ± 0.1 | 0.9 ± 0.1 | t = −1.45 | p = 0.15 | t = −3.55 | p = 0.001 |
| Neurocognitive measures; mean ± SE | |||||||
| WRAML2 – Number-letter | 12.1 ± 0.8 | 11.3 ± 0.6 | 13.1 ± 0.4 | t = 1.17 | p = 0.21 | t = 2.68 | p = 0.009 |
| WRAML2 – Verbal memory | 21.1 ± 0.9 | 22.1 ± 0.7 | 23.8 ± 0.7 | t = 2.31 | p = 0.02 | t = 1.60 | p = 0.12 |
| WRAML2 – Visual memory | 17.0 ± 1.1 | 16.3 ± 0.8 | 18.1 ± 0.6 | t = 0.91 | p = 0.37 | t = 1.79 | p = 0.08 |
| WRAML2 – Verbal working memory | 9.7 ± 0.5 | 9.5 ± 0.4 | 10.7 ± 0.3 | t = 1.64 | p = 0.11 | t = 2.32 | p = 0.02 |
| D-KEFS – Letter fluency | 10.8 ± 0.7 | 10.3 ± 0.4 | 11.0 ± 0.5 | t = 0.18 | p = 0.86 | t = 0.93 | p = 0.35 |
| D-KEFS – Category fluency | 12.0 ± 0.6 | 12.6 ± 0.6 | 12.8 ± 0.5 | t = 0.91 | p = 0.37 | t = 0.23 | p = 0.82 |
| D-KEFS – Category switching | 10.6 ± 0.7 | 9.7 ± 0.5 | 12.0 ± 0.5 | t = 1.52 | p = 0.13 | t = 3.00 | p = 0.004 |
| D-KEFS – Inhibition | 10.2 ± 0.5 | 10.9 ± 0.4 | 11.8 ± 0.3 | t = 2.71 | p = 0.009 | t = 1.52 | p = 0.13 |
| D-KEFS – Inhibition/switching | 10.5 ± 0.5 | 10.7 ± 0.4 | 11.1 ± 0.4 | t = 1.07 | p = 0.29 | t = 0.86 | p = 0.39 |
| D-KEFS – Tower test | 11.4 ± 0.3 | 11.2 ± 0.3 | 11.6 ± 0.3 | t = 0.34 | p = 0.74 | t = 0.78 | p = 0.44 |
Notes. FHx: family history of schizophrenia; ASz: antecedents of schizophrenia; TD: typically developing. Groups are not mutually exclusive (four children meeting both FHx and ASz criteria are retained in both risk groups). SE: standard error; BMI: body mass index; WRAML2: Wide Range Assessment of Memory and Learning 2nd Edition; D-KEFS: Delis–Kaplan Executive Function System; FE: Fisher's exact test.
Socioeconomic status based on the caregiver with the highest occupational status. Missing data: BMI (n = 2); psychosocial stress (n = 1).
2.4. Salivary cortisol assessment
Participants were provided with a verbal explanation and written instructions for collecting saliva samples at home (at the weekend or during the school holidays) using the passive drool procedure (http://www.salimetrics.com). Further details of our analysis protocol have been described previously (Belvederi Murri et al., 2012). In brief, six saliva samples were collected throughout the day on two consecutive days: at awakening, and at 15, 30, and 60 min after awakening, at 1200 h, and 2000 h. Participants were instructed to wake before 1000 h and collect the first sample immediately upon awakening, to avoid food consumption for 30 min prior to sample collection, and to refrain from strenuous exercise during the day. Samples were stored in the participant's home freezer until collection and subsequently frozen at −20 °C at the laboratory. After thawing and centrifugation at 3000 rpm for 15 min, cortisol levels were determined using the Salimetrics High Sensitivity Salivary Cortisol ELISA KIT (Salimetrics, Suffolk, UK), according to the recommended procedure. The analytical sensitivity was set to 0.33 nmol/l. Inter- and intra-assay coefficients ranged from 8% to 11% and 6% to 10%, respectively, which are consistent with values reported in other studies of high-risk youth (Collip et al., 2011; Walker et al., 2013).
Cortisol data were summarized using two Area Under the Curve (AUC) computations (Pruessner et al., 2003): (i) AUC with respect to the increase in cortisol levels following awakening (AUCi-CAR: calculated using values for awakening, and 15, 30, and 60 min after awakening), and (ii) AUC with respect to ground of cortisol levels during the day (AUCg-DAY: calculated using values for awakening, 1200 h, and 2000 h). Cortisol values were significantly correlated across testing days (p < 0.001 for each time-point); thus, cortisol data obtained on the first sampling day only were used for AUC computations, except when day 1 data were missing or when participants demonstrated better compliance with the sampling protocol on the second day (e.g., if participants reported waking after 1000 h on day 1 but not day 2).
