Abstract
Antibodies specific for the β1-adrenergic receptor are found in patients with chronic heart failure of various etiologies. From work presented in this issue of the JCI, we can now infer that these antibodies actually contribute to the pathogenesis of chronic heart failure. This commentary discusses mechanisms by which these antibodies may engender cardiomyopathy.
Do anti–β-adrenergic receptor (anti–β-AR) antibodies play a role in the pathogenesis of chronic systolic heart failure (CHF)? This question emerged almost 30 years ago (1), when antibodies with β-adrenergic stimulating (agonist) activity were discovered in the serum of patients with Chagas disease, one of the most common causes of CHF worldwide (2). Since that time, IgGs with agonist activity for the β1-adrenergic receptor (β1-AR) have been found in sera not only from patients with Chagas disease, but also from patients with idiopathic dilated cardiomyopathy (3) as well as ischemic (4) cardiomyopathy. Whether these antibodies merely correlate with myocardial inflammation that leads to CHF, result from myocardial inflammation, or actually contribute to the pathogenesis of CHF could not be ascertained — until now. In this issue of the JCI, Jahns et al. employed isogenic injections of anti–β1-AR antiserum in inbred rats to produce a cardiomyopathy that appears to be non-inflammatory (5). In so doing, these authors conclusively demonstrated that agonistic, anti–β1-AR IgG — by itself — is sufficient to engender the sort of myocardial dysfunction characteristic of CHF. This finding fundamentally advances our understanding of CHF. However, it should not really surprise us, because it represents a logical extension of diverse but congruent investigations conducted over several decades.
To provide historical and mechanistic perspectives for the elegant work of Jahns et al., we address several questions that relate their work to contemporary concepts of β1-AR pathophysiology: How might IgG activate the β1-AR, and how could chronic β1-AR activation result in cardiomyocyte toxicity? What molecular mechanisms regulate the β1-AR when it is chronically stimulated by IgG or other agonists, and how might these mechanisms affect the pathogenesis of CHF? Lastly, how can these perspectives elucidate the therapeutic efficacy of β-AR antagonists, or “beta blockers,” in CHF?
Activation of cardiac β1-ARs
The β1-AR constitutes approximately 80% of the cardiac β-AR complement. Like the β2-AR (with 54% overall homology), the β1-AR is a seven-membrane–spanning receptor, with three extracellular polypeptide sequences (“loops”) connecting the transmembrane α helices (Figure 1). With their intracellular domains, the β-ARs couple to the stimulatory heterotrimeric GTP-binding protein (Gs). Activation of the β1-AR requires a specific receptor conformation — one that is stabilized by agonist (and, apparently the binding of certain IgGs to the second extracellular loop; ref. 5). It also appears that β1-AR dimerization (6, 7) may be involved in receptor activation (and may underlie the agonist properties of anti-β1-AR antibodies observed in CHF patients and rats). Stimulation of β-ARs engenders a cascade of consequent activation: first Gs, then adenylyl cyclase (which forms cAMP), then the cAMP-dependent protein kinase (PKA). Activated PKA subsequently phosphorylates molecules critical for regulating sarcoplasmic [Ca2+] (8) — thereby increasing cardiomyocyte inotropy, chronotropy, and lusitropy. Activation of Gs can also increase L-type Ca2+ channel currents directly (9, 10) (Figure 1). Over the last few years, this time-honored scheme has been modified in ways that illuminate the β1-AR-specific findings of Jahns et al. (5).
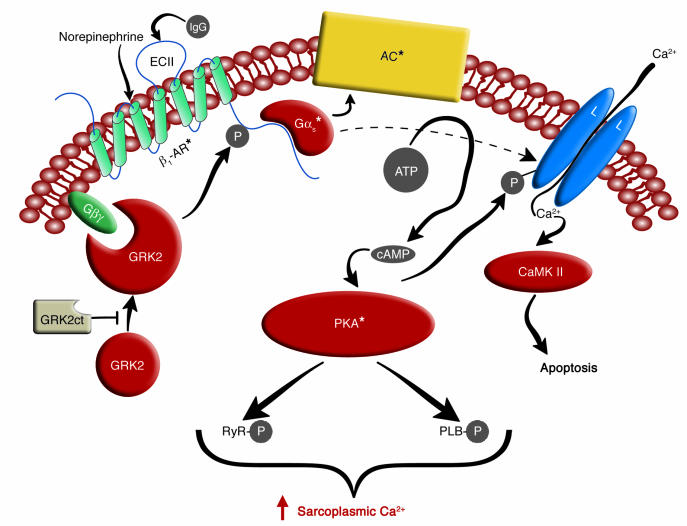
Figure 1.
