Abstract
Fetal circulation has characteristic features, being morphologically and functionally different from extrauterine circulation. The ductus arteriosus plays a fundamental role in directing the blood flow to fetal inferior body parts. Basically, the ductus arteriosus directs 80–85% of the right ventricular output arising from the superior vena cava, coronary sinus, and a small part from the inferior vena cava to descending aorta. Its histological structure is made up predominantly by a thick muscular layer, differently from the aorta and the pulmonary artery, which increases with gestational age. The fibers have a circumferential orientation, especially at the external layers, facilitating and making effective ductal constriction. These factors may generate lumen alterations which may cause fetal and neonatal complications, such as heart failure, hydrops, neonatal pulmonary hypertension, and even death. Classically, maternal administration of indomethacin and/or other antiinflammatory drugs interfere in prostaglandins metabolism, causing ductal constriction. However, many cases of fetal ductal constriction, as well as of persistent neonatal pulmonary artery hypertension, remain without an established etiology, being referred as “idiopathic.” In recent years, a growing body of evidence has shown that herbs, fruits, nuts, and a wide diversity of substances commonly used in daily diets have definitive effects upon the metabolic pathway of inflammation, with consequent inhibition of prostaglandins synthesis. This antiinflammatory action, especially of polyphenols, when ingested during the third trimester of pregnancy, may influence the dynamics of fetal ductus arteriosus flow. The goal of this review is to present these new observations and findings, which may influence dietary orientation during pregnancy. Birth Defects Research (Part C) 99:256–274, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: ductal constriction, polyphenols, pulmonary hypertension, prostaglandins, antiinflammatory substances
INTRODUCTION
Fetal circulation has characteristic features, being morphologically and functionally different from extrauterine circulation. From an anatomical standpoint, the ductus arteriosus is part of the right ventricular outflow tract. It plays a fundamental role in directing the blood flow to fetal inferior body parts. Basically, the ductus arteriosus directs 80–85% of the right ventricular output arising from the superior vena cava, coronary sinus, and a small part from the inferior vena cava to descending aorta (Trevett and Cotton, 2004). This vessel originates from the distal part of the sixth left aortic arch which connects the left pulmonary artery to dorsal aorta (Bergwerff et al., 1999; Stoller et al., 2012). Its histological structure is made up predominantly by a thick muscular layer, differently from the aorta and the pulmonary artery, which increases with gestational age. The fibers have a circumferential orientation, especially at the external layers, facilitating and making effective ductal constriction (Ho and Anderson, 1979). As a result of these histological characteristics, it is subject to the influence of many factors which mediate its patency. These factors may generate lumen alterations which may cause fetal and neonatal complications, such as heart failure, hydrops, neonatal pulmonary hypertension, and even death (Tarcan et al., 2004; Abdel Mohsen and Amin, 2013; Babaoglu et al., 2013). Classically, maternal administration of indomethacin and/or other antiinflammatory drugs interfere in prostaglandins metabolism, causing ductal constriction (Takami et al., 2005; Koren et al., 2006; Toyoshima et al., 2006a, b). However, many cases of fetal ductal constriction, as well as of persistent neonatal pulmonary artery hypertension, remain without an established etiology, being referred as “idiopathic” (Shima et al., 2011).
In recent years, a growing body of evidence has shown that herbs, fruits, nuts, and a wide diversity of substances commonly used in daily diet have definitive effects upon the metabolic pathway of inflammation, with consequent inhibition of prostaglandins synthesis (Martinez and Moreno, 2000; Di Paola et al., 2005). This antiinflammatory action, especially of polyphenols, when ingested during the third trimester of pregnancy, may influence the dynamics of fetal ductus arteriosus flow (Sridharan et al., 2009; Kapadia et al., 2010; Zielinsky et al., 2010a, b; Zielinsky et al., 2012a, b, c; Zielinsky et al., 2013; Vian et al., in press). This review has the purpose to approach this new evidence, which may influence dietary orientation during pregnancy.
FETAL DUCTUS ARTERIOSUS AND DUCTAL CONSTRICTION
Characteristics of Fetal Ductus Arteriosus
The ductus arteriosus originates from the distal portion of the left sixth aortic arch, which connects the left pulmonary artery to dorsal aorta (Bergwerff et al., 1999). Its histological structure is formed by an internal elastic membrane, a tunica media muscular layer, and an external adventitial layer. The muscular layer is predominant, which makes the ductal structure different from aorta and pulmonary artery, and increases with gestational age. It has a circumferential orientation, mainly at the external layers, which facilitates and makes effective ductal constriction (Ho and Anderson, 1979).
The ductus arteriosus is positioned between the pulmonary artery, near the emergency of left pulmonary artery, and the aorta, at the zone of the isthmus (Fig. 1). At the aortic-pulmonary plan, around 80–85% of the poorly saturated blood (50% oxygen) ejected by the right ventricle (RV) into the main pulmonary artery passes through the ductus arteriosus to the descending aorta (around 75 ml/min), and as a result will mix with the blood coming from the left ventricle (LV). Due to the high pulmonary vascular resistance, only 15–20% (near 15 ml/min) is directed to the lungs. Approximately 40–50% of the descending aorta flow passes through the umbilical arteries and returns to the placenta for the hematosis. The remaining blood will nourish the organs and the inferior body half (Yajima et al., 2013).
Figure 1.
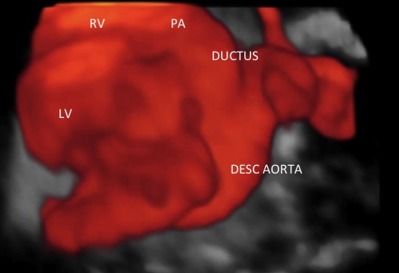
3D image of the normal ductus arteriosus obtained by spatio-temporal imaging correlation (STIC) and its anatomical relationships. LV: left ventricle; RV: right ventricle; PA: pulmonary artery; desc. aorta: descending aorta.
The ductus arteriosus shows a peculiar differentiation program to prepare itself for postnatal spontaneous closure (Bergwerff et al., 1999). There is a relationship between gestational age and the histological maturation of the ductus (Tada et al., 1985). The process of fetal intimal thickening starts at the second trimester of pregnancy and is characteristically a continuous process. This mechanism of intimal thickening seems to be linked to prostacyclin synthase (PGI2 synthase), which has a regulating role on ductal patency (Slomp et al., 1992). During ductus arteriosus closing, there are higher PGI2 synthase levels in smooth muscle cells at the sites of intimal thickening than in other places. These findings demonstrate the relationship between ductal morphology and the presence of PGI2 synthase (de Reeder et al., 1989; Majed and Khalil, 2012). Vascular remodeling also seems to be associated with dedifferentiation of the smooth muscle cells and to apoptosis present in the areas of tunica media and intimal layers (Slomp et al., 1997).
Hemodynamic alterations during the immediate neonatal period occur at the moment of cessation of placentary blood circulation, lung insufflation, pulmonary vasodilation, and foramen ovale closure. The sudden increase in systemic vascular resistance and the decrease in pulmonary vascular resistance generate a reverse flow through the ductus arteriosus and an abrupt increase in pulmonary flow. Some minutes after birth, 90% of the blood ejected by the RV is directed to the pulmonary arteries. With the decrease in pulmonary vascular resistance there is an increase in pulmonary blood flow, which culminates with ductal occlusion (Obladen, 2011; Hong et al., 2013). The functional closure of ductus arteriosus is initiated by a mechanism induced by the higher blood oxygen concentration (Coceani, 2013). This mechanism, albeit mediated by prostaglandins and endothelins, is intrinsic to smooth muscle cells (Michelakis et al., 2004). It is a potentially reversible phenomenon that occurs 8–72 hr after birth, secondary to muscular constriction. After this event, there is a remodeling of the vascular wall, with neointimal formation caused by proliferation and migration of smooth muscle cells from the tunica media to the subendothelial layers. This seems to be the final event of a process which initiates at the second trimester of pregnancy, starting with the accumulation of glycosaminoglycans at the subendothelial region (Slomp et al., 1992). Usually, the ductus arteriosus remains patent for some hours or days in the neonatal period.
Physiological closure of the ductus in the term neonate starts with a phase of functional obliteration secondary to the wall vessel muscular constriction. The closure is gradual and is completed more frequently in 10–15 hr after birth (Coceani and Baragatti, 2012). Observations in neonates show that the arterial duct start to close at the pulmonary arterial end, and then the constriction spreads to the aorta (Heymann and Rudolph, 1975). After completion of ductal occlusion, the arterial ligament is formed (Brezinka et al., 1993). If the ductus arteriosus remains patent in a term neonate, this is considered a pathological condition. The premature baby shows a delay in the remodeling process of the tunica media layer and is less responsive to oxygen, probably as a result of the immaturity of the structures (Tynan, 1993; Stoller et al., 2012).
Relaxing and Constrictive Factors on the Ductus Arteriosus
Since the ductus has a predominant muscular layer, its occlusion is influenced by a number of different constrictor and relaxing factors. Relaxing factors are prostaglandins, nitric oxide, and bradycinin, which cause liberation of prostaglandins and nitric oxide. Constrictive factors are oxygen, high doses of bradycinin and autonomic nervous system, both sympathetic and parasympathetic (Heymann and Rudolph, 1975). The vasoconstrictive effect is dose-dependent to several neurotransmitters, such as acetylcholine, histamine, serotonin, and catecholamine. With the increase in gestational age, the ductus becomes less sensitive to the dilating effects and more sensitive to constrictive factors (Heymann and Rudolph, 1975; Tarcan et al., 2004; Levin et al., 2005). Production of prostaglandins is dependent on two enzymes which act in different states, cyclo-oxygenase-1 (COX-1), expressed endogenously, and cyclo-oxygenase-2 (COX-2), locally induced during inflammatory processes (Takami et al., 2005; Majed and Khalil, 2012).
Prostaglandins have been extensively studied, with clear demonstration of its potent vasodilating action upon the ductus arteriosus. However, in recent years, new substances with constrictive and dilating effects on the ductus arteriosus have been described. The dilating action of 3- and 5-phosphodiesterase inhibitors upon fetal and neonatal ductus arteriosus in rodents has been reported (Momma et al., 2005; Ichikawa et al., 2012). This effect was shown to be more potent in fetuses than in neonates, suggesting that these substances could be useful in primary and secondary fetal ductal constriction, especially in preterm fetuses when compared to term fetuses (Toyoshima et al., 2006a, b). Other substances with dilating effects on the ductus were described, based on the knowledge of the role of endothelin receptors as messengers of postnatal ductal constriction (Coceani et al., 2000; Baragatti et al., 2011; Hong et al., 2013). It was shown that antagonists of endothelin receptors cause potent in vitro inhibition of the constrictive effect of cyclo-oxygenase inhibitors during fetal life, and of the postnatal physiological ductal constriction induced by oxygen (Takizawa et al., 2000; Momma et al., 2003).
Among the substances with known constrictive effect upon the ductus arteriosus, indomethacin, a cyclo-oxygenase inhibitor used in the treatment of premature labor, is one of the most extensively studied (Sharpe et al., 1975; Norton, 1997; Levy et al., 1999). Fetal ductal constriction may occur after few hours after maternal administration, and its action may last for several weeks. For this reason, the absence of signs of ductal constriction after 24–72 hr of usage does not exclude the diagnosis (Sharpe et al., 1975; Dudley and Hardie, 1985; Gordon and Samuels, 1995; Rasanen and Jouppila, 1995; Norton, 1997). Ductal sensitivity to indomethacin increases with gestational age, occurring in 5–10% of fetuses with less than 27 weeks, but reaching nearly 100% after 34 weeks, of gestation (Moise et al., 1988; Vermillion et al., 1997). In addition to indomethacin, it has been shown that several other nonsteroidal antiinflammatory drugs (NSAID), such as nimesulide (Paladini et al., 2005), diclofenac (Auer et al., 2004), aspirin, matamizole, ibuprofen (Schiessl et al., 2005), and many others also have the potential to cause constriction of ductus arteriosus. Selective COX-2 inhibitors, such as rofecoxib (Schiessl et al., 2005; Takami et al., 2005; Toyoshima et al., 2006a, b), have also shown a constrictive ductal effect on rat and sheep fetuses (Clyman et al., 1981a, b; Takahashi et al., 2000; Karadas et al., 2004). Sulindac, another prostaglandin inhibitor drug used in premature labor, was demonstrated to have a milder and more transient constrictive effect on fetal ductus than indomethacin (Rasanen and Jouppila, 1995). Glucocorticoids also show effects upon ductus arteriosus patency (Clyman et al., 1981a, b; Azancot-Benisty et al., 1995), but the pathophysiologic mechanism involved in the alteration of ductal tonus does not seem to be the same. There is apparent reduction in the ductal sensitivity to prostaglandin E2, which may be consequent to inhibition of the enzymatic liberation of arachidonic acid from phospholipids, a step in prostaglandin synthesis preceding cyclo-oxygenase (Hassid, 1982). Similar to other antiinflammatory drugs, the ductal effect of glucocorticoid is dose-dependent (Momma et al., 1981). Moreover, if associated to selective or nonselective NSAID, glucocorticoids have a synergistic action which increase frequency and severity of ductal constriction; its incidence may double, possibly due to glucocorticoid ability to decrease the ductal sensitivity to prostaglandin (Levy et al., 1999; Takami et al., 2005). Other experimentally tested substances with proven constrictive action on rat fetuses are retinoic acid (Moise et al., 1988; Levy et al., 1999; Wu et al., 2001; Takami et al., 2005), antagonists of prostanoid EP4 receptors (Momma et al., 2005; Fan et al., 2011; Chen et al., 2012a, b), and inhibitors of nitric oxide synthesis (L-name) (Takizawa et al., 2001; Keller et al., 2005; Reese et al., 2009; Hsu et al., 2010), the latter with proven effects in humans. Recently, a novel mechanism for sustaining postnatal ductal constriction induced by oxygen has been described, based on activation of the enzyme Rho-kinase (Kajimoto et al., 2007).