2.5. Psychosocial stress
Participants completed two psychosocial stress measures within 1 month of collecting salivary cortisol samples (mean lapse of time between saliva collection and psychosocial stress measure completion was ±3.2 days). As described previously (Cullen et al., 2014), an eight-item self-report measure assessed exposure to a range of child-appropriate negative life events (Heubeck and O'Sullivan, 1998), such as parental separation/divorce, death of someone close, and serious illness. This measure provided information on the total number of negative life events ever experienced and the average level of distress experienced in relation to each event, both at the time of the event and currently (each rated on a four-point scale, range: 0 ‘not at all’ to 3 ‘a lot’). Specifically, distress ratings were summed and divided by the total number of life events experienced to provide two average distress scores (previous and current) reflecting the average distress per event. Participants also completed a 37-item questionnaire adapted from Heubeck and O'Sullivan (1998) to assess exposure to school-related daily hassles (items spanned four domains: scholastic, peer, teacher, and home). Participants rated on a four-point scale how frequently they had experienced each hassle during the past 6 months (range: 0 ‘never’ to 3 ‘not at all’) and how distressed they were by this experience (range: 0 ‘not at all’ to 3 ‘a lot’). Frequency ratings were summed to provide a total daily hassle frequency score (reflecting the number of hassles experienced and how often they occurred) and distress ratings were summed and divided by the number of items endorsed to derive an overall daily hassle distress score (reflecting the average distress per item endorsed). The internal consistency of these two scales was examined in the total sample and found to be high (Cronbach's α = 0.88 and 0.92, respectively).
2.6. Neurocognitive measures
Exploratory analyses were conducted to examine the extent to which cortisol levels were associated with performance on memory and executive function tasks; these domains were examined due to their relationships with the hippocampus and medial prefrontal cortex, respectively, which are known to mediate HPA axis function (Herman et al., 2005). Participants completed subtests from the Wide Range Assessment of Memory and Learning 2nd Edition (Sheslow and Adams, 2003) and the Delis–Kaplan Executive Function System (Delis et al., 2001), described in Supplementary Table 1 (mean lapse of time between saliva collection and neurocognitive assessment was ±1.4 months). Standardized scores were derived for each subtest using manual-reported population norms.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.03.010.
2.7. Statistical analyses
Independent samples t-tests, Mann–Whitney U tests, chi-squared tests, and Fisher's exact tests were used to examine group differences on demographic variables. As AUCi-CAR and AUCg-DAY values were approximately normally distributed, linear regression analyses were conducted to examine the effect of risk status on both AUC measures. Standardized mean differences (d) were derived from unstandardized regression coefficients (Lipsey and Wilson, 2001), with; ‘small’, ‘medium’, and ‘large’ effect sizes denoted by values of 0.20, 0.50, and 0.80, respectively (Cohen, 1992). Within-group correlation analyses were conducted to examine the extent to which cortisol AUC values were associated with psychosocial stress and neurocognitive performance (Pearson's ‘r’ correlations were used for all analyses except for those examining the negative life event distress variables which were not normally distributed and were therefore examined using Spearman's rho ‘ρ’ correlations). Finally, independent samples t-tests, one-way ANOVA's, and correlation analyses (Pearson's ‘r’ and Spearman's rho ‘ρ’ for normally and non-normally distributed variables, respectively) were used to examine relationships between demographic variables and cortisol AUC measures. Linear regression analyses examining the effect of risk status on AUCi-CAR and AUCg-DAY values were repeated after adjusting for factors found to be associated with AUC values. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 20.
3. Results
3.1. Group differences in the CAR and diurnal cortisol
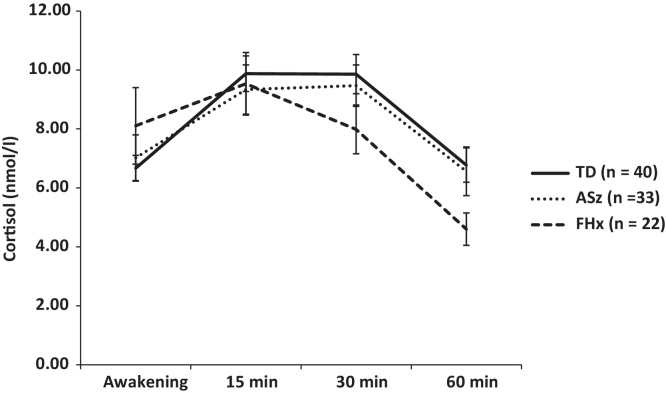
Mean AUCi-CAR values and cortisol values for the individual time points (awakening, and 15, 30, and 60 min after awakening) are presented by group in Table 2 and Fig. 1, respectively. Relative to the TD group, AUCi-CAR values were significantly lower in the FHx group (d = −0.73, p = 0.01), reflecting a blunted CAR among FHx children. In contrast, there was no significant effect of ASz status on AUCi-CAR values (d = −0.19, p = 0.42). This is evident by the visual inspection of the data in Fig. 1, which shows that FHx children have a smaller increase in cortisol levels between awakening and 15 min, and subsequently, a steeper decline in cortisol levels between 15 and 30 min compared to the TD group, whilst ASz children showed a similar pattern of post-awakening cortisol secretion as TD children. Post hoc tests within FHx subgroups showed that the magnitude of differences between FHx and TD children was twice as large among FHx children with a first-degree relative with schizophrenia (d = −1.09, p = 0.005) compared to those with an affected second-degree relative (d = −0.50, p = 0.14). AUCg-DAY values are also shown in Table 2. There were no significant group differences in cortisol levels during the day when either the FHx or ASz group were compared to the TD group (p ≥ 0.38). Post hoc tests performed on AUCg-DAY values indicated that the magnitude of differences between FHx and TD children was similar among FHx children with a first-degree relative with schizophrenia and those with an affected second-degree relative (d = 0.10 and 0.07, respectively; p ≥ 0.05).
Table 2.