Schema for β1-AR–mediated cardiomyocyte stimulation and β1-AR desensitization. The seven-membrane-spanning β1-AR is stimulated by the physiologic agonist norepinephrine or by IgG specific for the receptor’s second extracellular loop (ECII) (in CHF subjects). The stimulated β1-AR then activates the heterotrimeric Gs, which dissociates into its Gαs* and Gβγ substituents. The Gαs* activates both adenylyl cyclase (AC), which catalyzes cAMP formation, and the L-type calcium channel (L), which then permits Ca2+ to enter the cardiomyocyte. This Ca2+ can both augment contractility and, on a slower time scale, promote cardiomyocyte apoptosis by activating Ca2+/calmodulin kinase II (CaMK II). The cAMP produced by adenylyl cyclase activates PKA, which subsequently phosphorylates (P) numerous substrates important to sarcoplasmic [Ca2+] regulation: the L-type Ca2+ channel, the ryanodine receptor (RyR), and phospholamban (PLB). The net effect of this activity in the short term is to augment sarcoplasmic [Ca2+] and contractility. (In the long term, this activity engenders cardiomyocyte toxicity.) The activated β1-AR is desensitized when it is phosphorylated by PKA (not shown) and by GRK2, the cellular expression of which increases with chronic β1-AR stimulation. Translocation of GRK2 from the sarcoplasm to the sarcolemma requires Gβγ subunits, and this translocation is inhibited when GRK2ct is expressed heterologously in cardiomyocytes. Asterisks denote activated proteins.
Genetic and pharmacologic approaches have demonstrated that the β1-AR plays the predominant role in mediating cardiac inotropic and chronotropic responses to catecholamines (8). Cardiac preparations from β1-AR knockout mice fail to augment contractility or their rate of contraction in response to isoproterenol, which stimulates both β1- and β2-ARs (11). Remarkably, β1-AR knockout hearts have almost the same β2-AR density as cognate wild-type controls, and can demonstrate contractile responses equivalent to controls — when contractility is augmented directly via adenylyl cyclase rather than via β-ARs (11). Even isoproterenol-induced adenylyl cyclase activity in cardiac homogenates is mediated overwhelmingly via the β1-AR, in a manner disproportionate to the relative densities of β1- and β2-ARs (11). The failure of cardiac β2-ARs to promote cAMP production to a degree commensurate with their expression level may be attributable to a signaling property that the cardiac β2- and β1-AR do not share: the ability to activate both the inhibitory heterotrimeric G protein (Gi) and Gs (12, 13). (A β1-AR subtype-specific intracellular binding protein appears to prevent β1-AR/Gi coupling [ref. 14].) In human subjects, β2-AR–selective agonists have been used to augment left ventricular inotropy (15). However, a large fraction of this inotropic effect is mediated by β1-ARs, and the role of β2-ARs in this process may be largely to augment norepinephrine release from cardiac sympathetic neurons (15).
Excessive β1-AR activation yields cardiomyocyte toxicity
Excessive isoproterenol stimulation has long been known to produce cardiomyocyte toxicity, myocardial scarring, and CHF (16, 17). More recently, chronic administration of submaximal isoproterenol doses has also been shown to produce cardiomyopathy, independent of myocardial scarring (18). That this isoproterenol-induced cardiomyopathy results primarily from β1-AR activation can be inferred from the β1-AR knockout mouse studies discussed above (11), as well as from a host of in vitro studies with rodent cardiomyocytes (13, 19). Further evidence that chronic β1-AR hyperstimulation causes cardiomyocyte toxicity has emerged from studies with transgenic mice displaying modest (∼15-fold), cardiac-specific overexpression of the β1-AR: these mice not only possessed enhanced β1-AR activity, but also developed cardiomyopathy by age 4–9 months (20). In contrast, transgenic mice overexpressing the β2-AR at higher absolute levels failed to develop any cardiomyopathy by this age (21).
