Abstract
The amyloid β-peptide (Aβ peptide) is assumed to play a crucial and early role in the pathogenesis of Alzheimer disease. Thus, strategies for a pharmacotherapy aim at reducing Aβ peptide generation, which proteolytically derives from the amyloid precursor protein (APP). The main targets so far have been β- and γ-secretase, the two proteases that cleave APP at the N- and C-terminus of the Aβ peptide and are thus directly responsible for Aβ peptide generation. A different strategy, namely the activation of α-secretase, has barely been investigated for its therapeutic potential. α-Secretase cleaves within the Aβ peptide domain and thus precludes Aβ peptide generation. Now, new results demonstrate that activation of α-secretase indeed reduces Aβ peptide generation and toxicity in vivo.
Numerous laboratories are currently investigating β- and γ-secretase, the two amyloidogenic proteases that cleave the Aβ-peptide out of the amyloid precursor protein (APP). The reason is obvious. If we prevent these proteases from working, we will stop the progression of Alzheimer disease (AD). However, a rather old and almost forgotten idea, namely the activation of α-secretase, which cuts the amyloid β-peptide (Aβ peptide) into two nonamyloidogenic pieces, has now been reinvestigated. Compelling evidence that this strategy may work is now presented in a study by researchers in Germany and Belgium led by Falk Fahrenholz at the University of Mainz (1).
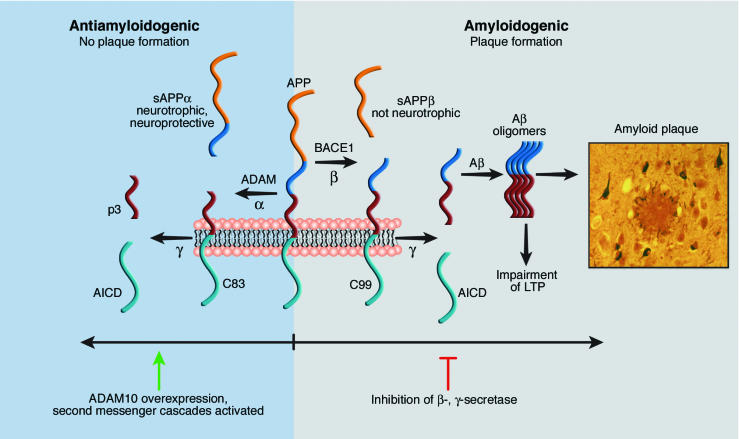
AD is the most prevalent neuro-degenerative disease, affecting about 20 million people worldwide (for an overview see ref. 2). The amyloid hypothesis of AD, which is now widely accepted, describes the pathogenesis of this disease as a cascade of several steps, from the initial generation of the Aβ peptide to cognitive impairment and neuronal loss (for overviews see refs. 3, 4). Whereas drugs are currently available that may slightly ameliorate late-stage symptoms such as cognitive deficits for a short time, no drugs are on the market that specifically target the cellular mechanisms of the disease, namely the proteolytic generation of the Aβ peptide from APP. APP is a type I membrane protein with unclear biological function. APP undergoes proteolytic processing in two different pathways (Figure 1). One is termed amyloidogenic because it leads to the generation of the Aβ peptide. The other one is referred to as antiamyloidogenic because it prevents Aβ peptide generation (2).
Figure 1.
Proteolytic processing of APP is divided into an amyloidogenic and an antiamyloidogenic pathway. Amyloidogenic pathway: Cleavage of APP by the protease β-secretase (BACE1) occurs at the N-terminus of the Aβ domain and yields the secreted sAPPβ as well as a C-terminal fragment of APP of 99 amino acids (C99). C99 is further cleaved within its transmembrane domain by γ-secretase, leading to the secretion of the Aβ peptide and the generation of the APP intracellular domain (AICD). The Aβ peptide is prone to aggregation. Aβ peptide oligomers are neurotoxic and lead to an impairment of long-term potentiation (LTP). Finally, large amounts of Aβ peptide are deposited in amyloid plaques, which are the characteristic pathological hallmarks of AD. The consecutive cleavage of APP by β- and γ-secretase constitutes the amyloidogenic pathway as it generates Aβ. Antiamyloidogenic pathway: Cleavage of APP by α-secretase within the Aβ peptide domain yields the neurotrophic and neuroprotective sAPPα. The α-secretase is a member of the ADAM family of metalloproteases. α-Cleavage of APP can be induced upon overexpression of ADAM10 or by the activation of second messenger cascades.