Cortisol awakening response and diurnal cortisol levels presented by group.
| Descriptive statistics |
Statistical analyses |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FHx (n = 22) | ASz (n = 33) | TD (n = 40) | FHx vs. TD |
ASz vs. TD |
|||||||
| d | B | 95% CI | p | d | B | 95% CI | p | ||||
| AUC measures; mean ± SE | |||||||||||
| AUCi-CAR (nmol min/l) | −33.8 ± 52.5 | 81.2 ± 39.0 | 121.6 ± 32.2 | −0.73 | −155.3 | −272.2 to −38.4 | 0.01 | −0.19 | −40.4 | −140.3 to 59.5 | 0.42 |
| AUCg-DAY (nmol h/l) | 34.5 ± 3.0 | 36.6 ± 3.1 | 33.5 ± 1.8 | 0.08 | 0.9 | −5.7 to 7.6 | 0.78 | 0.21 | 3.1 | −3.9 to 10.0 | 0.38 |
Notes. FHx: family history of schizophrenia; ASz: antecedents of schizophrenia; TD: typically developing. Groups are not mutually exclusive – four ASz + FHx cases are included in both groups. AUCi-CAR: area under the curve with respect to increase for the cortisol awakening response; AUCg-DAY: area under the curve with respect to ground for cortisol during the day; d: standardized mean difference; B: unstandardized beta coefficient; SE: standard error; CI: confidence interval.
Figure 1.
Mean cortisol levels (± standard error mean) at awakening and 15, 30, and 60 min post-awakening in children with a family history of schizophrenia (FHx), children presenting antecedents of schizophrenia (ASz), and typically developing children (TD).
3.2. Associations between psychosocial stress and cortisol
Consistent with our previous report in a larger, overlapping sample (Cullen et al., 2014), relative to the TD group, ASz and FHx children experienced a higher number of negative life events than the TD group (p = 0.007 and p = 0.08, respectively) and ASz children also reported that they more frequently experienced daily hassles and were more distressed by these experiences (p ≤ 0.001; Table 1). Within-group correlation analyses were conducted to examine the relationship between psychosocial stress and cortisol (Table 3). AUCi-CAR values were not significantly associated with the total number of negative life events experienced in any of the three groups (p ≥ 0.10). However, among FHx children, AUCi-CAR values were positively correlated with distress experienced in relation to negative life events at the time of the event (ρ = 0.51, p = 0.02) and with the level of distress experienced currently (ρ = 0.52, p = 0.02); in contrast, among TD children, AUCi-CAR values were negatively correlated with distress experienced at the time of the negative life event (ρ = −0.31, p = 0.05). These variables were not significantly correlated in ASz children. AUCi-CAR values were not associated with the frequency of daily hassles or with distress relating to these experiences (p ≥ 0.10). None of the psychosocial stress measures were correlated with AUCg-DAY values (p ≥ 0.10).
Table 3.
Correlations of cortisol with psychosocial stress and neurocognitive performance.
| Cortisol awakening response (AUCi-CAR) |
Diurnal cortisol (AUCg-DAY) |
|||||
|---|---|---|---|---|---|---|
| FHx (n = 22) | ASz (n = 33) | TD (n = 40) | FHx (n = 20) | ASz (n = 33) | TD (n = 40) | |
| Psychosocial stress | ||||||
| Total number of negative life events | 0.21 | −0.03 | −0.03 | 0.04 | 0.09 | 0.27 |
| Negative life event distress – previous | 0.51* | −0.16 | −0.31* | 0.06 | 0.30 | 0.11 |
| Negative life event distress – current | 0.52* | −0.05 | −0.27 | −0.05 | −0.12 | 0.20 |
| Frequency of daily hassles | 0.18 | 0.13 | −0.12 | 0.00 | 0.10 | 0.18 |
| Distress relating to daily hassles | 0.17 | 0.16 | 0.00 | 0.16 | −0.26 | 0.20 |
| Neurocognitive performance | ||||||
| WRAML2 – Number-letter | 0.29 | 0.19 | −0.22 | −0.62** | −0.01 | 0.16 |
| WRAML2 – Verbal memory | 0.46* | 0.04 | 0.03 | −0.47* | 0.17 | −0.12 |
| WRAML2 – Visual memory | 0.05 | −0.05 | 0.24 | −0.31 | 0.22 | −0.02 |
| WRAML2 – Verbal working memory | 0.08 | 0.09 | −0.09 | −0.36 | 0.26 | −0.06 |
| D-KEFS – Letter fluency | 0.23 | 0.41* | −0.14 | −0.43 | −0.30 | 0.27 |
| D-KEFS – Category fluency | 0.02 | 0.22 | 0.18 | −0.30 | 0.06 | 0.15 |
| D-KEFS – Category switching | 0.22 | −0.14 | 0.06 | 0.05 | 0.16 | 0.11 |
| D-KEFS – Inhibition | 0.27 | −0.16 | 0.01 | −0.06 | 0.09 | 0.03 |
| D-KEFS – Inhibition/switching | 0.27 | −0.06 | −0.03 | 0.02 | −0.05 | −0.04 |
| D-KEFS – Tower test | 0.18 | 0.22 | 0.10 | −0.55* | −0.27 | 0.17 |
Notes. FHx: family history of schizophrenia; ASz: antecedents of schizophrenia; TD: typically developing. Groups are not mutually exclusive – four ASz + FHx cases are included in both groups. AUCi-CAR: area under the curve with respect to increase for the cortisol awakening response; AUCg-DAY: area under the curve with respect to ground for cortisol during the day; WRAML2: Wide Range Assessment of Memory and Learning 2nd Edition; D-KEFS: Delis–Kaplan Executive Function System. Pearson's ‘r’ correlations were used for all analyses except for those examining the negative life event distress variables, which were examined using Spearman's rho ‘ρ’.
p ≤ 0.05,
p ≤ 0.01.