Role of Antiinflammatory Substances on the Genesis of Fetal Ductal Constriction
The action of NSAID results from inhibition of prostaglandin synthesis, by inactivation of cyclo-oxygenases. COX-1 and COX-2 are enzymes involved in prostaglandin, prostacyclin, and thromboxane biosynthesis (Majed and Khalil, 2012). This inhibitory mechanism interferes with the synthesis of prostaglandin G2, which is a precursor of prostaglandin E2 e F2 (Sharpe et al., 1975; Norton, 1997). The use of antiinflammatory drugs during pregnancy for the treatment of premature labor, pre-eclampsia or intrauterine growth restriction through prostaglandin biosynthesis inhibition has allowed the study of the relationship between ductal constriction and cyclo-oxygenase inhibitors. Indomethacin is the drug with prostaglandin inhibiting action most widely reported in the literature. Its inhibitory effect on cyclo-oxygenase is reversible, persisting until the drug is excreted (Gordon and Samuels, 1995; Vogel et al., 2010). The drug passage through the placentary barrier occurs freely during the second half of pregnancy, being minimal in early gestation (Moise et al., 1990). The response to indomethacin is individual in each fetus, and even in a twin pregnancy only one fetus could be affected, which suggests differences in ductus maturation (Hallak et al., 1991). The ductus arteriosus becomes more sensitive to indomethacin as gestational age increases, ductal constriction occurring in 5–10% in fetuses with less than 27 weeks, 15–20% in fetuses between 27 and 31 weeks, and 50% at week 32, and near 100% above 34 weeks. The occurrence of ductal constriction before week 27 is uncommon, but there are reports of cases with 22 weeks (Moise, 1993).
As already mentioned, many other nonsteroidal antiinflammatory compounds besides indomethacin are potentially involved in ductal constriction, such as nimesulide (Paladini et al., 2005), diclofenac (Auer et al., 2004), aspirin, metamizole, ibuprofen (Schiessl et al., 2005), and others (Koren et al., 2006). An experimental study in rats has suggested a gradation in the magnitude of the action of NSAID upon the fetal ductus, as the constrictive effect dose-dependent (Momma et al., 1984).
Glucocorticoids are synthetic hormones which mimic the endogenous action of cortisol, a hormone produced by the glomerular zone of the adrenal gland. Glucocorticoids also act on ductal patency. As occurs with the majority of other antiinflammatory substances, this effect is dose-dependent (Momma et al., 1981). There is enzymatic liberation of arachidonic acid, blocking prostaglandin synthesis (Hassid, 1982), and apparent reduction in sensitivity of the ductus to prostaglandin E2. Despite the tendency for premature closure of ductus arteriosus, the mechanism of action seems to be related to a primary alteration of the vessel, decreasing vascular reactivity to the relaxing effects of prostaglandin E2, without altering its synthesis (Clyman et al., 1981a, b; Cooper and Malik, 1984). The association of corticosteroids with indomethacin has shown a synergistic effect (Wasserstrum et al., 1989), and the incidence of ductal constriction doubles when these drugs are taken together, even though other studies have shown that the incidence of ductal constriction, with glucocorticoid in isolation similar to that of a control group (Levy et al., 1999) (Fig. 2).
Figure 2.
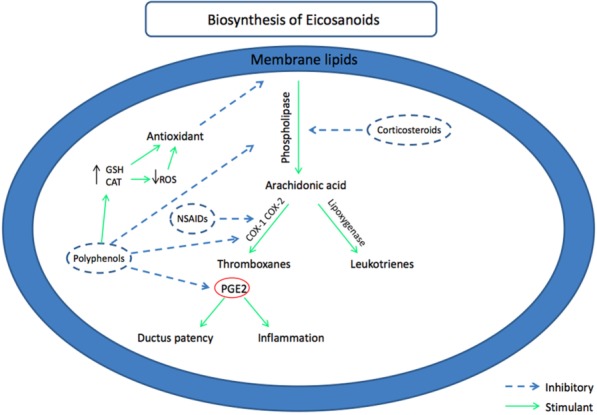
Biosynthesis of eicosanoids and their relationship to NSAID, polyphenols, and corticosteroids. COX-1: cyclo-oxygenase-1; COX-2: cyclo-oxygenase-2; PGE2: prostaglandin E-2; NSAID: nonsteroidal antiinflammatory drugs; ROS: reactive oxygen species; GSH: gluthatione; CAT: catalase.
Effects of Premature Ductus Arteriosus Constriction
Premature constriction of ductus arteriosus is followed by fetal hemodynamic repercussion. The higher resistance in the ductus generates blood flow turbulence, with an increase in systolic and diastolic velocities (DVs) and a decrease in ductal pulsatility index (PI). As a result, there is dilation of the pulmonary artery, right atrium, and RV, right to left bulging of the interventricular septum, tricuspid, and pulmonary insufficiency, and systolic and diastolic ventricular dysfunction (Mushiake et al., 2002; Paladini et al., 2005).
It has been demonstrated in a study in fetuses from 28 to 32 weeks of gestation after indomethacin administration that the drug shows a reversible constrictive effect on the ductus with significant reduction of PI and association with secondary disturbances, mainly in the RV, as a result of the increase in afterload, observed after about 4 hr, and normalization 24 hr after withdrawal of the substance. It was suggested that the hemodynamic alterations secondary to ductal constriction are right ventricular dilation and signs of heart failure, followed by concentric hypertrophy, a decrease in right ventricular chamber caused by mass increase and left ventricular compromise. These ventricular repercussions were more prominent in the presence of tricuspid regurgitation (Eronen et al., 1991; Eronen, 1993). Flow redirected through the foramen ovale results in left chamber volumetric overload (Harlass et al., 1989; Fyfe and Kline, 1990; Eronen, 1993). The aortic isthmus shows a rapid increase in its sectional area in response to local increased flow (Momma and Ando, 1994). This redistribution keeps peripheral perfusion and may explain the findings from clinical experience that show that severe ductal constriction is well tolerated for some days in the human fetus.
Past experimental studies used to speculate that constriction of ductus arteriosus resulted in an increase of the tunica media layer of pulmonary arteries, and generates a secondary increase in intrauterine vascular pulmonary resistance (Levin et al., 1978a, b). The hemodynamic alterations in ductal constriction may be related to pulmonary vascular alterations. In the vast majority of studies directed to increase the knowledge about fetal and neonatal pulmonary arterial hypertension, fetal ductal constriction is the experimental model of choice. In a classical study, administration of indomethacin to fetal lambs was followed by fetal ductal constriction and pulmonary hypertension (Levin et al., 1979). Blocking of prostaglandin biosynthesis probably has a direct effect in pulmonary arterioles in mammalian fetuses (Starling and Elliott, 1974; Cassin et al., 1979). The sustained increase in right ventricular afterload is capable of producing morphological, functional, and hemodynamic modifications, with chronic histological and degenerative alterations of the right ventricular myocardium (Harada et al., 1997a, b). Severe ductal constriction may interfere in placentary flow and myocardial performance, and may lead to fetal death. If ductal constriction is less severe or chronic, fetal pulmonary arterial hypertension may be a consequence of excessive development of the arteriolar smooth muscular layer and constriction of the pulmonary arterioles. Predominance of the increased thickness of the muscular layer and of the aerial pathway mass is a feature less described in neonatal pulmonary hypertension consequent to other causes (Murphy et al., 1981, 1984). In cases related to maternal drug usage, ventricular dysfunction may reverse after its suspension. However, if this picture is not treated, it may be followed by endocardial ischemia and right ventricular papillary muscles dysfunction, and later on by heart failure, hydrops, and potentially death (Moise, 1993). Intrauterine ductal constriction may cause transient or permanent tricuspid regurgitation and neonatal myocardial ischemia (Levin et al., 1976, 1978a, b).
In clinical practice, in cases with severe ductal constriction after prostaglandin inhibitory drugs, the suspension of its usage may result in a decrease of ductal velocities and an increase in PI within 24 hr, with posterior normalization of hemodynamic consequences (Vogel et al., 2010). Mild cases may be approached with just a decrease in the administered substance concentration, but in every fetus serial echocardiographic follow-up is recommended (Norton, 1997).
The evidence of fetal cardiac dysfunction was described as a characteristic feature of fetal closure of ductus arteriosus (Macones and Robinson, 1997). In severe cases, interruption of pregnancy may be indicated, with neonatal cardiopulmonary resuscitation. The clinical course after birth depends on the severity of intrauterine right ventricular cardiac insufficiency and to the response to elevation in pulmonary vascular resistance (Hofstadler et al., 1996).
Long-term prognosis is still uncertain, but when early evolution is favorable, there are usually no late complications. After the occurrence of fetal heart failure, right ventricular functional abnormalities may persist throughout the neonatal period, even in those patients with a benign outcome.
Diagnosis and Clinical Repercussion of Premature Ductus Arteriosus Constriction
Utilization of echocardiographic and Doppler techniques has allowed that the diagnosis of fetal ductal constriction, formerly possible only in necropsy, could be made in prenatal life (Harada et al., 1997a, b). Fetuses at risk for development of premature constriction of ductus arteriosus could be monitored and submitted to early intervention when necessary.
Echocardiographic diagnosis of fetal ductal constriction is based on the presence, at color Doppler, of turbulent flow in the ductus (Figs. 3 and 4), with increased systolic velocity (SV) (higher than 1.4 m/s), increased DV (higher than 0.30 m/s), and decreased PI (below 2.2). In the first publications, the cutoff point for the PI was described as 1.9 (Huhta et al., 1987), but more recent studies have considered a somewhat higher limit (Mielke and Benda, 2000; Zielinsky et al., 2012a, b, c). The PI is independent of the ultrasound angle and is useful in the differential diagnosis when there is increased ductal flow without concomitant constriction. This situation may occur when the increased SV is caused by an increase in right ventricular output. The PI does not change with gestational age and should be used to define the diagnosis (Tulzer et al., 1991). When there is total occlusion of the ductus arteriosus, absence of transductal flow is considered diagnostic (Fig. 5). With the increase in afterload secondary to ductal constriction, the fetal heart initially shows proliferative growing and, at later stages, hyperplasia is substituted to apoptosis and a hypertrophic response (van den Hoff et al., 2004). There is a characteristic increase in right to left diameters ratio, an increase in pulmonary artery to aorta ratio, and interventricular septum bulging toward the LV (Rasanen and Jouppila, 1995) (Fig. 6).
Figure 3.
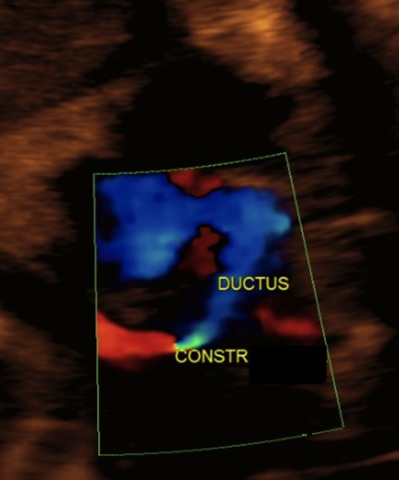
Ductal constriction in a 32 weeks fetus exposed to high concentration of polyphenols. Notice the severe narrowing of the ductus, with turbulent flow across the vessel at color flow mapping.
Figure 4.
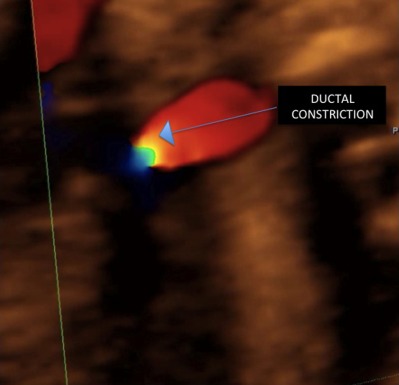
Ductal constriction (same case of Fig. 3). There is a pinpoint obstruction of the ductus, with important turbulent flow at color mapping (arrow).
Figure 5.
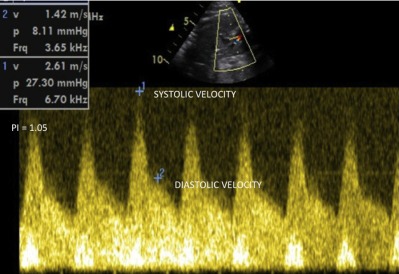
Pulsed Doppler tracing of severe ductal constriction (same case of Figs. 3 and 4). There is a high systolic ductal velocity (2.61 m/s), a very high DV (1.42 m/s), and a very low PI (PI = 1.05).
Figure 6.
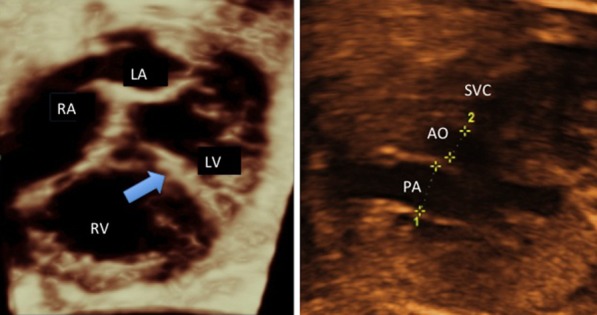
2D echocardiogram in ductal constriction (same case of Figs. 5). Left: four-chamber view showing an increase in right to left ventricular dimensions ratio end a leftward bulging of the interventricular septum (arrow). Right: three-vessel view, demonstrating a pulmonary to aorta diameters disproportion.