In the amyloidogenic pathway, APP is first cleaved by the β-secretase, BACE1, at the N-terminus of the Aβ domain (5). This cleavage generates the soluble sAPPβ and a C-terminal fragment, which undergoes a second cleavage by a protease called γ-secretase. γ-Secretase cleaves within the transmembrane domain of APP and is a heteromeric protein complex consisting of presenilin, nicastrin, PEN-2, and APH-1 (for a review see ref. 5). The fact that mice deficient in either protease do not generate the Aβ peptide clearly implicates BACE1 and the γ-secretase complex as the amyloidogenic proteases in vivo and makes them suitable drug targets for AD (5).
In contrast, the antiamyloidogenic pathway starts with APP cleavage by α-secretase, which cuts within the Aβ domain and thus precludes Aβ peptide generation. Following α-cleavage, the C-terminal APP fragment undergoes γ-cleavage, leading to the generation of the p3 peptide (6) (Figure 1), which seems to be benign, since it is not found in the amyloid plaques characteristic of AD. α-Secretase is a member of the ADAM (a disintegrin and metalloprotease) family of proteases (for a review see ref. 7) and is either ADAM10 (8), ADAM17/TACE (9), or even ADAM9 (10). At present, it is unclear whether only one of them or all three together constitute the physiologically relevant α-secretase.
Since the combined action of β- and γ-secretase leads to Aβ peptide generation, the inhibition of their activity is considered to be a highly promising approach to treat AD and is being pursued by a number of pharmaceutical companies. However, the development of specific β- and γ-secretase inhibitors that are able to cross the blood-brain barrier seems to be a particular challenge. BACE1 seems to have a rather unusual large active cleft, which makes the generation of selective inhibitors difficult (11). The suitability of inhibiting γ-secretase has been called into question by findings that this protease is involved in physiologically highly important signaling mechanisms required for cell fate decisions (3, 12). Although these problems may be circumvented at some point, it seems to be increasingly important to search for alternative targets. One such approach may be the facilitation of α-secretase cleavage of APP, an idea based on the original findings of Nitsch and colleagues (13). Since α-secretase cleaves within the Aβ peptide domain, its activation may even have the double advantage of not only precluding the neurotoxic Aβ peptide formation but also generating the putatively neuroprotective sAPPα (14, 15). This approach has so far received little attention but is particularly tempting, since BACE1 and α-secretase compete for the ectodomain cleavage of APP (16). Thus, it is conceivable to shift APP cleavage from the amyloidogenic β-secretase to the antiamyloidogenic α-secretase cleavage. Indeed, in cultured cells a number of pharmacological agents can stimulate the α-secretase cleavage of APP at the expense of β-cleavage, thereby reducing Aβ peptide generation (for a review see ref. 7). Likewise, overexpression of ADAM proteases such as ADAM10 (8) or ADAM17/TACE (17) also increases APP α-cleavage. However, evidence is still lacking that an increased expression or activity of ADAM proteases is antiamyloidogenic in vivo. This is mainly due to the embryonic and perinatal lethality of ADAM10 (18) and ADAM17 (19) knockout mice, respectively, which prevented the functional analysis of these proteases in older mice at an age when amyloid plaques are forming. To circumvent these difficulties, Postina and colleagues (1) chose a different approach to test the potential antiamyloidogenic role of α-secretase in vivo. As a natural extension of their original work, which identified ADAM10 as one of the α-secretases (8), they chose to overexpress bovine ADAM10 selectively in neurons. Additionally, a dominant-negative ADAM10 mutant was overexpressed, which inhibits the endogenous APP α-cleavage in cultured cells (8) and should thus reduce the antiamyloidogenic APP processing in mice. Both transgenic lines were crossed with the AD mouse model generated in van Leuven’s laboratory in Belgium (20). In this model, human APP is overexpressed in neurons. Overexpression of human APP results in enhanced generation of the Aβ peptide, which at the end is deposited in the mouse brain in the same way as in human brains. Furthermore, enhanced Aβ peptide production also results in memory deficits (20). By crossing this AD mouse model with ADAM10 transgenic mice, Postina et al. have now elegantly shown that overexpressed ADAM10 acts as at least one of the α-secretases in vivo and thus is indeed antiamyloidogenic (1). ADAM10 overexpression increased sAPPα secretion and reduced Aβ peptide generation. Although the reduction in Aβ peptide generation was not dramatic, it was sufficient to almost completely prevent amyloid plaque formation in the mouse brain. In contrast, the dominant-negative ADAM10 mutant had the opposite effect of wild-type ADAM10. It inhibited APP α-cleavage, slightly increased Aβ peptide generation, and increased both the number and the size of amyloid plaques in the mouse brain. The rather small effects on total Aβ peptide generation