3.3. Associations between neurocognitive function and cortisol
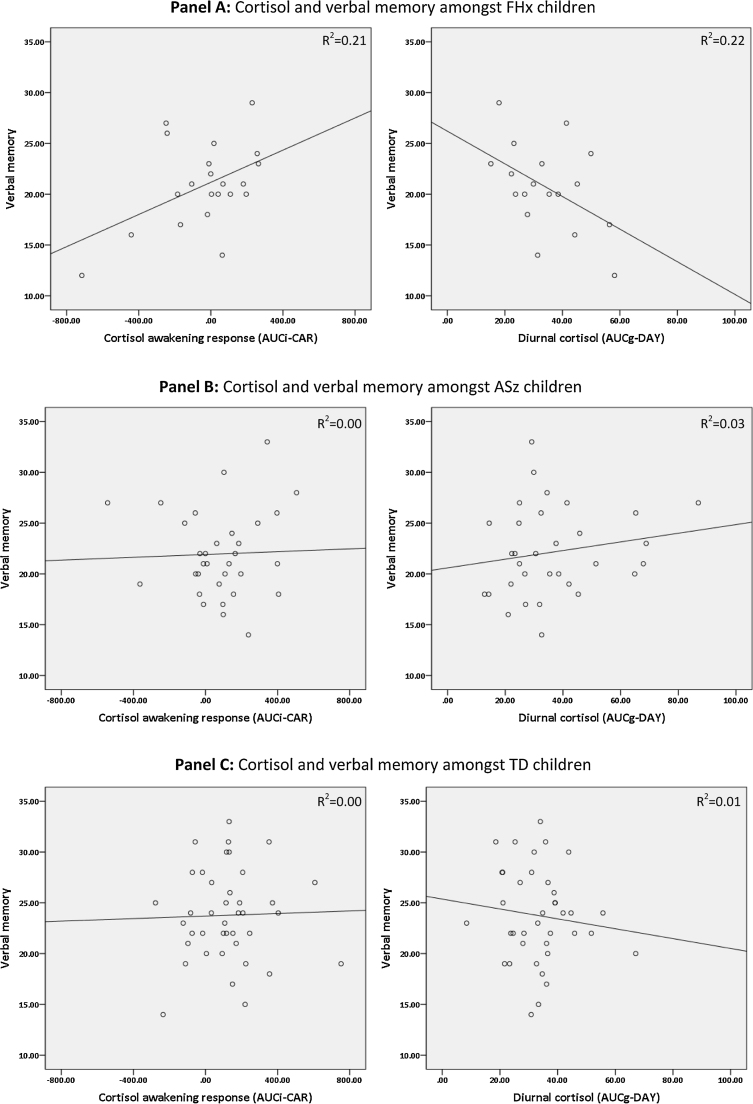
In line with our previous work examining neurocognitive function in ASz and FHx children at age 9–12 years (Cullen et al., 2010; Dickson et al., 2014), both ASz and FHx groups showed poorer performance on neurocognitive tests at age 11–14 years compared to the TD group (Table 1). Specifically, ASz children obtained significantly lower scores on the number-letter (p = 0.009), verbal working memory (p = 0.02), and verbal fluency – category switching subtests (p = 0.004), whilst FHx children obtained lower scores on the verbal memory index (p = 0.02) and the colour-word interference – inhibition subtest (p = 0.009). Correlations between cortisol AUC measures and neurocognitive performance are presented in Table 3. AUCi-CAR values were positively correlated with letter fluency among ASz children (r = 0.41, p = 0.02) and with verbal memory in the FHx group (r = 0.46, p = 0.04), but were not significantly associated with any neurocognitive measure in the TD group. In contrast, among FHx children, AUCg-DAY values were negatively correlated with verbal memory (r = −0.47, p = 0.05) and scores on the number-letter subtest (r = −0.62, p = 0.008) and towers test (r = −0.55, p = 0.02). The contrasting relationships between verbal memory and cortisol AUC values are further illustrated in Fig. 2.
Figure 2.
The relationship between verbal memory performance and the cortisol awakening response (AUCi-CAR) and diurnal cortisol (AUCg-DAY) in children with a family history of schizophrenia (FHx: Panel A), children presenting antecedents of schizophrenia (ASz: Panel B), and typically developing children (TD: Panel C).
3.4. Demographic correlates of salivary cortisol
Neither AUCi-CAR nor AUCg-DAY values were significantly associated with age, sex, ethnicity, pubertal status, tobacco use, time of awakening, or sampling month (p ≥ 0.1). However, there was a significant main effect of socioeconomic status on AUCg-DAY values (F[2,82] = 6.31, p = 0.003); post hoc tests indicated that children classed in the highest socioeconomic category (i.e., whose parents were employed in higher managerial, administrative, and professional occupations) had significantly lower AUCg-DAY values relative to children in the intermediate socioeconomic category (p = 0.001). We therefore repeated the AUCg-DAY analyses described above whilst adjusting for socioeconomic status, but observed no change to the pattern of results (i.e., there was still no significant effect of FHx or of ASz status on AUCg-DAY values). Additionally, the results were unchanged after excluding one participant from the ASz group who was being treated with stimulant medications (i.e., ASz children did not differ significantly to the TD group on either AUCi-CAR or AUCg-DAY values).