Right ventricular systolic and diastolic function is impaired in fetuses with ductal constriction, assessed by different methods (Harada et al., 1997a, b; Mori et al., 2001). The hemodynamic compromise is considered mild when there is mild or no tricuspid and/or pulmonary regurgitation, with normal chambers diameters; moderate in the presence of tricuspid regurgitation with right ventricular dilation without hypertrophy and/or impaired contractility (Fig. 7), and severe when the tricuspid and/or pulmonary insufficiency is important or there is functional pulmonary atresia, right ventricular dilation with ventricular parietal hypertrophy, and alteration in right ventricular contractile function. The compromise is also considered severe when there is total ductal occlusion or, alternatively, in the presence of a PI lower than 1.0, associated to any degree of hemodynamic repercussion (Harada et al., 1997a, b; Toyoshima et al., 2006a, b). Since the constrictive effect upon the ductus arteriosus is predominantly dose-dependent (Momma et al., 1984), it is usual the resolution of hemodynamic alterations after suspension of the causing substances without development of fetal or neonatal cardiac dysfunction (Rudolph, 1981; Moise et al., 1988; Mari et al., 1989; Rasanen and Jouppila, 1995; Respondek et al., 1995). Even in the presence of a severe ductal constriction after maternal utilization of drugs with prostaglandin inhibiting effect, withdrawal of their use may show reversal of the increased SV and DV within 24 hr, with improvement of the hemodynamic alterations (Rasanen and Jouppila, 1995). In some more severe cases, interruption of pregnancy may be necessary, sometimes with immediate cardiopulmonary neonatal resuscitation. Despite not having been established, the association between duration of fetal ductal constriction and the prevalence and severity of neonatal pulmonary hypertension (Tarcan et al., 2004) is obviously important to remove the cause as soon as possible, to allow early recovery.
Figure 7.
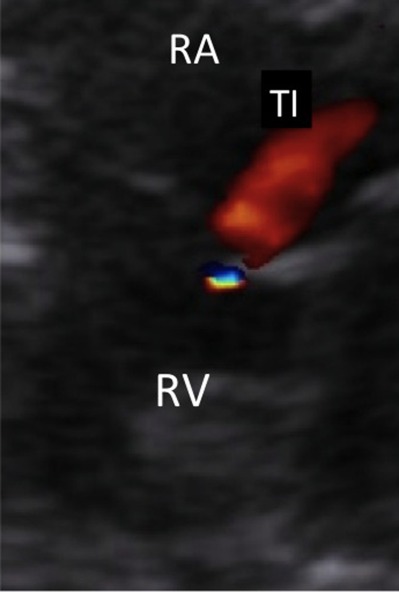
Doppler color flow mapping of a tricuspid regurgitant jet as a result of severe right ventricular hypertension secondary to ductal constriction (same case of Figs. 3–6). RV: right ventricle; RA: right atrium; TI: tricuspid insufficiency.
The decision to interrupt pregnancy should take into account fetal pulmonary maturity, the severity of clinical and echocardiographic manifestations of ductal constriction, and the presence or not of a progressive pattern. In the immediate neonatal period, the physiologic ductal closure associated to hemodynamic changes usual to this period allows normalization of the cardio-circulatory alterations secondary to the increased right ventricular overload. However, as already mentioned, the prolonged increase in right ventricular pressure, when transmitted to the lungs, may cause a reactive pulmonary arteriolar vasoconstriction with secondary pulmonary artery hypertension, which will need intensive treatment (Coceani, 1993). Since persistent pulmonary hypertension of the neonate without cardiac abnormalities occurs in approximately 1/1000 liveborns, and around 23% of the cases do not have a known definitive etiology, very probably many of these cases are secondary to undetected fetal premature constriction of ductus arteriosus (Van Marter et al., 1996). Thus, ductal constriction should always be considered an etiological possibility in “idiopathic” neonatal persistent pulmonary hypertension. This disorder carries a bad prognosis and is characterized by postnatal persistence of increased pulmonary vascular resistance, cyanosis due to right-to-left shunts through the foramen ovale and ductus, decreased pulmonary blood flow, and severe hypoxemia (Walsh-Sukys, 1993; Abman, 1999). Persistent pulmonary hypertension of the newborn has been associated to antenatal exposure to NSAID (Manchester et al., 1976; Csaba et al., 1978; Goudie and Dossetor, 1979; Rubaltelli et al., 1979; Wilkinson et al., 1979; Turner and Levin, 1984; Besinger et al., 1991; Norton et al., 1993; Alano et al., 2001; Tarcan et al., 2004; Hernández-Díaz, 2007), even though a recent case–control study could not confirm this risk (Van Marter et al., 2013). In fetal lambs, mechanical occlusion of the fetal ductus arteriosus reproduces the hemodynamic and structural features of persistent pulmonary hypertension of the newborn. Experimental prenatal exposition to NSAID has demonstrated alterations similar to those found in ductal constriction, with increased thickness of the smooth muscle layer of the pulmonary arterial vasculature (Sharpe et al., 1975; Heymann and Rudolph, 1976; Levin et al., 1978a, b; Lock et al., 1980).
BIOLOGICAL EFFECTS OF POLYPHENOL-RICH SUBSTANCES AND THEIR ROLE IN PROSTAGLANDIN INHIBITION
Polyphenols
Polyphenols are chemical structures present in all the superior vegetal organisms. More than 8000 structures are known, and they act on pigmentation, growing, reproduction, and resistance of plants against diseases (Velioglu et al., 1998). There are flavonoid and nonflavonoid polyphenols.
Flavonoids represent the major family and are the basic structures of tannins or proanthocyanidins. Tannins and its flavonoids are the best known polyphenols in alimentation, because of their presence in beer and wine, but a wide variety of polyphenols are present in a great number of foods and beverages. Many studies have investigated the cynetic and extension of absorption of polyphenols by mensuration of plasma concentration and urinary or plasmatic excretion (Bravo, 1998).
Flavonoids are the most abundant polyphenols in the human diet, and their consumption has triggered the interest of consumers and food industries for many reasons, but mainly because of their biological activity in systems relevant to human health (Scalbert and Williamson, 2000). This biological activity is related to antiinflammatory and antioxidant effects (Majewska et al., 2011), based on its interference in the inflammatory cascade, with inhibition of prostaglandin synthesis and mediation of nitric oxide synthetase (Fukumoto and Mazza, 2000; Nijveldt et al., 2001).
Polyphenols with greater importance and literature references are catechins mainly in green and black tea, resveratrol in wine and black grapes, chlorogenic acid in coffee and teas and flavonoids present in fruits and vegetables (USDA, 2007).
The principal alimentary sources with higher concentration in polyphenols are herbal teas, mate tea, dark chocolate, fruits, natural juices, vegetables, olive and soy oils, and red wine. Among fruits, the highest concentration of flavonoids is orange, red and purple grapes strawberry and other berries, and black prune and its derivatives. Vegetables with higher polyphenol purple onion concentration are purple onion, green spices, tomato, and derivatives. These foods show a concentration above 30 mg of flavonoids per 100 g of food, representing an amount above the 75th percentile of the USDA database (USDA, 2007).
Antiinflammatory and Antioxidant Actions of Polyphenols
In recent years, many investigational studies have been trying to ascertain the real therapeutic effect of substances found in nature and commonly used by the general population. Several of these substances have nowadays their antiinflammatory and antioxidant effects (Heijnen et al., 2001; Chun et al., 2003) scientifically and unequivocally demonstrated upon the chain of production of oxidative stress related to inflammatory mediators such as COX-2 and prostaglandin E2, metalloproteinases, and others. Substances rich in polyphenols are among the most widely used for a variety of reasons, even during pregnancy. The antiinflammatory and antioxidant effects of these substances are secondary to inhibition of the metabolic route of prostaglandin, especially of COX-2, preventing the transformation of arachidonic acid into prostaglandin (Takami et al., 2005; Koren et al., 2006; Toyoshima et al., 2006a, b). The literature reports on the mechanism of antioxidant and antiinflammatory action of polyphenols, which are beneficial to a large portion of the population, and the scientific evidence of their ethnomedicinal effect show that a large number of molecules derived from functional foods and plants have been isolated and even introduced successfully in the international pharmaceutical industry (Akkol et al., 2012). It has been demonstrated unambiguously that the polyphenols decrease oxidative stress (including in pregnancy) (Chen et al., 2012a, b), plasma triglycerides and cholesterol levels (Andujar et al., 2012), blood pressure (Mathew et al., 2012; Hodgson et al., 2013a, b), the consequences of gastric hypersecretion (D'Argenio et al., 2008), the development of some neoplasms (Malik et al., 2003; Hadi et al., 2007; Khan et al., 2012) and atherosclerosis (Romain et al., 2012; Widmer et al., 2013), the manifestations of aging (Andrade and Assuncao, 2012), and Alzheimer's disease (Valls-Pedret et al., 2012), and various other health problems. Polyphenols such as quercitin and kaempferol, among many others, are present in many foods and their antiinflammatory and antinociceptive activities have been shown to be as or more powerful than those of indomethacin (Kupeli et al., 2007a, b; Valls-Pedret et al., 2012).
Green tea, for example, is a compound of young leaves from the plant Camellia sinensis (Lorenzi H, Matos FJA, 2002). Approximately 30–40% of the leaves' solid extract is composed of polyphenols, mainly catechins. Among the most important catechins present in green tea are epicatechin, gallate-3-epicatechin, epigallocatechin, and, predominantly, gallate-3-epigallocatechin, with contains 7 g per 100 g of dry leaves. Several in vitro studies, both in animals and in humans, have demonstrated their antioxidant, anticarcinogenic, antiinflammatory, probiotic, and antimicrobial actions secondary to inhibition of endogenous inflammatory response, dependent on the interference on the prostaglandin synthesis pathway (Zhang et al., 2000; Smith and Dou, 2001; Chen et al., 2002; Lorenzi, 2002; Di Paola et al., 2005). Black tea has also been shown to be rich in catechins, and the tea compound involving theaflavin has been shown to act on nitric oxide and on the liberation of arachidonic acid. It has already been clearly demonstrated that tea drinkers could benefit from the protective cardiovascular effects exerted by this polyphenol-rich substance (Duffy et al., 2001a, b).
Resveratrol, a polyphenol compound found in grape rind, grape juice, and red wine, is known by its antioxidant, antithrombotic, antiinflammatory, and anticarcinogenic actions (Kris-Etherton et al., 2002). Several studies have demonstrated the effect of resveratrol upon the nervous system as well as on the liver and the cardiovascular system. One of the possible mechanisms that explain its biological activity is related to a decrease in liberation of arachidonic acid, thus affecting induction of COX-2, with a consequent reduction in prostaglandin synthesis (Subbaramaiah et al., 1998; Martinez and Moreno, 2000).
Mate tea, a typical regional beverage very rich in polyphenols, widely consumed in South America, mainly Paraguay, Brazil, Argentina, and Uruguay, is obtained from the dried and minced leaves of Ilex paraguariensis. Many studies have demonstrated its potent antineoplasic, antiinflammatory, and antioxidant effects, due to the action of its polyphenolic compounds (Bixby et al., 2005).
Orange juice has been shown to have important antioxidant activity as a result of a high content of flavonoids, especially quercitin, and the ability of the phytochemical substance to interact with biomembranes. It was speculated that the daily consumption of orange juice might be useful in providing additional protection against cellular oxidation in vivo (Ito et al., 2005).
Dark chocolate shows high concentration of flavonoids and has antiinflammatory properties. It has been demonstrated to have an inverse association with C-reactive protein, in amounts as low as 20 g every 3 days, suggesting that the regular intake of dark chocolate may reduce inflammatory processes (di Giuseppe et al., 2008). Since flavonoids modify the production of proinflammatory cytokines, the synthesis of eicosanoids, the activation of platelets, and nitric oxide-mediated mechanisms, a growing body of evidence is available to support a potent action of cocoa flavonoids in inflammation (Selmi et al., 2008).
Many other substances rich in polyphenols present in nature commonly used in daily routine by the general population have also shown definite antiinflammatory effects, secondary to inhibition of the prostaglandin synthesis pathway. Examples are boldine, with antiinflammatory and antithermic activities (Backhouse et al., 1994), propolis, with antiinflammatory action in asthmatic patients (Khayyal et al., 2003), passion fruit, with cytotoxic, antiinflammatory, and scar-promoting effects (Khayyal et al., 2003; Silva et al., 2006; Montanher et al., 2007), tomato and ginseng, also with antiinflammatory action on COX-2 (Jin et al., 2007) salvia, with antiinflammatory effects on acute and chronic processes (Bozin et al., 2007; Celik and Isik, 2008), chamomile, with moderate antioxidant and antimicrobial activity and significant in vitro antiplatelet actions (Della Loggia et al., 1986; Pozharitskaya et al., 2007a, b), and very many others, with variable concentrations of polyphenol substances presenting antiinflammatory and antioxidant effects, all of them by interfering with prostaglandin synthesis (Fig. 2).
Polyphenols and Pregnancy
A number of food and beverages such as herbal teas, grape and derivatives, orange, chocolate, fruits, and many others, with high concentrations of polyphenols, are freely consumed throughout gestation. Despite the positive effects of polyphenols on general health, as discussed in the previous sections, other studies from our group and others point toward the indication that maternal consumption of polyphenol-rich foods in late pregnancy, specifically in the third trimester, may be harmful to fetal health, as a result of the antiinflammatory and antioxidant effects of these substances on the ductus arteriosus, due to the inhibition of prostaglandin synthesis (Sridharan et al., 2009; Kapadia et al., 2010; Zielinsky et al., 2010a, b; Zielinsky et al., 2012a, b, c; Zielinsky et al., 2013; Vian et al., in press). These findings will be discussed on the next topics.