as compared to amyloid deposition suggest that relatively minor changes in Aβ peptide production may be sufficient to induce AD pathology. This provides hope, since secretase modifiers (regardless of whether they are inhibitors of β- and γ-secretase or activators of α-secretase) may only be required at rather low dose. Most importantly, the study by Postina and colleagues also provides evidence that overexpression of ADAM10 not only reduces amyloid plaque formation but also alleviates the deficits in spatial learning and synaptic plasticity observed in the control animals (1), suggesting that an activation of α-secretase cleavage may also improve cognitive status in humans. On the molecular level, however, it remains unclear whether this beneficial effect is due to the reduced amyloid burden, the increased generation of the neuroprotective sAPPα, or both. Moreover, it could even be caused by the increased secretion of other protein substrates of ADAM10. However, the mice expressing ADAM10 that were initially generated (before being crossed to the APP-expressing mice) did not show any obvious phenotypic changes compared to control animals, which is good news, given that ADAM10 is also involved in the cleavage of membrane proteins other than APP, such as Notch (18), EGF, and β-cellulin (21).
To fully evaluate the therapeutic potential of activation of α-secretase, additional studies are needed. In contrast to the transgenic mice overexpressing ADAM10 from shortly after birth, humans would not be treated until later in life. Thus, it will be important to use mice with an inducible ADAM10 expression to address the question whether ADAM10 still lowers Aβ peptide generation and rescues memory deficits when overexpressed later in life, when the first plaques already have formed. A direct transfer of the murine results into patients would require a gene therapy approach to overexpress ADAM10 in neurons. Since this may not be easily feasible in humans, it is of utmost importance to explore other ways to increase the expression and activity of ADAM proteases in vivo. From tissue culture studies it is well known that the ADAM cleavage of APP can be stimulated through an activation of second messenger cascades (for an overview see ref. 7). However, at present little is known about the underlying cellular pathways and mechanisms. Therefore, the identification of regulatory genes and chemical compounds that selectively affect ADAM-protease activity may lead to new drug targets and new possibilities for pharmacological intervention in AD. However, one has to keep in mind that activation of the second messenger cascades is a rather nonselective approach which again may result in unwanted side effects. The work by Fahrenholz and colleagues is a major step forward in exploring the therapeutic potential of α-secretase targeting.
Footnotes
See the related article beginning on page 1456.
Nonstandard abbreviations used: a disintegrin and metalloprotease (ADAM); Alzheimer disease (AD); amyloid β-peptide (Aβ peptide); amyloid precursor protein (APP).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Postina R, et al. A disintegrin-metal-loproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 2004;113:1456–1464. doi:10.1172/JCI200420864. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts myloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 3.Citron M. beta-Secretase inhibition for the treatment of Alzheimer’s disease - promise and challenge. Trends Pharmacol. Sci. 2004;25:92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Haass C. Take five-BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J. 2004;23:483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haass C, et al. b-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem. 1993;268:3021–3024. [PubMed] [Google Scholar]
- 7.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 8.Lammich S, et al. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxbaum JD, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 10.Koike H, et al. Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 1999;343:371–375. [PMC free article] [PubMed] [Google Scholar]
- 11.Hong L, et al. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290:150–153. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- 12.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 13.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa K, et al. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C- terminal heparin-binding domain. J. Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 15.Meziane H, et al. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J. Biol. Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- 17.Slack BE, Ma LK, Seah CC. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-alpha converting enzyme. Biochem. J. 2001;357:787–794. doi: 10.1042/0264-6021:3570787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann D, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 19.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 20.Moechars D, et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 21.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell. Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]