4. Discussion
In the first study to examine the CAR in children at elevated risk for psychosis, children with a family history of schizophrenia, but not those presenting multiple antecedents of schizophrenia, were characterized by a blunted CAR relative to their typically developing peers. However, neither FHx nor ASz children were characterized by elevated diurnal cortisol relative to the TD group. The CAR was positively correlated with distress relating to negative life events among FHx children, but negatively correlated in the TD group. Furthermore, among FHx and ASz children, greater abnormality of HPA axis function (that is, higher diurnal cortisol levels and a more blunted CAR) was associated with poorer performance on tests of verbal memory and executive function. These results further support the notion that at least some HPA axis changes precede illness onset, rather than being a subsequent epiphenomenon, and might participate in the development of neurocognitive abnormalities.
Our finding that FHx children were characterized by a blunted CAR relative to the TD group converges with two recent studies which observed a blunted CAR in patients with first episode psychosis compared to healthy controls (Mondelli et al., 2010a; Pruessner et al., 2013). The absence of a blunted CAR among ASz children contrasts with our previous work showing that ASz children present several neurobiological features that characterize adults with schizophrenia, including functional brain abnormality following commission of behavioural errors (Laurens et al., 2010), structural brain abnormalities encompassing the temporal lobe (Cullen et al., 2013), and involuntary dyskinetic movement abnormalities (Macmanus et al., 2012). One possible explanation is that the blunted CAR reflects a genetically mediated effect (although it is also possible that this effect may be driven by other environmental factors not examined in this study). For example, a study of individuals at increased risk for post-traumatic stress disorder (PTSD) on account of having parents with the disorder observed that offspring showed the same pattern of HPA axis abnormalities as individuals with PTSD (i.e., lower cortisol levels) even though they had not been exposed to trauma themselves (Yehuda et al., 2000). The fact that the effect of FHx status was twice as large among those with a first-degree relative with schizophrenia as among those with a second-degree relative is consistent with this hypothesis. Furthermore, data from a twin study indicates that the CAR is influenced by genetic factors whilst cortisol levels later in the day are not (Wust et al., 2000). A recent review concluded that elevated cortisol levels were more consistently observed among UHR youth compared to individuals with a family history of psychosis (Aiello et al., 2012), suggesting that different mechanisms may contribute to distinct HPA axis abnormalities. This “genetic model”, however, does not exclude the possibility of delivering psychosocial interventions aimed at preventing the development of further HPA axis abnormalities and reducing the risk of psychosis. For example, success has recently been observed in a trial of a novel school-based psychosocial intervention designed to enable adolescents to deal more effectively with stress, which led to a reduction in both cortisol levels and the risk of depression at a 3-month follow-up (Lupien et al., 2013).
Neither FHx nor ASz children, however, were characterized by elevated diurnal cortisol levels, which contrasts with previous observations among young adult relatives of patients with psychosis (Collip et al., 2011), youth at UHR (Sugranyes et al., 2012; Walker et al., 2013) and adolescents with SPD (Walker et al., 2001; Mittal et al., 2007). This may be due to our participants being younger (mean age: 13.1 years) than high-risk youth in previous studies (mean age range: 14.1–28.5 years). Longitudinal studies indicate that cortisol levels during the day increase during late childhood and adolescence (Walker et al., 2001; Shirtcliff et al., 2012); thus, the elevation in daytime cortisol levels observed in high-risk youth relative to their healthy peers may not emerge (or may not be detectable) until later in adolescence.
We identified a dissociation in the relationship between psychosocial stress and cortisol among FHx and TD children; specifically, distress relating to negative life events was positively correlated with the CAR in the FHx group but negatively correlated among TD children. Interestingly, a dissociation in stress-cortisol relationships was observed in a study of patients with first episode psychosis which reported a negative correlation between diurnal cortisol and the number of recent stressful events in the patient group but a positive correlation among healthy controls (Mondelli et al., 2010a). Few studies of high-risk youth have measured psychosocial stress and cortisol concurrently. One study of young adult siblings of patients with psychosis observed that siblings showed an increase in cortisol after experiencing negative events during the day, but that healthy controls did not (Collip et al., 2011). Thus, one possible explanation for the dissociation we observed is that psychosis (and psychosis vulnerability) may influence the effect of psychosocial stress on HPA axis function. This may relate to how these stressful events are appraised. The degree to which stressors are viewed as potentially controllable or uncontrollable has been associated with higher and lower morning cortisol levels, respectively (Miller et al., 2007). Thus, it is possible that FHx children may feel that they are personally responsible for the negative life events they experience, whilst TD children might view these events as being outside of their control. Our finding that cortisol levels were not correlated with daily hassles contrasts with a study of UHR youth in which a positive association was observed between morning cortisol levels and the number of hassles experienced during the past month (Thompson et al., 2007). One possible explanation for the lack of association in our study is that daily hassles were not always assessed on the day of cortisol collection. For example, cortisol levels have been found to be higher on days when individuals experience a greater number of stressors compared to stress-free days (Stawski et al., 2013).