ALTERATIONS ON FETAL DUCTUS ARTERIOSUS FLOW FOLLOWING MATERNAL INTAKE OF POLYPHENOL-RICH SUBSTANCES
Experimental Studies
Experimental study of the role of maternal consumption of green tea, mate tea, and grape juice on fetal ductal constriction
To test the hypothesis that there is a cause-and-effect relationship between maternal exposure of polyphenol-rich substances in late pregnancy and fetal ductal constriction, we administered concentrated amounts of mate tea, green tea, and grape juice to 13 near-term (more than 120 days of pregnancy) ewes as the only source of liquid. Four received mate tea, four green tea, and five grape juice. In all fetuses receiving mate tea and green tea, echocardiographic evidence of ductal constriction was shown after 1 week, with significant increases in systolic and diastolic ductal velocities and decreases in PI. The heart specimens of all five fetal lambs exposed for 1 week to grape juice showed morphological and histological features of ductal constriction, with increased right ventricular diameter, right ventricular hypertrophy, and increased avascular zone thickness at the ductal wall. Four control fetal lambs fed a habitual diet did not show echocardiographic or histological alterations. It was concluded that mate tea, green tea, and grape juice, substances with high content in polyphenols, caused ductus arteriosus constriction in this experimental ovine model (Zielinsky et al., 2007). The pathophysiological explanation was that polyphenols present in the administered maternal diet acted in the metabolic cascade of the prostaglandin synthesis and caused ductal constriction and its consequences.
Fetal ductal constriction caused by maternal ingestion of green tea in late pregnancy: an experimental study
The aim of this study was to test the hypothesis that experimental maternal intake of green tea in late pregnancy causes fetal ductus arteriosus constriction, probably because of prostaglandin inhibition. Twelve fetal lambs (pregnancy > 120 days) were assessed before and after maternal administration of green tea (n = 8) or water (n = 4; controls) as the only source of liquid. After 1 week, echocardiography showed signs of constriction of the ductus arteriosus in all fetuses from mothers ingesting green tea, with an increase in mean SV (from 0.70 ± 0.19 m/s to 0.92 ± 0.15 m/s, 31.4%, p = 0.001) and mean DV (0.19 ± 0.05 m/s to 0.31 ± 0.01 m/s, 63.1%, p < 0.001), a decrease of PI (2.2 ± 0.4 to 1.8 ± 0.3, 22.2%, p = 0.003), and an increase of mean right ventricular/left ventricular diameter ratio (0.89 ± 0.14 to 1.43 ± 0.23, 60.6%, p < 0.001). In the four control fetuses, there were no significant changes. All lambs exposed to green tea also showed dilated and hypertrophic RVs at autopsy, which were not present in control fetuses. Histological analysis showed a significantly larger mean thickness of the medial avascular zone of the ductus arteriosus in fetuses exposed to green tea than in controls (747.6 ± 214.6 mm vs. 255.3 ± 97.9 mm, p < 0.001). This study in fetal lambs showed a cause-and-effect relationship between experimental maternal exposure of green tea and fetal ductus arteriosus constriction in late pregnancy, supporting the findings of the preliminary study (Zielinsky et al., 2012a, b, c).
Experimental assessment of ductus arteriosus flow, oxidative stress, and polyphenol excretion after maternal polyphenol-rich diet in late pregnancy
Since we had already established that maternal consumption of polyphenol-rich foods interferes with fetal ductus arteriosus flow in humans, probably mediated by prostaglandin E2 inhibition, and that restriction of polyphenols in maternal diet reverses ductal constriction, as discussed later in this manuscript, we aimed to assess experimentally the interrelations of ductal dynamics, oxidative damage, and polyphenol excretion after a controlled diet of polyphenol-rich foods in pregnant sheep. Six pregnant sheep with more than 120 days of gestational age were fed for 2 weeks with a standardized amount of polyphenol-rich foods (basal intake + 3100 mg/day). Fetal ductal systolic and diastolic flow velocities, and PI obtained by Doppler echocardiography, lipid peroxidation represented by plasma thiobarbituric acid reactive substances (TBARS), and urinary excretion of total polyphenols were assessed, before and 14 days after dietary intervention. Multiple comparison tests were used. A significant increase of ductal systolic and diastolic velocities (SV; DV) and a decrease in PI was shown after 14 days of intervention, when compared to the basal state (SV: 1.34 ± 0.01 vs. 0.75 ± 0.05 m/s, p < 0.001, DV: 0.28 ± 0.02 vs. 0.18 ± 0.01 m/s, p < 0.001, PI: 2.04 ± 0.11 vs. 2.54 ± 0.07, p < 0.001), indicating fetal ductal constriction. Urinary total polyphenol excretion increased significantly after intervention (687.47 ± 106.47 vs. 316.79 ± 30.31 mg gallic acid equivalent (GAE)/g creatinine, p < 0.001). A decrease in lipid peroxidation was shown, determined by TBARS levels (15.50 ± 0.20 mM), and by non-proteic reduced thiols after treatment. There was an increase in enzyme catalase and glutathione peroxidase after dietary intervention. Despite having no involvement on lipid damage in ductal constriction, carbanilide proteins caused an increase in proteic damage. The vasoconstrictor and antiinflammatory effects were demonstrated by a decrease in nitric oxide after polyphenol consumption. Oxidative stress was associated with echocardiographic parameters of ductal constriction, through proteic damage with SV (r = 0.629, p = 0.028), DV (r = 0.905, p = 0.0001), and PI (r= −0.772, p = 0.003). Moreover, ductal SV was correlated with catalase (r = 0.672, p = 0.033) and ductal flow PI, inversely with glutathione peroxidase (r = −0.629, p = 0.05). Ductal constriction was also negatively associated to inflammatory parameters, as systolic and DVs correlated to nitric oxide (r = −0.853, p = 0.0004 and r = −0.705, p = 0.010, respectively), and likewise ductal PI (r = 0.599, p = 0.039). In addition, both antiinflammatory and antioxidant mechanisms (nitric oxide with glutathione peroxidase, and nitric oxide with catalase) were correlated (r = −0.755, p = 0.004 and r = −0.812, p = 0.001, respectively), confirming that both effects could be attributed to polyphenols. In conclusion, elevated experimental maternal polyphenol consumption in ewes induced fetal ductal constriction with increased urinary excretion of total polyphenols and alterations in biomarkers of oxidative stress, characterizing the antioxidant, and antiinflammatory actions of polyphenols (Zielinsky et al., 2012a, b, c; Bubols et al., in press).
Clinical Studies
Development and validation of a food frequency questionnaire for consumption of polyphenol-rich foods in pregnant women
All clinical studies performed to evaluate the effects of maternal intake polyphenol-rich foods during the third trimester of pregnancy on fetal ductus arteriosus depend on the adequate assessment of its concentration, and there were no validated instruments to quantify total polyphenols in pregnant women. Thus, the aim of this study was to evaluate the reproducibility and validity of a food frequency questionnaire (FFQ) with 52 items, to assess the intake of polyphenol-rich foods in pregnant women in Brazil. This cross-sectional study included 120 pregnant women who participated in nutritional interviews in two moments. The intake of polyphenols estimated by the developed FFQ was compared with the average of two 24-hr recalls (24HR), with the average intake measured by a 3-day food diary (D3days) and with the urinary excretion of total polyphenols. The triangular method was applied to calculate Pearson's correlation coefficients, intraclass correlation and Bland–Altman plots for the FFQ, using an independent biochemical marker, in addition to classification by quarters of consumption. The questionnaires were log transformed, adjusted for body mass index and gestational age. The adjustment for energy was applied only at 24HR and D3days. Analysis of the reproducibility between the FFQ showed a very high correlation (r = 0.72; p < 0.05). A low but significant association was observed between the FFQ and urinary excretion (0.23; p = 0.01), as is usual in this kind of cooperation. The association between the dietary survey methods varied from moderate to very high (r = 0.36 to r = 0.72; p < 0.001). In conclusion, this questionnaire showed reproducibility and validity for the quantification of consumption of total polyphenols in pregnant women (Vian et al., in press).
Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics
We hypothesized that polyphenols or flavonoids present in food and beverages commonly consumed by pregnant women could influence ductal flow dynamics, probably by inhibition of prostaglandin synthesis, and thus be a risk factor for ductal constriction. With that in mind, we compared ductal flow behavior and right ventricular size in third-trimester fetuses exposed and not exposed to polyphenol-rich foods and beverages via maternal consumption, to test the hypothesis that maternal consumption of polyphenol-rich foods during the third trimester interferes with fetal ductal dynamics. In a prospective analysis, Doppler ductal velocities and right-to-left ventricular dimensions ratio of 102 normal fetuses exposed to polyphenol-rich foods (daily estimated maternal consumption above the 75th percentile, or 1089 mg, as previously determined) were compared with 41 normal unexposed fetuses (polyphenol ingestion below the 25th percentile, or 127 mg). In the exposed fetuses, ductal velocities were higher (systolic: 0.96 ± 0.23 m/s; diastolic: 0.17 ± 0.05 m/s) and right-to-left ventricular ratio was higher (1.23 ± 0.23) than in unexposed fetuses (systolic: 0.61 ± 0.18 m/s, p < 0.001; diastolic: 0.11 ± 0.04 m/s, p =0.011; right-to-left ventricular ratio: 0.94 ± 0.14, p < 0.001). We concluded that since it was shown that polyphenol-rich foods intake in late gestation may trigger alterations in fetal ductal dynamics, changes in perinatal dietary orientation should be recommended, with the purpose to decrease maternal polyphenol ingestion (Zielinsky et al., 2010a, b).
Reversal of fetal ductal constriction after maternal restriction of polyphenol-rich foods: an open clinical trial
Ductal constriction has long been related to inhibition of the prostaglandin synthesis pathway, mainly as a result of maternal intake of nonsteroidal antiinflammatory in the third trimester of pregnancy. Constriction of fetal ductus arteriosus is a risk factor for pulmonary hypertension in the newborn period, with its known severe consequences. For several years, many reports have been discussing the high prevalence of ductus constriction in the absence of a known trigger effect. It has been discussed that other extrinsic factors, in addition to NSAID, could be involved in the genesis of this important clinical situation. We and others have already suggested that maternal ingestion of polyphenol-rich foods in late pregnancy, such as herbal teas, grape juice, dark chocolate, and others, could be associated to fetal ductal constriction. Experimental studies in fetal lambs have supported this hypothesis, showing a cause and effect relationship of maternal consumption of green tea and other polyphenol-rich substances with constriction of fetal ductus arteriosus in the animal model. In the human setting, we have demonstrated that fetuses exposed to a maternal diet rich in polyphenols in the third trimester show higher ductal velocities and lower pulsatility indexes, as well as larger RVs, than those exposed to minimal amounts of these substances. The rationale for understanding the behavior of fetal ductal arteriosus flow dynamics after maternal ingestion of polyphenols in late pregnancy is that these substances have definite antiinflammatory and antioxidant effects, largely reported in the literature, based on the inhibition of COX-2 or other components of the metabolic cascade resulting in prostaglandins biosynthesis. These actions are similar to that involved in prostaglandin inhibition caused by NSAID. Thus, the purpose of this study was to test the hypothesis that fetuses with constriction of ductus arteriosus and no history of maternal ingestion of NSAID, but whose mothers have used PRF in the third trimester, show complete reversal of the ductal constrictive effect and its hemodynamics consequences after maternal dietary intervention aimed at restriction of these substances. A controlled clinical trial of 51 third trimester fetuses with ductal constriction with no history of NSAID intake was designed. All mothers were submitted to a FFQ and were oriented to PRF withdrawal, being reassessed after 3 weeks. Doppler parameters were assessed before and after discontinuation of these substances. A control group of 26 third trimester normal fetuses, with no ductus arteriosus constriction, in which no dietary intervention was offered, was reviewed after 3 weeks. Student's t test and Wilcoxon's test were used. Mean gestational age was 32 ± 3 weeks (28–37 weeks). After discontinuation of polyphenol-rich foods for three weeks or more, by means of a detailed nutritional intervention, with adequate substitution of essential nutrients, 48/51 fetuses (96%) showed complete reversal of ductal constriction, with decreases in mean ductal SV (1.74 ± 0.20 m/s to 1.31 ± 0.34 m/s, p < 0.001), mean DV (0.33 ± 0.09 m/s to 0.21 ± 0.07 m/s, p < 0.001), and mean right to left ventricular dimension ratio (1.37 ± 0.26 to 1.12 ± 0.17, p < 0.001), and an increase in mean ductal PI (1.98 ± 0.36 to 2.46 ± 0.23, p < 0.001). Median daily maternal consumption of polyphenol-rich foods was 286 mg per day and decreased after orientation to 0 mg per day, p < 0.001. In the control group, with gestational age of 32 ± 4 weeks (29–37 weeks), there were no significant differences in median daily maternal consumption of polyphenol-rich foods, mean ductal SV, DV, PI, and right ventricular to left ventricular diameter ratio. This behavior is similar to that already widely reported upon the withdrawal of NSAID in fetuses with constriction of ductus arteriosus caused by those pharmacological agents, when there is habitual regression of the disorder. The original conclusion of this controlled clinical trial was that reduction of maternal polyphenol intake during pregnancy, especially in the third trimester, is followed by complete reversal of ductal constriction, which may reduce the risk of neonatal hypertension and influence maternal dietary habits in late pregnancy (Zielinsky et al., 2012a, b, c).