The particular signaling pathways responsible for β1-AR–induced cardiomyocyte toxicity remain somewhat enigmatic. Increasing cardiomyocyte PKA activity by as little as 2.4-fold can engender CHF (22), and cardiomyocyte overexpression of Gαs engenders CHF (23). However, overexpression of adenylyl cyclase type VI (which also augments cardiomyocyte cAMP levels) not only avoids CHF but also can alleviate CHF in the Gαq-overexpressing mouse (24). In addition, β1-AR–promoted cardiomyocyte apoptosis can result from Ca2+/calmodulin-dependent protein kinase activity, independently from the PKA pathway (19). The diverse studies delineating molecular mechanisms responsible for β1-AR–promoted cardiomyocyte toxicity have been reviewed recently (8). In light of these data, it is intriguing that levels of plasma norepinephrine (which activates the β1-AR, like the anti–β1-AR IgG of Jahns et al. [ref. 5]) have been directly and independently associated with CHF mortality in human subjects (25).
Regulatory mechanisms for subduing the β1-AR signaling system
In the face of persistent β1-AR hyperstimulation (either in CHF or experimental systems), both receptor-based and non-receptor counter-regulatory mechanisms are engaged in the cardiomyocyte — perhaps to bridle cellular toxicity. These mechanisms result in approximately 50% downregulation of the β1-AR itself (26) (through mechanisms that appear to involve PI3K [ref. 27]), a decrease in adenylyl cyclase activity, and upregulation of the multifunctional Gi (which can inhibit adenylyl cyclase) (23). In addition, myocardium from CHF patients demonstrates a two- to threefold upregulation of G protein–coupled receptor kinase-2 (GRK2) (26), which phosphorylates and desensitizes the β1-AR (28, 29). This upregulation of GRK2 even precedes the onset of left ventricular failure in mice with transgenic myocardial overexpression of calsequestrin (30). Although these “desensitizing” mechanisms are insufficient to prevent CHF, some of them may, paradoxically, contribute to CHF pathophysiology. Perhaps the most thoroughly studied of these cases is the role of GRK2.
Relieving excessive β1-AR desensitization
Inhibition of cardiomyocyte GRK2 activity has been shown to ameliorate CHF in several mouse models, including deficiency of muscle LIM protein (31) and myocardial calsequestrin overexpression (32). GRK2 inhibition in these studies was achieved by transgenic myocardial overexpression of a polypeptide that comprises the carboxyl-terminal third of GRK2. This molecule, termed GRK2ct, inhibits GRK2 activity on receptors by binding to the heterotrimeric G protein βγ subunits required for GRK2 recruitment to the receptors (33) (Figure 1). Because “GRK2ct therapy” presumably does not alter the fundamental cardiomyocyte problems leading to myocardial dysfunction in these mouse models, its success points to the possibility that CHF-related enhancement of cardiomyocyte GRK2 activity may itself be maladaptive, and contribute to the pathogenesis of CHF. Remarkably, GRK2ct-expressing myocardium demonstrates attenuation of CHF-related GRK2 upregulation (31), β1-AR downregulation (31), and β1-AR/adenylyl cyclase desensitization (31, 32). In interpreting these data, however, it is important to note that GRK2ct binds a large variety of Gβγ subunits as well as phosphatidylinositol 3,5-bisphosphate (33). These binding activities could, beyond inhibiting GRK2, also contribute to the effects observed with GRK2ct (27, 34).
β-AR antagonists: possible mechanisms underlying therapeutic efficacy
Improvements in myocardial contractility, exercise tolerance, and mortality have been observed in CHF patients treated chronically with the β-AR antagonists metoprolol, bisoprolol, and carvedilol (35). This clinical improvement cannot depend upon reversal of β1-AR downregulation, since carvedilol does not promote such a reversal (35). Moreover, this clinical improvement seems unlikely to be mediated through β1-ARs at all, since it can be observed under conditions precluding agonist stimulation of β1-ARs (36). However, probably because it diminishes toxic hyperstimulation of the β1-AR by elevated sympathetic tone, prolonged β-AR antagonist therapy reduces cardiomyocyte apoptosis in CHF (37) and effects a partial recovery of CHF-associated derangements in gene expression (38, 39) and PKA-mediated hyperphosphorylation of proteins (40, 41), including those constituting the signaling and Ca2+-handling machinery downstream of the β1-AR. These “receptor-distal” mechanisms can enhance myocardial performance despite ongoing β-AR antagonist occupancy (36).