The current study is the first to examine the relationship between HPA axis function and neurocognitive performance among individuals at elevated risk for psychosis. Moderate-to-large impairments across a range of neurocognitive domains have been observed consistently in patients with first episode psychosis relative to healthy controls (Mesholam-Gately et al., 2009), and prospective studies indicate that some of these impairments are present in children who go on to develop the disorder in later life (Dickson et al., 2012). The current study suggests that the deficits in memory and executive function that we have observed in ASz and FHx children (Cullen et al., 2010; Dickson et al., 2014), and which are also known to characterize UHR youth (Fusar-Poli et al., 2012), are associated with greater abnormality of HPA axis function. Specifically, a more blunted CAR was associated with poorer verbal memory and letter fluency among FHx and ASz children, respectively; moreover, in the FHx group only, higher diurnal cortisol was correlated with poorer performance on the number-letter subtest and towers test, and lower verbal memory index scores. In contrast, HPA axis function was not associated with neurocognitive performance among TD children. These findings are consistent with those of a recent study of patients with first episode psychosis in which a more blunted CAR was associated with poorer verbal memory and processing speed in patients but not healthy controls (Aas et al., 2011). A study of patients with chronic schizophrenia reported that higher diurnal cortisol levels were correlated with poorer memory and executive function (Walder et al., 2000). Further, a recent study reported that elevated cortisol levels were associated with reduced hippocampal volume among individuals with first episode psychosis (Mondelli et al., 2010b). Thus, one explanation for our findings is that stress-induced HPA axis function may have a direct effect on neurocognitive performance (i.e., via the effects of cortisol on the brain structures supporting these functions). Alternatively, common neurodevelopmental mechanisms may influence both HPA axis function and neurocognitive performance.
4.1. Limitations
The current study is limited by the relatively small participant groups. Whilst the number of children at elevated risk for schizophrenia was sufficiently large for us to be able to detect HPA axis abnormalities among those with a family history of illness, our ability to detect significant effects in the ASz group may be particularly limited in light of the fact that the proportion of individuals in this group who will go on to develop schizophrenia (i.e., true positives in whom HPA axis abnormalities might be expected) may be low. Indeed, given that ASz children are not currently seeking help for their symptoms, the psychosis transition rate in this group may be lower than is observed among UHR youth. However, the non help-seeking nature of this group may be considered a potential strength of the study, as it reduces the possibility that any HPA axis abnormalities are merely due to distress associated with emerging illness. The inclusion of children with a second-degree relative with schizophrenia in the FHx group is likely to have underestimated the effect of putative genetic liability. However, this allowed us to investigate the possibility that those with greater genetic liability would show more pronounced HPA axis abnormalities. Indeed, post hoc analyses confirmed that the magnitude of differences in the CAR between FHx and TD children was twice as large among those with a first-degree relative as in those with an affected second-degree relative. The fact that the groups were not matched on key demographic variables might also be considered a potential limitation of the study. Compared to the TD group, ASz and FHx children were more likely to be of black or other ethnicity and tended to be from lower socioeconomic backgrounds. However, this pattern is consistent with the elevated rates of psychosis observed among African-Caribbean and black African adults in the UK (Kirkbride et al., 2012) and with population-based studies showing that low socioeconomic status in childhood is associated with increased risk of psychosis (Wicks et al., 2005; Corcoran et al., 2009). A large number of correlation analyses were conducted with no corrections applied to reduce the risk of type 1 errors, so it is possible that some of the significant correlations observed may have arisen by chance. These investigations are exploratory in nature, and must be interpreted with caution. A further limitation relates to the lapse of time between salivary cortisol collection and neurocognitive assessments; however, the groups did not differ in the lapse of time between assessments and so this would not have contributed to the different patterns of association observed across the groups. Finally, we were not able to examine the relationship between HPA axis function and more severe forms of psychosocial stress such as childhood maltreatment, which has been implicated in the development of psychosis (Schafer and Fisher, 2011) and associated with a blunted cortisol response to stress (Ouellet-Morin et al., 2011). Thus, childhood maltreatment may have been able to explain the blunted CAR observed among FHx children. This is especially likely to be the case as individuals with a family history of psychosis are at elevated risk for exposure to childhood physical abuse (Fisher et al., 2014).
4.2. Conclusions
The current study adds to the growing body of evidence suggesting that individuals on the trajectory to psychosis are characterized by abnormal HPA axis function. Albeit longitudinal studies are needed to further understand the relevance of these abnormalities, these findings, and those of aforementioned studies, support the targeting of the HPA axis as a strategy for early intervention aiming to reduce the risk of psychosis.
Role of funding source
None.
Conflict of interest
None declared.
Acknowledgments
This work was supported by funding to KRL from a National Institute for Health Research (NIHR) Career Development Fellowship (CDF/08/01/015); a Bial Foundation Research Grant (35/06); a NARSAD Young Investigator Award (2005); and the British Medical Association Margaret Temple Award for schizophrenia research (2006). AEC was supported by the Waterloo Foundation (164/1719) and HLF was supported by a Medical Research Council (MRC) Population Health Scientist award (G1002366). All authors are affiliated with the NIHR Specialist Biomedical Research Centre (BRC) for Mental Health at the South London and Maudsley National Health Service Foundation Trust and Institute of Psychiatry, King's College London, United Kingdom. The authors thank the children and caregivers who participated in the study, and the researchers and students who contributed to data collection.
Footnotes
Schizotypal (personality) disorder is classified as a schizophrenia spectrum disorder in both the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) and the International Classification of Disorders Version 10 (World Health Organization, 1992).
Contributor Information
Alexis E. Cullen, Email: alexis.cullen@kcl.ac.uk.
Kristin R. Laurens, Email: kristin.laurens@kcl.ac.uk.