Restriction of polyphenols and fetal ductal flow in normal pregnancies: an open clinical trial
Since we had have demonstrated reversal of fetal ductal constriction after dietary maternal restriction of polyphenol-rich foods, due to its inhibitory action on prostaglandin synthesis, we tested the hypothesis that normal third trimester fetuses also improve ductus arteriosus dynamics after maternal restriction of polyphenols. We designed a controlled clinical trial with 46 fetuses with gestational age equal to or above 28 weeks submitted to two Doppler echocardiographic studies with an interval of at least 2 weeks, as the examiners were blinded to maternal dietary habits. A validated FFQ was applied and a diet based on polyphenol-poor foods (less than 30 mg/100 mg) was recommended. A control group of 26 third trimester fetuses was submitted to the same protocol. Statistics used t test for independent samples. Mean gestational age was 33 ± 2 weeks. Mean daily maternal estimated polyphenol intake was 1277 mg, decreasing to 126 mg after dietary orientation (p = 0.0001). Significant decreases in systolic (SV) and diastolic (DV) ductal velocities, and RV/LV diameters ratio, as well as increase in ductal pulsatility index (PI) were observed [SV = 1.2 ± 0.4 m/s (0.7–1.6) to 0.9 ± 0.3 m/s (0.6–1.3) (p = 0.018); DV = 0.21 ± 0.09 m/s (0.15–0.32) to 0.18 ± 0.06 m/s (0.11–0.25) (p = 0.016); RV/LV ratio = 1.3 ± 0.2 (0.9–1.4) to 1.1 ± 0.2 (0.8–1.3) (p = 0.004); ductal PI = 2.2 ± 0.03 (2.0–2.7) to 2.4 ± 0.4 (2.2–2.9) (p = 0.04)]. In the control group, with gestational age of 32 ± 4 weeks, there were no significant differences in DMPI, mean SDV, DDV, PI, and RV/LV ratio.
This study demonstrated that, as already reported for fetuses with ductal constriction, dietary intervention for restricting for a period of 2 weeks or more the intake of foods rich in polyphenols by pregnant women in the third trimester improves ductus arteriosus flow dynamics and decreases the RV size in normal fetuses. Ductal constriction is a noncategorical, “yes or no” phenomenon, but rather a continuous spectrum with increasing severity related to the clinical manifestations of right ventricular overload, tricuspid and/or pulmonary regurgitation, and Doppler-echocardiographic findings of increased systolic and mainly diastolic ductal flow velocities as well as decreased ductal PI. Therefore, it seems logical to consider that the initial changes, even though still not filling in the classic criteria of constriction, can develop into more serious forms, exceeding the established diagnostic cutoff points. The sample assessed in this study comprised normal fetuses, with exclusion of those who already had a diagnosis of ductal constriction, to demonstrate how the nutritional guidance can decrease the potential risk for development of the disease. This new data can influence obstetric monitoring and guidance of the eating habits of pregnant women at late pregnancy (Zielinsky et al., 2013).
Case reports and other studies relating maternal polyphenol ingestion to ductal constriction
Premature constriction of the fetal ductus arteriosus following the maternal consumption of camomile herbal tea
The authors have observed two cases of premature ductal constriction associated with the maternal consumption of camomile tea, both of which without history of NSAID. In the first patient, withdrawal of the camomile from the diet resulted in complete reversal of the echocardiographic signs of ductal constriction, and in the second, diagnosed at 35 weeks with signs of severe ductal constriction, pregnancy was interrupted, with good neonatal outcome. The authors advocate caution with the use of camomile tea in late pregnancy, particularly if other prostaglandin antagonists are used concomitantly (Sridharan et al., 2009).
Prenatal closure of the ductus arteriosus and maternal ingestion of anthocyanins
The authors report a case of prenatal ductus arteriosus closure after maternal ingestion of MonaVie, a juice blend containing the cyclooxygenase and nitric oxide synthase inhibitors, anthocyanins and proanthocyanidins. A G2P0Ab1 woman had an uncomplicated first and second trimester and normal 20-week fetal ultrasound. At 37 weeks, she developed polyhydramnios; a fetal echocardiogram showed right atrial and ventricular enlargement with right ventricular dysfunction. Immediately after birth, there was pulmonary hypertension by echocardiogram with ductal complete closure, severe right ventricular hypertrophy and dysfunction, and marked right-to-left atrial shunting. Improvement occurred over 3 weeks with the neonate tolerating room air and a follow-up echocardiogram showing minimal atrial shunting and improved RV function. This report shows an association between MonaVie ingestion throughout pregnancy and prenatal ductal closure resulting in cardiac dysfunction and pulmonary hypertension at birth. The authors propose that placental transport of and recommend pregnant women not to ingest dietary supplements containing proanthocyanidins and anthocyanins until their metabolism and placental transport have been examined (Kapadia et al., 2010).
Functional foods for the fetus?
In this case report, the authors present a case of premature closure of the ductus arteriosus associated to maternal consumption of functional foods containing anthocyanins. A 36-year-old primigravid woman was referred to their hospital because of fetal pleural effusion at 38 weeks of gestation. Her prenatal course had been uneventful, and fetal ultrasound at 35 weeks of gestation showed no abnormalities. She had no significant medical history and declined medications, including NSAID. A detailed dietary history was taken, which revealed that she had ingested concentrated prune berry (40 g/day) and violet vegetable juice (200 ml/day) as functional foods for the last 10 days. Detailed ultrasound demonstrated a large-for-date fetus, with a moderate amount of ascites and pleural effusion. Fetal echocardiography revealed a dilated and hypertrophic RV with decreased movement. Color flow Doppler echocardiography depicted no flow signal documented into the dilated pulmonary trunk, indicating functional pulmonary atresia. As the ductus was not detected on ultrasound, the fetus was diagnosed to have heart failure due to premature ductal closure. A baby was delivered by emergency cesarean section because of the risk of progressive heart failure. The neonate was cyanotic and was cared for under mechanical ventilation in the intensive care unit. Echocardiography immediately after birth demonstrated low-grade right ventricular dysfunction with the ductal closure. The mother had started taking prune extracts and violet vegetable juice at 36 weeks of gestation for the first time and continued until delivery. Prune extracts are the dried fruits of certain cultivars of Prunus domestica, and have recently been promoted as antioxidants. They contain anthocyanins, and in vitro assays have shown that prunes have the highest antioxidative capacity among dried fruits. The violet vegetable juice the patient ingested is a blend of 18 vegetables and nine fruits that contains 20 mg/200 ml anthocyanins. Therefore, prune extracts and violet vegetable juice could trigger ductal closure, although placental transport of anthocyanins is not fully understood. The authors also conclude that special care must be taken when consuming functional foods in late pregnancy (Tanaka et al., 2011).
Idiopathic severe constriction of the fetal ductus arteriosus: a possible underestimated pathophysiology
A case of idiopathic fetal ductal constriction is reported, in which the diagnosis was confirmed by ultrasound at 38 weeks of gestation. The baby was born by Cesarean section, and her postnatal course was mild; transient tachypnea requiring only several days of supplemental oxygen with spontaneous regression of the abnormal echocardiographic findings by 3 months of age. The authors comment that idiopathic intrauterine constriction/closure of the ductus arteriosus, which is distinct from that secondary to maternal exposure to NSAID, such as indomethacin, or structural cardiac defect, is an uncommon event that often results in severe fetal–neonatal morbidity and mortality. They also state that fetal ductus arteriosus constriction may be underestimated, particularly with a negative history of maternal drug exposure and mild postnatal clinical presentation, and they discuss our belief that a maternal polyphenol-rich diet may influence fetal ductal flow dynamics and be a risk factor for constriction or even closure. They also comment on our findings that fetuses exposed to maternal polyphenol-rich diets have a narrowing tendency in the ductus arteriosus and larger dimensions on the right side of the heart, when compared to unexposed fetuses in a prospective analysis using fetal echocardiographic evaluation. Their conclusion is that pregnant women and their clinicians should be more alert of dietary orientation during pregnancy and take preventive actions, implying reduced maternal polyphenol intake (Shima et al., 2011).
Idiopathic constriction of the fetal ductus arteriosus: three cases and review of the literature
This series describes diagnosis of fetal ductus arteriosus constriction of “unknown” etiology in three cases, their prenatal management, and outcomes. They state that constriction of the ductus arteriosus can be diagnosed prenatally with careful interrogation of the ductal arch using pulsed Doppler sonography and complete fetal echocardiography. They discuss that premature closure of the ductus arteriosus can lead to progressive right heart dysfunction with tricuspid regurgitation, congestive heart failure, fetal hydrops, and intrauterine death, and that close monitoring is mandatory to rule out development of right heart failure and to determine the intervention time. The authors comment that ductus arteriosus constriction has been associated with maternal consumption of polyphenol-rich beverages, and that constriction caused by these substances is reversible, with its discontinuation resulting in echocardiographic improvement in most cases. They conclude that a history of taking NSAID or polyphenol-rich foods should be sought (Enzensberger et al., 2012).
Ongoing Studies and Next Steps
Association of neonatal pulmonary hypertension and maternal diet rich in polyphenols in late pregnancy
This project is designed as a case–control study, in which the study factor is the maternal intake of polyphenol-rich substances during the third trimester (consumption above the 75th percentile) and the outcome the presence of neonatal pulmonary hypertension. For each case, three paired controls will be studied, in which the maternal consumption of polyphenols is below the 25th percentile, to test the hypothesis that pulmonary hypertension is more frequent in the presence of a higher polyphenol consumption, as a result of fetal ductal constriction.
Role of oxidative stress, inflammation, prostaglandin levels, and maternal polyphenol consumption in regression of fetal ductal constriction: a controlled clinical trial
This ongoing study aims to establish the correlations between biomarkers of oxidative stress, inflammation cascade, prostaglandin levels, and maternal polyphenol consumption in the presence of fetal ductal constriction and at the end of at least 2 weeks of maternal dietary intervention, after the expected reversal of the disorder. A control group without signs of fetal ductal constriction follows the same protocol, but without the dietary intervention.
A double-blind randomized clinical trial placebo-controlled of polyphenol supplementation in non-pregnant women at childbearing age
The purpose of this study is to test the hypothesis that polyphenol supplementation to non-pregnant women at childbearing age interfere with prostaglandin synthesis, decreasing its levels, and at the same time decreasing markers of inflammation and oxidative stress.
Experimental studies on the mechanism of fetal construction
This ongoing study in rodents aims to explore the molecular basis of fetal ductal constriction after administration of polyphenol-rich substances. The hypothesis to be tested, using Western Blot, is that acute administration of polyphenols inhibits the expression of COX-2 on the fetal vasculature, resulting in ductal constriction.
A multicenter international registry of reversal of fetal ductus arteriosus constriction after maternal nutritional intervention to decrease consumption of polyphenol-rich foods in late pregnancy
To follow up with our published study from a single institution, this registry would increase the strength of evidence studying a large number of patients around the world using the same methodology, preceded by local validation of the FFQ in each center, to take into account variations in polyphenol concentrations in different soils, fruits, and plants.
FINAL CONSIDERATIONS
When the level of evidence regarding the recommendation of avoidance of polyphenol-rich substances by pregnant women, at the third trimester, is critically analyzed, an important question naturally arises: why not to have performed or to perform a randomized clinical trial to obtain the strongest possible evidence of this action, at the apex of the pyramid? Such a study, in simple terms, would try to resolve the research problem of the value of nutritional intervention (restriction of polyphenols in maternal diet) against no intervention, in the presence of fetal ductal constriction without history of prenatal exposure to NSAID. In this hypothetical trial, the study factor would be the restriction of maternal intake of polyphenol-rich foods in fetuses with ductal constriction in late pregnancy and the outcome the improvement of fetal echocardiographic signs of ductal constriction, a decrease in systolic and diastolic ductal velocities, an increase in PI, a decrease in right to left ventricular diameters ratio, and pulmonary artery to aorta dimensions ratio, of flow turbulence, sepal bulging, and tricuspid regurgitation. Would there be equipoise in a randomized clinical trial with the proposed intervention and outcomes (Freedman, 1987; Hellman and Hellman, 1991; Joffe and Miller, 2012; Petticrew et al., 2013; Sheehan et al., in press)? In other words, would it be ethical to perform a randomized clinical trial to assess the benefit of polyphenol restriction in maternal diet at the third trimester in the presence of ductal constriction? The answer to that question, considering the conceptual model to obtain a state of equipoise, is “no”! Such a state of equipoise needs the triangular interrelationship of the three points: (1) definite benefit of the study to society; (2) doubt of the investigator about the intervention effectiveness; (3) safety of all the subjects in the two arms of the clinical trial (Freedman, 1987; Gifford, 2007; van der Graaf and van Delden, 2011, 2012; Adibe and St. Peter, 2012). Even though there is a clear benefit to society in defining if maternal nutritional intervention, decreasing maternal intake of polyphenols in late pregnancy, improves fetal ductal constriction, the two remaining points of the triangle of the conceptual model of equipoise are not fulfilled. The uncertainty about the effectiveness of maternal restriction of polyphenols is no longer present, based on the previous published studies herein disclosed and, most importantly, safety in the two arms of a randomized clinical trial, in which one of them would not receive a clearly effective intervention, cannot be established. The deleterious effects of ductal constriction upon fetal hemodynamics and the risk of pulmonary arterial hypertension secondary to this functional disorder are well known. There are no reports in the literature of spontaneous reversal of ductal constriction, without removal of the causal factor. How to submit the control group to the risk of keeping the ductus constricted, with all its potential complications? In summary, a randomized clinical trial to assess the effect of maternal dietary intervention in fetuses with ductal constriction, with the level of evidence accumulated today, do not fulfill the equipoise principles, and for this reason cannot be considered ethical.