As a specific example of how mechanisms distal to the receptors can affect cardiomyocyte function, let us again consider the role of GRK2 in CHF. Bisoprolol (42) and carvedilol (43) have been used in animals to reduce GRK expression. (We should note that bisoprolol was used by Jahns et al. to abolish the adenylyl cyclase response to anti–β1-AR IgG [ref. 5].) Because GRK2 can desensitize a multitude of heptahelical receptors (33), reduced GRK2 levels could enhance signal transduction, and thus inotropy, evoked by heptahelical receptors other than the (antagonist-occupied) β1-AR, such as endothelin receptors (44). From the perspective of this hypothesis, it is intriguing that metoprolol and GRK2ct reduced calsequestrin-associated cardiomyopathy in a synergistic manner (32).
Perspectives
From decades of CHF investigations, we should indeed have expected that agonistic, anti–β1-AR antibodies would promote the development of heart failure. Now that Jahns et al. have provided an important proof of principle (5), we have another excellent reason to increase the clinical use of β-AR antagonist therapy in CHF of all causes. Whether immunoadsorption of anti–β1-AR antibodies (45) will provide CHF patients with benefits beyond those obtainable from β-AR antagonists alone, however, remains to be determined.
Acknowledgments
R.J. Lefkowitz is an Investigator of the Howard Hughes Medical Institute. This work was also supported in part by NIH grants HL-64744 (N.J. Freedman), HL-16037, and HL-70631 (R.J. Lefkowitz).
Footnotes
See the related article beginning on page 1419.
Nonstandard abbreviations used: β-adrenergic receptor (β-AR); β1-adrenergic receptor (β1-AR); chronic systolic heart failure (CHF); cyclic AMP–dependent protein kinase (PKA); G protein–coupled receptor kinase-2 (GRK2); GRK2 carboxyl-terminal polypeptide (GRK2ct); inhibitory heterotrimeric G protein (Gi); stimulatory heterotrimeric GTP-binding protein (Gs).
Conflict of interest: R.J. Lefkowitz is cofounder of Norak, a company developing an inhibitor to GRK2.
References
- 1.Sterin-Borda L, et al. Effect of Chagasic sera on the rat isolated atrial preparation: immunological, morphological and function aspects. Cardiovasc. Res. 1976;10:613–622. doi: 10.1093/cvr/10.6.613. [DOI] [PubMed] [Google Scholar]
- 2.Hagar JM, Rahimtoola SH. Chagas’ heart disease. Curr. Probl. Cardiol. 1995;20:825–924. [PubMed] [Google Scholar]
- 3.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ. Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Jahns R, et al. Autoantibodies activating human β1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–654. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 5.Jahns R, et al. Direct evidence for a β1-adrenergic receptor–directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Invest. 2004;113:1419–1429. doi:10.1172/JCI200420149. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 7.Hebert TE, et al. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 8.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 9.Mattera R, et al. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989;243:804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- 10.Lader AS, et al. Cardiac Gsα overexpression enhances L-type calcium channels through an adenylyl cyclase independent pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9669–9674. doi: 10.1073/pnas.95.16.9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrer DK, et al. Targeted disruption of the mouse β1-adrenergic receptor gene: developmental and cardiovascular effects. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao RP, et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Xiao RP, Balke CW. Na+/Ca2+ exchange linking β2-adrenergic Gi signaling to heart failure: associated defect of adrenergic contractile support. J. Mol. Cell. Cardiol. 2004;36:7–11. doi: 10.1016/j.yjmcc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Hu LA, et al. GIPC interacts with the β1-adrenergic receptor and regulates β1-adrenergic receptor-mediated ERK activation. J. Biol. Chem. 2003;278:26295–26301. doi: 10.1074/jbc.M212352200. [DOI] [PubMed] [Google Scholar]
- 15.Newton GE, Azevedo ER, Parker JD. Inotropic and sympathetic responses to the intracoronary infusion of a β2-receptor agonist: a human in vivo study. Circulation. 1999;99:2402–2407. doi: 10.1161/01.cir.99.18.2402. [DOI] [PubMed] [Google Scholar]