References
- Aas M., Dazzan P., Mondelli V., Toulopoulou T., Reichenberg A., Di Forti M., Fisher H.L., Handley R., Hepgul N., Marques T., Miorelli A., Taylor H., Russo M., Wiffen B., Papadopoulos A., Aitchison K.J., Morgan C., Murray R.M., Pariante C.M. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol. Med. 2011;41:463–476. doi: 10.1017/S0033291710001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew-Blais J., Seidman L.J. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn. Neuropsychiatry. 2013;18:44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello G., Horowitz M., Hepgul N., Pariante C.M., Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with at risk mental state. Psychoneuroendocrinology. 2012;37:1600–1613. doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Belvederi Murri M., Pariante C.M., Dazzan P., Hepgul N., Papadopoulos A.S., Zunszain P., Di Forti M., Murray R.M., Mondelli V. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Borges S., Gayer-Anderson C., Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38:603–611. doi: 10.1016/j.psyneuen.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Brunelin J., d’Amato T., van Os J., Cochet A., Suaud-Chagny M.F., Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr. Res. 2008;100:206–211. doi: 10.1016/j.schres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A., Acebo C. A self-administered rating scale for pubertal development. J. Adolesc. Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Collip D., Nicolson N.A., Lardinois M., Lataster T., van Os J., Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol. Med. 2011;41:2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Corcoran C., Perrin M., Harlap S., Deutsch L., Fennig S., Manor O., Nahon D., Kimhy D., Malaspina D., Susser E. Effect of socioeconomic status and parents’ education at birth on risk of schizophrenia in offspring. Soc. Psychiatry Psychiatr. Epidemiol. 2009;44:265–271. doi: 10.1007/s00127-008-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen A.E., De Brito S.A., Gregory S.L., Murray R.M., Williams S.C., Hodgins S., Laurens K.R. Temporal lobe volume abnormalities precede the prodrome: a study of children presenting antecedents of schizophrenia. Schizophr. Bull. 2013;39:1318–1327. doi: 10.1093/schbul/sbs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen A.E., Dickson H., West S.A., Morris R.G., Mould G.L., Hodgins S., Murray R.M., Laurens K.R. Neurocognitive performance in children aged 9–12 years who present putative antecedents of schizophrenia. Schizophr. Res. 2010;121:15–23. doi: 10.1016/j.schres.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Cullen A.E., Fisher H.L., Roberts R.E., Pariante C.M., Laurens K.R. Daily stressors and negative life events in children at elevated risk of developing schizophrenia. Br. J. Psychiatry. 2014 doi: 10.1192/bjp.bp.113.127001. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J.H. The Psychological Corporation; San Antonio: 2001. The Delis–Kaplan Executive function System: Examiners Manual. [Google Scholar]
- Dickson H., Cullen A.E., Reichenberg A., Hodgins S., Campbell D.D., Morris R.G., Laurens K.R. Cognitive impairment among children at-risk for schizophrenia. J. Psychiatr. Res. 2014;50:92–99. doi: 10.1016/j.jpsychires.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Dickson H., Laurens K.R., Cullen A.E., Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol. Med. 2012;42:743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- Fisher H.L., McGuffin P., Boydell J., Fearon P., Craig T.K., Dazzan P., Morgan K., Doody G.A., Jones P.B., Leff J., Murray R.M., Morgan C. Interplay between childhood physical abuse and familial risk in the onset of psychotic disorders. Schizophr. Bull. 2014 doi: 10.1093/schbul/sbt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Deste G., Smieskova R., Barlati S., Yung A.R., Howes O., Stieglitz R.D., Vita A., McGuire P., Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Ostrander M.M., Mueller N.K., Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Heubeck B., O'Sullivan C. An exploration into the nature, frequency and impact of school hassles in the middle school years. Aust. Psychol. 1998;33:130–137. [Google Scholar]
- Kirkbride J.B., Errazuriz A., Croudace T.J., Morgan C., Jackson D., Boydell J., Murray R.M., Jones P.B. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PLoS ONE. 2012;7:e31660. doi: 10.1371/journal.pone.0031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens K.R., Hobbs M.J., Sutherland M., Green M.J., Mould G.L. Psychotic-like experiences in a community sample of 8,000 children aged 9–11 years: an item response theory analysis. Psychol. Med. 2012;47:1495–1506. doi: 10.1017/S0033291711002108. [DOI] [PubMed] [Google Scholar]
- Laurens K.R., Hodgins S., Maughan B., Murray R.M., Rutter M.L., Taylor E.A. Community screening for psychotic-like experiences and other putative antecedents of schizophrenia in children aged 9–12 years. Schizophr. Res. 2007;90:130–146. doi: 10.1016/j.schres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Laurens K.R., Hodgins S., Mould G.L., West S.A., Schoenberg P.L., Murray R.M., Taylor E.A. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol. Psychiatry. 2010;67:238–245. doi: 10.1016/j.biopsych.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Laurens K.R., Hodgins S., Taylor E., Murray R.M. Is earlier intervention for schizophrenia possible?: Identifying antecedents of schizophrenia in children aged 9–12 years. In: David A.S., McGuffin P., Kapur S., editors. Schizophrenia: The Final Frontier. Psychology Press; London: 2011. [Google Scholar]
- Lipsey M.W., Wilson D.B. Sage Publications; Thousand Oaks, CA: 2001. Practical meta-analysis. [Google Scholar]