The evidence already available recommends (Class II, level A) a note of caution with regard to the consumption by women in the third trimester of pregnancy of foods with high concentrations of polyphenols, to avoid triggering constriction of ductus arteriosus, with its potential harmful consequences, such as fetal and neonatal heart failure and pulmonary arterial hypertension of the newborn.
Acknowledgments
The authors acknowledge the participation, in many parts of the studies here described, of Alexandre Bestetti, Alexandre Naujorks, Ana Zilio, André Busato, Andressa Oliveira, Angela Arnt, Antonio Piccoli, Carolina Barbisan, Caroline Klein, Felipe Martignon, Fernanda Lin, Fernanda Swarowski, Guilherme Bubols, Honório Menezes, Izabele Vian, João Luiz Manica, Julia Silva, Kenya Lampert, Letícia Almeida, Lucas Aita, Luciano Bender, Luiz Henrique Nicoloso, Marcelo Alievi, Marcelo Pizzato, Marina Moraes, Marinez Barra, Mauro Lopes, Patrícia Pizzato, Renato Frajndlich, and Solange Garcia.
REFERENCES
- Abdel Mohsen AH, Amin AS. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of Al-minya university hospital in egypt. J Clin Neonatol. 2013;2:78–82. doi: 10.4103/2249-4847.116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abman SH. Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn. Pediatr Rev. 1999;20:e103–109. [PubMed] [Google Scholar]
- Adibe OO, St. Peter SD. Equipoise, ethics, and the necessity of randomized trials in surgery. Arch Surg. 2012;147:899–900. doi: 10.1001/archsurg.2012.1796. [DOI] [PubMed] [Google Scholar]
- Akkol EK, Das S, Sarker SD, Nahar L. The treatment of inflammation, pain, and Fever using medicinal plants. Adv Pharmacol Sci. 2012;2012:476985. doi: 10.1155/2012/476985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano MA, Ngougmna E, Ostrea EM, Jr, Konduri GG. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001;107:519–523. doi: 10.1542/peds.107.3.519. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Assuncao M. Protective effects of chronic green tea consumption on age-related neurodegeneration. Curr Pharm Des. 2012;18:4–14. doi: 10.2174/138161212798918986. [DOI] [PubMed] [Google Scholar]
- Andujar I, Recio MC, Giner RM, Rios JL. Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev. 2012;2012:906252. doi: 10.1155/2012/906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer M, Brezinka C, Eller P, et al. Prenatal diagnosis of intrauterine premature closure of the ductus arteriosus following maternal diclofenac application. Ultrasound Obstet Gynecol. 2004;23:513–516. doi: 10.1002/uog.1038. [DOI] [PubMed] [Google Scholar]
- Azancot-Benisty A, Benifla JL, Matias A, et al. Constriction of the fetal ductus arteriosus during prenatal betamethasone therapy. Obstet Gynecol. 1995;85(5 Pt 2):874–876. doi: 10.1016/0029-7844(94)00445-j. [DOI] [PubMed] [Google Scholar]
- Babaoglu K, Cakiroglu Y, Altun G, et al. Intrauterine idiopathic severe ductal constriction diagnosed by fetal echocardiography: a cause of hydrops fetalis. Anadolu Kardiyol Derg. 2013;13:496–497. doi: 10.5152/akd.2013.150. [DOI] [PubMed] [Google Scholar]
- Backhouse N, Delporte C, Givernau M, et al. Anti-inflammatory and antipyretic effects of boldine. Agents Actions. 1994;42:114–117. doi: 10.1007/BF01983475. [DOI] [PubMed] [Google Scholar]
- Baragatti B, Ciofini E, Scebba F, et al. Cytochrome P-450 3A13 and endothelin jointly mediate ductus arteriosus constriction to oxygen in mice. Am J Physiol Heart Circ Physiol. 2011;300:H892–901. doi: 10.1152/ajpheart.00907.2010. [DOI] [PubMed] [Google Scholar]
- Bergwerff M, DeRuiter MC, Gittenberger-de Groot AC. Comparative anatomy and ontogeny of the ductus arteriosus, a vascular outsider. Anat Embryol (Berl) 1999;200:559–571. doi: 10.1007/s004290050304. [DOI] [PubMed] [Google Scholar]
- Besinger RE, Niebyl JR, Keyes WG, Johnson TR. Randomized comparative trial of indomethacin and ritodrine for the long-term treatment of preterm labor. Am J Obstet Gynecol. 1991;164:981–986; discussion 986–988. doi: 10.1016/0002-9378(91)90569-d. [DOI] [PubMed] [Google Scholar]
- Bixby M, Spieler L, Menini T, Gugliucci A. Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: a comparative study with green tea and wines using a protein nitration model and mammalian cell cytotoxicity. Life Sci. 2005;77:345–358. doi: 10.1016/j.lfs.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Brezinka C, Gittenberger-de Groot AC, Wladimiroff JW. The fetal ductus arteriosus, a review. Zentralbl Gynakol. 1993;115:423–432. [PubMed] [Google Scholar]
- Bubols G, Zielinsky P, Piccoli A, Jr, et al. Inflammation and oxidative stress are involved in the polyphenol-induced ductus arteriosus constriction in pregnant sheep (in press) [DOI] [PubMed]
- Cassin S, Tyler T, Leffler C, Wallis R. Pulmonary and systemic vascular responses of perinatal goats to prostaglandins E1 and E2. Am J Physiol. 1979;236:H828–832. doi: 10.1152/ajpheart.1979.236.6.H828. [DOI] [PubMed] [Google Scholar]
- Celik I, Isik I. Determination of chemopreventive role of Foeniculum vulgare and Salvia officinalis infusion on trichloroacetic acid-induced increased serum marker enzymes lipid peroxidation and antioxidative defense systems in rats. Nat Prod Res. 2008;22:66–75. doi: 10.1080/14786410701590426. [DOI] [PubMed] [Google Scholar]
- Chen B, Tuuli MG, Longtine MS, et al. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab. 2012a;302:E1142–1152. doi: 10.1152/ajpendo.00003.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, O'Mara PW, Poole SD, et al. Isoprostanes as physiological mediators of transition to newborn life: novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr Res. 2012b;72:122–128. doi: 10.1038/pr.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Wheeler DS, Malhotra V, et al. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26:233–241. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun OK, Kim DO, Lee CY. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem. 2003;51:8067–8072. doi: 10.1021/jf034740d. [DOI] [PubMed] [Google Scholar]
- Clyman RI, Ballard PL, Sniderman S, et al. Prenatal administration of betamethasone for prevention of patient ductus arteriosus. J Pediatr. 1981a;98:123–126. doi: 10.1016/s0022-3476(81)80557-4. [DOI] [PubMed] [Google Scholar]
- Clyman RI, Mauray F, Roman C, et al. Glucocorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. J Pediatr. 1981b;98:126–128. doi: 10.1016/s0022-3476(81)80558-6. [DOI] [PubMed] [Google Scholar]
- Coceani F. Control mechanisms for the ductus arteriosus and the perinatal pulmonary circulation. J Lipid Mediat. 1993;6:473–476. [PubMed] [Google Scholar]
- Coceani F. Oxygen sensing in the ductus arteriosus: endothelin still a player. Circ Res. 2013;112:e154. doi: 10.1161/CIRCRESAHA.113.301615. [DOI] [PubMed] [Google Scholar]
- Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36:92–97. doi: 10.1053/j.semperi.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Coceani F, Liu YA, Seidlitz E, et al. Deletion of the endothelin-A-receptor suppresses oxygen-induced constriction but not postnatal closure of the ductus arteriosus. J Cardiovasc Pharmacol. 2000;36(5 Suppl 1):S75–77. doi: 10.1097/00005344-200036051-00025. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Malik KU. Effect of glucocorticoids on vascular reactivity to vasoactive hormones in rat isolated kidney: lack of relationship to prostaglandins. Br J Pharmacol. 1984;82:679–688. doi: 10.1111/j.1476-5381.1984.tb10807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaba IF, Sulyok E, Ertl T. Relationship of maternal treatment with indomethacin to persistence of fetal circulation syndrome. J Pediatr. 1978;92:484. doi: 10.1016/s0022-3476(78)80454-5. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Mazzone G, Tuccillo C. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr. 2008;100:1228–1236. doi: 10.1017/S0007114508988747. [DOI] [PubMed] [Google Scholar]
- de Reeder EG, Gittenberger-de Groot AC, van Munsteren JC. Distribution of prostacyclin synthase, 6-keto-prostaglandin F1 alpha, and 15-hydroxy-prostaglandin dehydrogenase in the normal and persistent ductus arteriosus of the dog. Am J Pathol. 1989;135:881–887. [PMC free article] [PubMed] [Google Scholar]
- Della Loggia R, Tubaro A, Dri P. The role of flavonoids in the antiinflammatory activity of Chamomilla recutita. Prog Clin Biol Res. 1986;213:481–484. [PubMed] [Google Scholar]
- di Giuseppe R, Di Castelnuovo A, Centritto F, et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 2008;138:1939–1945. doi: 10.1093/jn/138.10.1939. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Mazzon E, Muia C, et al. Green tea polyphenol extract attenuates lung injury in experimental model of carrageenan-induced pleurisy in mice. Respir Res. 2005;6:66. doi: 10.1186/1465-9921-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DK, Hardie MJ. Fetal and neonatal effects of indomethacin used as a tocolytic agent. Am J Obstet Gynecol. 1985;151:181–184. doi: 10.1016/0002-9378(85)90008-0. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Keaney JF, Jr, Holbrook M, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001a;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Vita JA, Holbrook M, et al. Effect of acute and chronic tea consumption on platelet aggregation in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001b;21:1084–1089. doi: 10.1161/01.atv.21.6.1084. [DOI] [PubMed] [Google Scholar]
- Enzensberger C, Wienhard J, Weichert J, et al. Idiopathic constriction of the fetal ductus arteriosus: three cases and review of the literature. J Ultrasound Med. 2012;31:1285–1291. doi: 10.7863/jum.2012.31.8.1285. [DOI] [PubMed] [Google Scholar]
- Eronen M. The hemodynamic effects of antenatal indomethacin and a beta-sympathomimetic agent on the fetus and the newborn: a randomized study. Pediatr Res. 1993;33:615–619. doi: 10.1203/00006450-199306000-00017. [DOI] [PubMed] [Google Scholar]
- Eronen M, Pesonen E, Kurki T, et al. The effects of indomethacin and a beta-sympathomimetic agent on the fetal ductus arteriosus during treatment of premature labor: a randomized double-blind study. Am J Obstet Gynecol. 1991;164(1 Pt 1):141–146. doi: 10.1016/0002-9378(91)90644-7. [DOI] [PubMed] [Google Scholar]
- Fan F, Ma A, Guan Y, et al. Effect of PGE2 on DA tone by EP4 modulating Kv channels with different oxygen tension between preterm and term. Int J Cardiol. 2011;147:58–65. doi: 10.1016/j.ijcard.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Fyfe DA, Kline CH. Fetal echocardiographic diagnosis of congenital heart disease. Pediatr Clin North Am. 1990;37:45–67. doi: 10.1016/s0031-3955(16)36831-6. [DOI] [PubMed] [Google Scholar]
- Gifford F. Pulling the plug on clinical equipoise: a critique of Miller and Weijer. Kennedy Inst Ethics J. 2007;17:203–226; discussion 227–246. doi: 10.1353/ken.2007.0020. [DOI] [PubMed] [Google Scholar]
- Gordon MC, Samuels P. Indomethacin. Clin Obstet Gynecol. 1995;38:697–705. doi: 10.1097/00003081-199538040-00004. [DOI] [PubMed] [Google Scholar]
- Goudie BM, Dossetor JF. Effect on the fetus of indomethacin given to suppress labour. Lancet. 1979;2:1187–1188. doi: 10.1016/s0140-6736(79)92411-5. [DOI] [PubMed] [Google Scholar]
- Hadi SM, Bhat SH, Azmi AS, et al. Oxidative breakage of cellular DNA by plant polyphenols: a putative mechanism for anticancer properties. Semin Cancer Biol. 2007;17:370–376. doi: 10.1016/j.semcancer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Hallak M, Reiter AA, Ayres NA, Moise KJ., Jr Indomethacin for preterm labor: fetal toxicity in a dizygotic twin gestation. Obstet Gynecol. 1991;78(5 Pt 2):911–913. [PubMed] [Google Scholar]
- Harada K, Rice MJ, McDonald RW, et al. Doppler echocardiographic evaluation of ventricular diastolic filling in fetuses with ductal constriction. Am J Cardiol. 1997a;79:442–446. doi: 10.1016/s0002-9149(96)00783-7. [DOI] [PubMed] [Google Scholar]
- Harada K, Rice MJ, Shiota T, et al. Two-dimensional echocardiographic evaluation of ventricular systolic function in human fetuses with ductal constriction. Ultrasound Obstet Gynecol. 1997b;10:247–253. doi: 10.1046/j.1469-0705.1997.10040247.x. [DOI] [PubMed] [Google Scholar]
- Harlass FE, Duff P, Brady K, Read J. Hydrops fetalis and premature closure of the ductus arteriosus: a review. Obstet Gynecol Surv. 1989;44:541–543. doi: 10.1097/00006254-198907000-00009. [DOI] [PubMed] [Google Scholar]
- Hassid A. Regulation of prostaglandin biosynthesis in cultured cells. Am J Physiol. 1982;243:C205–211. doi: 10.1152/ajpcell.1982.243.5.C205. [DOI] [PubMed] [Google Scholar]
- Heijnen CG, Haenen GR, Vekemans JA, Bast A. Peroxynitrite scavenging of flavonoids: structure activity relationship. Environ Toxicol Pharmacol. 2001;10:199–206. doi: 10.1016/s1382-6689(01)00083-7. [DOI] [PubMed] [Google Scholar]