- 16.Rona G, Chappel G, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Arch. Pathol. 1959;67:443–455. [PubMed] [Google Scholar]
- 17.Beznak M, Hacker P. Hemodynamics during the chronic stage of myocardial damage caused by isoproterenol. Can. J. Physiol. Pharmacol. 1964;42:269–274. doi: 10.1139/y64-030. [DOI] [PubMed] [Google Scholar]
- 18.Woodiwiss AJ, et al. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation. 2001;103:155–160. doi: 10.1161/01.cir.103.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Zhu WZ, et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 2003;111:617–625. doi:10.1172/JCI200316326. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt S, Hein L, Wiesmann F, Lohse M. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liggett SB, et al. Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 22.Antos CL, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ. Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 23.Vatner SF, Vatner DE, Homcy CJ. β-adrenergic receptor signaling: an acute compensatory adjustment — inappropriate for the chronic stress of heart failure? Insights from Gsα overexpression and other genetically engineered animal models. Circ. Res. 2000;86:502–506. doi: 10.1161/01.res.86.5.502. [DOI] [PubMed] [Google Scholar]
- 24.Roth DM, et al. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 25.Rector TS, Cohn JN. Prognosis in congestive heart failure. Annu. Rev. Med. 1994;45:341–350. doi: 10.1146/annurev.med.45.1.341. [DOI] [PubMed] [Google Scholar]
- 26.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 27.Nienaber JJ, et al. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J. Clin. Invest. 2003;112:1067–1079. doi:10.1172/JCI200318213. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman NJ, et al. Phosphorylation and desensitization of the human β1-adrenergic receptor: involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J. Biol. Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 29.Rockman HA, et al. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 30.Cho MC, et al. Defective β-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. J. Biol. Chem. 1999;274:22251–22256. doi: 10.1074/jbc.274.32.22251. [DOI] [PubMed] [Google Scholar]
- 31.Rockman HA, et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac βARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 34.Peppel K, et al. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells attenuates mitogenic signaling via G protein-coupled and platelet-derived growth factor receptors. Circulation. 2000;102:793–799. doi: 10.1161/01.cir.102.7.793. [DOI] [PubMed] [Google Scholar]
- 35.Bristow MR. β-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 36.Metra M, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J. Am. Coll. Cardiol. 2002;40:1248–1258. doi: 10.1016/s0735-1097(02)02134-4. [DOI] [PubMed] [Google Scholar]
- 37.Sabbah HN, et al. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J. Am. Coll. Cardiol. 2000;36:1698–1705. doi: 10.1016/s0735-1097(00)00913-x. [DOI] [PubMed] [Google Scholar]
- 38.Gaussin V, et al. Common genomic response in different mouse models of β-adrenergic-induced cardiomyopathy. Circulation. 2003;108:2926–2933. doi: 10.1161/01.CIR.0000101922.18151.7B. [DOI] [PubMed] [Google Scholar]
- 39.Lowes BD, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N. Engl. J. Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 40.Reiken S, et al. β-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 41.Reiken S, et al. β-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 42.Ping P, et al. Reduced β-adrenergic receptor activation decreases G-protein expression and β-adrenergic receptor kinase activity in porcine heart. J. Clin. Invest. 1995;95:1271–1280. doi: 10.1172/JCI117777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 44.Beyer ME, Nerz S, Kazmaier S, Hoffmeister HM. Effect of endothelin-1 and its combination with adenosine on myocardial contractility and myocardial energy metabolism in vivo. J. Mol. Cell. Cardiol. 1995;27:1989–1997. doi: 10.1016/0022-2828(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 45.Mobini R, et al. Hemodynamic improvement and removal of autoantibodies against β1-adrenergic receptor by immunoadsorption therapy in dilated cardiomyopathy. J. Autoimmun. 2003;20:345–350. doi: 10.1016/s0896-8411(03)00042-8. [DOI] [PubMed] [Google Scholar]