- Lupien S.J., Ouellet-Morin I., Trepanier L., Juster R.P., Marin M.F., Francois N., Sindi S., Wan N., Findlay H., Durand N., Cooper L., Schramek T., Andrews J., Corbo V., Dedovic K., Lai B., Plusquellec P. The DeStress for success program: effects of a stress education program on cortisol levels and depressive symptomatology in adolescents making the transition to high school. Neuroscience. 2013;249:74–87. doi: 10.1016/j.neuroscience.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Mackie C.J., Castellanos-Ryan N., Conrod P.J. Developmental trajectories of psychotic-like experiences across adolescence: impact of victimization and substance use. Psychol. Med. 2011;41:47–58. doi: 10.1017/S0033291710000449. [DOI] [PubMed] [Google Scholar]
- Macmanus D., Laurens K.R., Walker E.F., Brasfield J.L., Riaz M., Hodgins S. Movement abnormalities and psychotic-like experiences in childhood: markers of developing schizophrenia? Psychol. Med. 2012;42:99–109. doi: 10.1017/S0033291711001085. [DOI] [PubMed] [Google Scholar]
- Maxwell M.E. National Institute of Mental Health; St. Louis, MO: 1992. Family Interview for Genetic Studies. [Google Scholar]
- McVie S., Bradshaw P. The University of Edinburgh; Edinburgh: 2005. Adolescent Smoking, Drinking and Drug Use, The Edinburgh Study of Youth Transitions and Crime. [Google Scholar]
- Mesholam-Gately R.I., Giuliano A.J., Goff K.P., Faraone S.V., Seidman L.J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Zhou E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mittal V.A., Dhruv S., Tessner K.D., Walder D.J., Walker E.F. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol. Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mondelli V., Dazzan P., Hepgul N., Di Forti M., Aas M., D’Albenzio A., Di Nicola M., Fisher H., Handley R., Marques T.R., Morgan C., Navari S., Taylor H., Papadopoulos A., Aitchison K.J., Murray R.M., Pariante C.M. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr. Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Pariante C.M., Navari S., Aas M., D’Albenzio A., Di Forti M., Handley R., Hepgul N., Marques T.R., Taylor H., Papadopoulos A.S., Aitchison K.J., Murray R.M., Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr. Res. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics . National Statistics; 2010. Neighbourhood Statistics by Local Authority. [Google Scholar]
- Ouellet-Morin I., Odgers C.L., Danese A., Bowes L., Shakoor S., Papadopoulos A.S., Caspi A., Moffitt T.E., Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol. Psychiatry. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner M., Vracotas N., Joober R., Pruessner J.C., Malla A.K. Blunted cortisol awakening response in men with first episode psychosis: relationship to parental bonding. Psychoneuroendocrinology. 2013;38:229–240. doi: 10.1016/j.psyneuen.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Schafer I., Fisher H.L. Childhood trauma and psychosis – what is the evidence? Dialog. Clin. Neurosci. 2011;13:360–365. doi: 10.31887/DCNS.2011.13.2/ischaefer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D., Adams W. Wide range Inc.; Delaware: 2003. Wide Range Assessment of Memory and Learning – Second Edition (WRAML2) [Google Scholar]
- Shirtcliff E.A., Allison A.L., Armstrong J.M., Slattery M.J., Kalin N.H., Essex M.J. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev. Psychobiol. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stateva M., Minton J., Beckett C., Doolan M., Ford T., Kallitsoglou A., Scott S. Challenges recruiting families with children at risk of anti-social behaviour into intervention trials: lessons from the Helping Children Achieve (HCA) study. J. Child. Serv. 2012;7:285–302. [Google Scholar]
- Stawski R.S., Cichy K.E., Piazza J.R., Almeida D.M. Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G., Thompson J.L., Corcoran C.M. HPA-axis function, symptoms, and medication exposure in youths at clinical high risk for psychosis. J. Psychiatr. Res. 2012;46:1389–1393. doi: 10.1016/j.jpsychires.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.N., Phillips L.J., Komesaroff P., Yuen H.P., Wood S.J., Pantelis C., Velakoulis D., Yung A.R., McGorry P.D. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. J. Psychiatr. Res. 2007;41:561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Vreeburg S.A., Kruijtzer B.P., van Pelt J., van Dyck R., DeRijk R.H., Hoogendijk W.J., Smit J.H., Zitman F.G., Penninx B.W. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34:1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Walder D.J., Walker E.F., Lewine R.J. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol. Psychiatry. 2000;48:1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker E., Mittal V., Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker E.F., Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E.F., Trotman H.D., Pearce B.D., Addington J., Cadenhead K.S., Cornblatt B.A., Heinssen R., Mathalon D.H., Perkins D.O., Seidman L.J., Tsuang M.T., Cannon T.D., McGlashan T.H., Woods S.W. Cortisol levels and risk for psychosis: initial findings from the North American Prodrome Longitudinal Study. Biol. Psychiatry. 2013;74:410–417. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.F., Walder D.J., Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev. Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wicks S., Hjern A., Gunnell D., Lewis G., Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am. J. Psychiatry. 2005;162:1652–1657. doi: 10.1176/appi.ajp.162.9.1652. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organisation; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]
- Wust S., Federenko I., Hellhammer D.H., Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Wang L., Lv H., Yu J., Xun Z., Yang G. Abnormality of glycometabolism related factors in non-psychotic offspring of schizophrenic patients. Psychiatry Res. 2012;198:183–186. doi: 10.1016/j.psychres.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Bierer L.M., Schmeidler J., Aferiat D.H., Breslau I., Dolan S. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am. J. Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.