- Hellman S, Hellman DS. Of mice but not men. Problems of the randomized clinical trial. N Engl J Med. 1991;324:1585–1589. doi: 10.1056/NEJM199105303242208. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Van Marter L, Werler MM, et al. Risk factors for persistent pulmonary hypertension of the newborn. Pedatrics. 2007;120:e272–e282. doi: 10.1542/peds.2006-3037. [DOI] [PubMed] [Google Scholar]
- Heymann MA, Rudolph AM. Control of the ductus arteriosus. Physiol Rev. 1975;55:62–78. doi: 10.1152/physrev.1975.55.1.62. [DOI] [PubMed] [Google Scholar]
- Heymann MA, Rudolph AM. Effects of acetylsalicylic acid on the ductus arteriosus and circulation in fetal lambs in utero. Circ Res. 1976;38:418–422. doi: 10.1161/01.res.38.5.418. [DOI] [PubMed] [Google Scholar]
- Ho SY, Anderson RH. Anatomical closure of the ductus arteriosus: a study in 35 specimens. J Anat. 1979;128(Pt 4):829–836. [PMC free article] [PubMed] [Google Scholar]
- Hodgson JM, Croft KD, Woodman RJ, et al. Black tea lowers the rate of blood pressure variation: a randomized controlled trial. Am J Clin Nutr. 2013a;97:943–950. doi: 10.3945/ajcn.112.051375. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Woodman RJ, Puddey IB, et al. Short-term effects of polyphenol-rich black tea on blood pressure in men and women. Food Funct. 2013b;4:111–115. doi: 10.1039/c2fo30186e. [DOI] [PubMed] [Google Scholar]
- Hofstadler G, Tulzer G, Altmann R, et al. Spontaneous closure of the human fetal ductus arteriosus—a cause of fetal congestive heart failure. Am J Obstet Gynecol. 1996;174:879–883. doi: 10.1016/s0002-9378(96)70317-4. [DOI] [PubMed] [Google Scholar]
- Hong Z, Kutty S, Toth PT, et al. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res. 2013;112:802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JH, Oishi P, Wiseman DA, et al. Nitric oxide alterations following acute ductal constriction in the fetal lamb: a role for superoxide. Am J Physiol Lung Cell Mol Physiol. 2010;298:L880–887. doi: 10.1152/ajplung.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhta JC, Moise KJ, Fisher DJ, et al. Detection and quantitation of constriction of the fetal ductus arteriosus by Doppler echocardiography. Circulation. 1987;75:406–412. doi: 10.1161/01.cir.75.2.406. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Yokoyama U, Iwamoto M, et al. Inhibition of phosphodiesterase type 3 dilates the rat ductus arteriosus without inducing intimal thickening. Circ J. 2012;76:2456–2464. doi: 10.1253/circj.cj-12-0215. [DOI] [PubMed] [Google Scholar]
- Ito H, Gonthier MP, Manach C, et al. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br J Nutr. 2005;94:500–509. doi: 10.1079/bjn20051522. [DOI] [PubMed] [Google Scholar]
- Jin UH, Park SG, Suh SJ, et al. Inhibitory effect of Panax notoginseng on nitric oxide synthase, cyclo-oxygenase-2 and neutrophil functions. Phytother Res. 2007;21:142–148. doi: 10.1002/ptr.2018. [DOI] [PubMed] [Google Scholar]
- Joffe S, Miller FG. Equipoise: asking the right questions for clinical trial design. Nat Rev Clin Oncol. 2012;9:230–235. doi: 10.1038/nrclinonc.2011.211. [DOI] [PubMed] [Google Scholar]
- Kajimoto H, Hashimoto K, Bonnet SN, et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- Kapadia V, Embers D, Wells E, et al. Prenatal closure of the ductus arteriosus and maternal ingestion of anthocyanins. J Perinatol. 2010;30:291–294. doi: 10.1038/jp.2009.140. [DOI] [PubMed] [Google Scholar]
- Karadas B, Kaya T, Bagcivan I, et al. Comparison of effects of cyclooxygenase inhibitors on myometrial contraction and constriction of ductus arteriosus in rats. Eur J Pharmacol. 2004;485:289–298. doi: 10.1016/j.ejphar.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Keller RL, Tacy TA, Fields S, et al. Combined treatment with a nonselective nitric oxide synthase inhibitor (l-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatr Res. 2005;58:1216–1221. doi: 10.1203/01.pdr.0000183659.20335.12. [DOI] [PubMed] [Google Scholar]
- Khan HY, Zubair H, Ullah MF, et al. A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols. Curr Drug Targets. 2012;13:1738–1749. doi: 10.2174/138945012804545560. [DOI] [PubMed] [Google Scholar]
- Khayyal MT, el-Ghazaly MA, el-Khatib AS, A, et al. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol. 2003;17:93–102. doi: 10.1046/j.1472-8206.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Koren G, Florescu A, Costei AM, et al. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother. 2006;40:824–829. doi: 10.1345/aph.1G428. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113((Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Kupeli E, Tatli II, Akdemir ZS, Yesilada E. Bioassay-guided isolation of anti-inflammatory and antinociceptive glycoterpenoids from the flowers of Verbascum lasianthum Boiss. ex Bentham. J Ethnopharmacol. 2007a;110:444–450. doi: 10.1016/j.jep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kupeli E, Tatli II, Akdemir ZS, Yesilada E. Estimation of antinociceptive and anti-inflammatory activity on Geranium pratense subsp. finitimum and its phenolic compounds. J Ethnopharmacol. 2007b;114:234–240. doi: 10.1016/j.jep.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Levin DL, Fixler DE, Morriss FC, Tyson J. Morphologic analysis of the pulmonary vascular bed in infants exposed in utero to prostaglandin synthetase inhibitors. J Pediatr. 1978a;92:478–483. doi: 10.1016/s0022-3476(78)80453-3. [DOI] [PubMed] [Google Scholar]
- Levin DL, Hyman AI, Heymann MA, Rudolph AM. Fetal hypertension and the development of increased pulmonary vascular smooth muscle: a possible mechanism for persistent pulmonary hypertension of the newborn infant. J Pediatr. 1978b;92:265–269. doi: 10.1016/s0022-3476(78)80022-5. [DOI] [PubMed] [Google Scholar]
- Levin DL, Mills LJ, Weinberg AG. Hemodynamic, pulmonary vascular, and myocardial abnormalities secondary to pharmacologic constriction of the fetal ductus arteriosus. A possible mechanism for persistent pulmonary hypertension and transient tricuspid insufficiency in the newborn infant. Circulation. 1979;60:360–364. doi: 10.1161/01.cir.60.2.360. [DOI] [PubMed] [Google Scholar]
- Levin DL, Rudolph AM, Heymann MA, Phibbs RH. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976;53:144–151. doi: 10.1161/01.cir.53.1.144. [DOI] [PubMed] [Google Scholar]
- Levin M, Goldbarg S, Lindqvist A, et al. ATP depletion and cell death in the neonatal lamb ductus arteriosus. Pediatr Res. 2005;57:801–805. doi: 10.1203/01.PDR.0000157791.95954.56. [DOI] [PubMed] [Google Scholar]
- Levy R, Matitiau A, Ben Arie A, et al. Indomethacin and corticosteroids: an additive constrictive effect on the fetal ductus arteriosus. Am J Perinatol. 1999;16:379–383. doi: 10.1055/s-1999-6814. [DOI] [PubMed] [Google Scholar]
- Lock JE, Olley PM, Soldin S, Coceani F. Indomethacin-induced pulmonary vasoconstriction in the conscious newborn lamb. Am J Physiol. 1980;238:H639–651. doi: 10.1152/ajpheart.1980.238.5.H639. [DOI] [PubMed] [Google Scholar]
- Lorenzi H, Matos FJ. Plantas medicinais no Brasil: nativas e exóticas. Nova Odessa: 2002. Instituto Planturum 512. [Google Scholar]
- Macones GA, Robinson CA. Is there justification for using indomethacin in preterm labor? An analysis of neonatal risks and benefits. Am J Obstet Gynecol. 1997;177:819–824. doi: 10.1016/s0002-9378(97)70275-8. [DOI] [PubMed] [Google Scholar]
- Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012;64:540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M, Skrzycki M, Podsiad M, Czeczot H. Evaluation of antioxidant potential of flavonoids: an in vitro study. Acta Pol Pharm. 2011;68:611–615. [PubMed] [Google Scholar]
- Malik A, Azam S, Hadi N, Hadi SM. DNA degradation by water extract of green tea in the presence of copper ions: implications for anticancer properties. Phytother Res. 2003;17:358–363. doi: 10.1002/ptr.1149. [DOI] [PubMed] [Google Scholar]
- Manchester D, Margolis HS, Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1976;126:467–469. doi: 10.1016/0002-9378(76)90640-2. [DOI] [PubMed] [Google Scholar]
- Mari G, Moise KJ, Jr, Deter RL, et al. Doppler assessment of the pulsatility index of the middle cerebral artery during constriction of the fetal ductus arteriosus after indomethacin therapy. Am J Obstet Gynecol. 1989;161(6 Pt 1):1528–1531. doi: 10.1016/0002-9378(89)90918-6. [DOI] [PubMed] [Google Scholar]
- Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865–870. doi: 10.1016/s0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- Mathew AS, Capel-Williams GM, Berry SE, Hall WL. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum Nutr. 2012;67:351–357. doi: 10.1007/s11130-012-0318-9. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Mielke G, Benda N. Reference ranges for two-dimensional echocardiographic examination of the fetal ductus arteriosus. Ultrasound Obstet Gynecol. 2000;15:219–225. doi: 10.1046/j.1469-0705.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- Moise KJ., Jr Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168:1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- Moise KJ, Jr, Huhta JC, Sharif DS, et al. Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl J Med. 1988;319:327–331. doi: 10.1056/NEJM198808113190602. [DOI] [PubMed] [Google Scholar]
- Moise KJ, Jr, Ou CN, Kirshon B, et al. Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol. 1990;162:549–554. doi: 10.1016/0002-9378(90)90427-9. [DOI] [PubMed] [Google Scholar]
- Momma K, Ando M. In situ morphology of fetal aortic isthmus following ductal constriction in rats. Fetal Diagn Ther. 1994;9:53–61. doi: 10.1159/000263907. [DOI] [PubMed] [Google Scholar]
- Momma K, Hagiwara H, Konishi T. Constriction of fetal ductus arteriosus by non-steroidal anti-inflammatory drugs:study of additional 34 drugs. Prostaglandins. 1984;28:527–536. doi: 10.1016/0090-6980(84)90241-7. [DOI] [PubMed] [Google Scholar]
- Momma K, Nakanishi T, Imamura S. Inhibition of in vivo constriction of fetal ductus arteriosus by endothelin receptor blockade in rats. Pediatr Res. 2003;53:479–485. doi: 10.1203/01.PDR.0000049516.70216.2E. [DOI] [PubMed] [Google Scholar]
- Momma K, Nishihara S, Ota Y. Constriction of the fetal ductus arteriosus by glucocorticoid hormones. Pediatr Res. 1981;15:19–21. doi: 10.1203/00006450-198101000-00005. [DOI] [PubMed] [Google Scholar]
- Momma K, Toyoshima K, Takeuchi D, et al. In vivo constriction of the fetal and neonatal ductus arteriosus by a prostanoid EP4-receptor antagonist in rats. Pediatr Res. 2005;58:971–975. doi: 10.1203/01.pdr.0000182182.49476.24. [DOI] [PubMed] [Google Scholar]
- Montanher AB, Zucolotto SM, Schenkel EP, Frode TS. Evidence of anti-inflammatory effects of Passiflora edulis in an inflammation model. J Ethnopharmacol. 2007;109:281–288. doi: 10.1016/j.jep.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Mori Y, Rice MJ, McDonald RW, et al. Evaluation of systolic and diastolic ventricular performance of the right ventricle in fetuses with ductal constriction using the Doppler Tei index. Am J Cardiol. 2001;88:1173–1178. doi: 10.1016/s0002-9149(01)02056-2. [DOI] [PubMed] [Google Scholar]
- Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98:962–967. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr. 1984;104:758–762. doi: 10.1016/s0022-3476(84)80962-2. [DOI] [PubMed] [Google Scholar]
- Mushiake K, Motoyoshi F, Kinoshita Y, et al. Severe heart failure due to ductal constriction caused by maternal indomethacin. Pediatr Int. 2002;44:174–176. doi: 10.1046/j.1328-8067.2001.01512.x. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Norton ME. Teratogen update: fetal effects of indomethacin administration during pregnancy. Teratology. 1997;56:282–292. doi: 10.1002/(SICI)1096-9926(199710)56:4<282::AID-TERA7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Norton ME, Merrill J, Cooper BA, et al. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- Obladen M. History of the ductus arteriosus: 1. Anatomy and spontaneous closure. Neonatology. 2011;99:83–89. doi: 10.1159/000308367. [DOI] [PubMed] [Google Scholar]
- Paladini D, Marasini M, Volpe P. Severe ductal constriction in the third-trimester fetus following maternal self-medication with nimesulide. Ultrasound Obstet Gynecol. 2005;25:357–361. doi: 10.1002/uog.1873. [DOI] [PubMed] [Google Scholar]
- Petticrew M, McKee M, Lock K, et al. In search of social equipoise. BMJ. 2013;347:f4016. doi: 10.1136/bmj.f4016. [DOI] [PubMed] [Google Scholar]
- Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH* method. J Sep Sci. 2007a;30:1250–1254. doi: 10.1002/jssc.200600532. [DOI] [PubMed] [Google Scholar]
- Pozharitskaya ON, Ivanova SA, Shikov AN, et al. Separation and evaluation of free radical-scavenging activity of phenol components of green, brown, and black leaves of Bergenia crassifolia by using HPTLC-DPPH* method. J Sep Sci. 2007b;30:2447–2451. doi: 10.1002/jssc.200700178. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Jouppila P. Fetal cardiac function and ductus arteriosus during indomethacin and sulindac therapy for threatened preterm labor: a randomized study. Am J Obstet Gynecol. 1995;173:20–25. doi: 10.1016/0002-9378(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Reese J, O'Mara PW, Poole SD, et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2009;88:89–96. doi: 10.1016/j.prostaglandins.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek M, Weil SR, Huhta JC. Fetal echocardiography during indomethacin treatment. Ultrasound Obstet Gynecol. 1995;5:86–89. doi: 10.1046/j.1469-0705.1995.05020086.x. [DOI] [PubMed] [Google Scholar]
- Romain C, Gaillet S, Carillon J, et al. Vineatrol and cardiovascular disease: beneficial effects of a vine-shoot phenolic extract in a hamster atherosclerosis model. J Agric Food Chem. 2012;60:11029–11036. doi: 10.1021/jf303549t. [DOI] [PubMed] [Google Scholar]
- Rubaltelli FF, Chiozza ML, Zanardo V, Cantarutti F. Effect on neonate of maternal treatment with indomethacin. J Pediatr. 1979;94:161. doi: 10.1016/s0022-3476(79)80390-x. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. The effects of nonsteroidal antiinflammatory compounds on fetal circulation and pulmonary function. Obstet Gynecol. 1981;58(5 Suppl):63S–67S. [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(8S Suppl):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Schneider KT, Zimmermann A, et al. Prenatal constriction of the fetal ductus arteriosus—related to maternal pain medication? Z Geburtshilfe Neonatol. 2005;209:65–68. doi: 10.1055/s-2005-864116. [DOI] [PubMed] [Google Scholar]
- Selmi C, Cocchi CA, Lanfredini M, et al. Chocolate at heart: the anti-inflammatory impact of cocoa flavanols. Mol Nutr Food Res. 2008;52:1340–1348. doi: 10.1002/mnfr.200700435. [DOI] [PubMed] [Google Scholar]
- Sharpe GL, Larsson KS, Thalme B. Studies on closure of the ductus arteriosus. XII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins. 1975;9:585–596. doi: 10.1016/0090-6980(75)90064-7. [DOI] [PubMed] [Google Scholar]
- Sheehan M, Timlin C, Peach K, et al. Position statement on ethics, equipoise and research on charged particle radiation therapy. J Med Ethics. 2013 doi: 10.1136/medethics-2012-101290. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Shima Y, Ishikawa H, Matsumura Y, et al. Idiopathic severe constriction of the fetal ductus arteriosus: a possible underestimated pathophysiology. Eur J Pediatr. 2011;170:237–240. doi: 10.1007/s00431-010-1295-3. [DOI] [PubMed] [Google Scholar]
- Silva JR, Campos AC, Ferreira LM, et al. [Extract of Passiflora edulis in the healing process of gastric sutures in rats: a morphological and tensiometric study] Acta Cir Bras. 2006;21(Suppl 2):52–60. doi: 10.1590/s0102-86502006000800009. [DOI] [PubMed] [Google Scholar]
- Slomp J, Gittenberger-de Groot AC, Glukhova MA, et al. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol. 1997;17:1003–1009. doi: 10.1161/01.atv.17.5.1003. [DOI] [PubMed] [Google Scholar]
- Slomp J, van Munsteren JC, Poelmann RE, et al. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis. 1992;93:25–39. doi: 10.1016/0021-9150(92)90197-o. [DOI] [PubMed] [Google Scholar]
- Smith DM, Dou QP. Green tea polyphenol epigallocatechin inhibits DNA replication and consequently induces leukemia cell apoptosis. Int J Mol Med. 2001;7:645–652. doi: 10.3892/ijmm.7.6.645. [DOI] [PubMed] [Google Scholar]
- Sridharan S, Archer N, Manning N. Premature constriction of the fetal ductus arteriosus following the maternal consumption of camomile herbal tea. Ultrasound Obstet Gynecol. 2009;34:358–359. doi: 10.1002/uog.6453. [DOI] [PubMed] [Google Scholar]
- Starling MB, Elliott RB. The effects of prostaglandins, prostaglandin inhibitors, and oxygen on the closure of the ductus arteriosus, pulmonary arteries and umbilical vessels in vitro. Prostaglandins. 1974;8:187–203. doi: 10.1016/0090-6980(74)90042-2. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Demauro SB, Dagle JM, Reese J. Current perspectives on pathobiology of the ductus arteriosus. J Clin Exp Cardiolog. 2012;8:pii:S8–001. doi: 10.4172/2155-9880.S8-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Chung WJ, Michaluart P, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- Tada T, Wakabayashi T, Nakao Y, et al. Human ductus arteriosus. A histological study on the relation between ductal maturation and gestational age. Acta Pathol Jpn. 1985;35:23–34. [PubMed] [Google Scholar]
- Takahashi Y, Roman C, Chemtob S, et al. Cyclooxygenase-2 inhibitors constrict the fetal lamb ductus arteriosus both in vitro and in vivo. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1496–1505. doi: 10.1152/ajpregu.2000.278.6.R1496. [DOI] [PubMed] [Google Scholar]
- Takami T, Momma K, Imamura S. Increased constriction of the ductus arteriosus by dexamethasone, indomethacin, and rofecoxib in fetal rats. Circ J. 2005;69:354–358. doi: 10.1253/circj.69.354. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Horikoshi E, Shen MH, et al. Effects of TAK-044, a nonselective endothelin receptor antagonist, on the spontaneous and indomethacin- or methylene blue-induced constriction of the ductus arteriosus in rats. J Vet Med Sci. 2000;62:505–509. doi: 10.1292/jvms.62.505. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Kihara T, Kamata A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and L-NAME in fetal rats. Biol Neonate. 2001;80:64–67. doi: 10.1159/000047122. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Miyakoshi K, Yamada M, et al. Functional foods for the fetus? Acta Obstet Gynecol Scand. 2011;90:1172–1173. doi: 10.1111/j.1600-0412.2011.01167.x. [DOI] [PubMed] [Google Scholar]
- Tarcan A, Gurakan B, Yildirim S, et al. Persistent pulmonary hypertension in a premature newborn after 16 hours of antenatal indomethacin exposure. J Perinat Med. 2004;32:98–99. doi: 10.1515/JPM.2004.019. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the fetal and postnatal ductus arteriosus by inhibition of phosphodiesterase 3 in rats. Biol Neonate. 2006a;89:251–256. doi: 10.1159/000089954. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Takeda A, Imamura S, et al. Constriction of the ductus arteriosus by selective inhibition of cyclooxygenase-1 and −2 in near-term and preterm fetal rats. Prostaglandins Other Lipid Mediat. 2006b;79:34–42. doi: 10.1016/j.prostaglandins.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Trevett TN, Jr, Cotton J. Idiopathic constriction of the fetal ductus arteriosus. Ultrasound Obstet Gynecol. 2004;23:517–519. doi: 10.1002/uog.980. [DOI] [PubMed] [Google Scholar]
- Tulzer G, Gudmundsson S, Sharkey AM, et al. Doppler echocardiography of fetal ductus arteriosus constriction versus increased right ventricular output. J Am Coll Cardiol. 1991;18:532–536. doi: 10.1016/0735-1097(91)90611-c. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levin DL. Prostaglandin synthesis inhibition in persistent pulmonary hypertension of the newborn. Clin Perinatol. 1984;11:581–589. [PubMed] [Google Scholar]
- Tynan M. The ductus arteriosus and its closure. N Engl J Med. 1993;329:1570–1572. doi: 10.1056/NEJM199311183292111. [DOI] [PubMed] [Google Scholar]
- USDA. Database for the flavonoid content of selected foods release. 2007. Available at: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02-1.pdf.
- Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29:773–782. doi: 10.3233/JAD-2012-111799. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Deprez RH, Ruijter JM, et al. Increased cardiac workload by closure of the ductus arteriosus leads to hypertrophy and apoptosis rather than to hyperplasia in the late fetal period. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:193–202. doi: 10.1007/s00210-004-0955-0. [DOI] [PubMed] [Google Scholar]
- van der Graaf R, van Delden JJ. Equipoise should be amended, not abandoned. Clin Trials. 2011;8:408–416. doi: 10.1177/1740774511409600. [DOI] [PubMed] [Google Scholar]
- van der Graaf R, van Delden JJ. On what we will lose in giving up on clinical equipoise: a reply to Miller. Clin Trials. 2012;9:628–629. doi: 10.1177/1740774512453221. [DOI] [PubMed] [Google Scholar]
- Van Marter LJ, Hernandez-Diaz S, Werler MM, et al. Nonsteroidal antiinflammatory drugs in late pregnancy and persistent pulmonary hypertension of the newborn. Pediatrics. 2013;131:79–87. doi: 10.1542/peds.2012-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marter LJ, Leviton A, Allred EN, et al. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiinflammatory drug consumption during pregnancy. Pediatrics. 1996;97:658–663. [PubMed] [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. [Google Scholar]
- Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997;177:256–259; discussion 259–261. doi: 10.1016/s0002-9378(97)70184-4. [DOI] [PubMed] [Google Scholar]
- Vian I, Zielinsky P, Zilio AM, et al. Development and validation of a food frequency questionnaire for consumption of polyphenol-rich foods in pregnant women. Matern Child Nutr. 2013 doi: 10.1111/mcn.12025. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M, Wilkins-Haug L, McElhinney DB, et al. Reversible ductus arteriosus constriction due to maternal indomethacin after fetal intervention for hypoplastic left heart syndrome with intact/restrictive atrial septum. Fetal Diagn Ther. 2010;27:40–45. doi: 10.1159/000268290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh-Sukys MC. Persistent pulmonary hypertension of the newborn. The black box revisited. Clin Perinatol. 1993;20:127–143. [PubMed] [Google Scholar]
- Wasserstrum N, Huhta JC, Mari G, et al. Betamethasone and the human fetal ductus arteriosus. Obstet Gynecol. 1989;74:897–900. [PubMed] [Google Scholar]
- Widmer RJ, Freund MA, Flammer AJ, et al. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur J Nutr. 2013;52:1223–1231. doi: 10.1007/s00394-012-0433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AR, Aynsley-Green A, Mitchell MD. Persistent pulmonary hypertension and abnormal prostaglandin E levels in preterm infants after maternal treatment with naproxen. Arch Dis Child. 1979;54:942–945. doi: 10.1136/adc.54.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GR, Jing S, Momma K, Nakanishi T. The effect of vitamin A on contraction of the ductus arteriosus in fetal rat. Pediatr Res. 2001;49:747–754. doi: 10.1203/00006450-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Yajima I, Colombo S, Puig I, et al. A subpopulation of smooth muscle cells, derived from melanocyte-competent precursors, prevents patent ductus arteriosus. PLoS One. 2013;8:e53183. doi: 10.1371/journal.pone.0053183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159:169–173. doi: 10.1016/s0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- Zielinsky P, Bubols G, Piccoli AL, et al. 2012a. Experimental assessment of ductus arteriosus flow, oxidative stress and polyphenol excretion after maternal polyphenol-rich diet in late pregnancy. 22nd World Congress on Ultrasound in Obstetrics and Gynecology, 2012, Copenhagen—Ultrasound in Obstetrics & Gynecology (Print) 40:16.
- Zielinsky P, Manica JL, Piccoli AL, Jr, et al. Experimental study of the role of maternal consumption of green tea, mate tea and grape juice on fetal ductal constriction. 17th World Congress on Ultrasound in Obstetrics and Gynecology, 2007—Ultrasound in Obstetrics and Gynecology. 2007;30:515. [Google Scholar]
- Zielinsky P, Manica JL, Piccoli AL, Jr, et al. Fetal ductal constriction caused by maternal ingestion of green tea in late pregnancy: an experimental study. Prenat Diagn. 2012b;32:921–926. doi: 10.1002/pd.3933. [DOI] [PubMed] [Google Scholar]
- Zielinsky P, Piccoli AL, Jr, Manica JL, Nicoloso LH. New insights on fetal ductal constriction: role of maternal ingestion of polyphenol-rich foods. Expert Rev Cardiovasc Ther. 2010a;8:291–298. doi: 10.1586/erc.09.174. [DOI] [PubMed] [Google Scholar]
- Zielinsky P, Piccoli AL, Jr, Manica JL, et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J Perinatol. 2010b;30:17–21. doi: 10.1038/jp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinsky P, Piccoli AL, Jr, Manica JL, et al. Reversal of fetal ductal constriction after maternal restriction of polyphenol-rich foods: an open clinical trial. J Perinatol. 2012c;32:574–579. doi: 10.1038/jp.2011.153. [DOI] [PubMed] [Google Scholar]
- Zielinsky P, Piccoli AL, Jr, Vian I, et al. Maternal restriction of polyphenols and fetal ductal dynamics in normal pregnancy: an open clinical trial. Arq Bras Cardiol. 2013;101:217–225. doi: 10.5935/abc.20130166. [DOI] [PMC free article] [PubMed] [Google Scholar]


