Abstract
Objectives
Oestrogen has been proven to significantly enhance osteogenic potency, while oestrogen deficiency usually leads to impaired osteogenic differentiation of mesenchymal stem cells. However, little is known concerning direct effects of oestrogen on differentiation of human dental pulp stem cells (DPSCs).
Materials and methods
In this study, human DPSCs were isolated and treated with 10−7 m 17β‐oestradiol (E2). Alkaline phosphatase (ALP) assay and alizarin red staining were performed.
Results
Alkaline phosphatase and alizarin red showed that E2 treatment significantly enhanced ALP activity and mineralization ability of DPSCs, but had no effect on cell proliferation. Real‐time RT‐PCR and western blot assay demonstrated that odonto/osteogenic markers (ALP, RUNX2/RUNX2, OSX/OSX, OCN/OCN and DSPP/DSP) were significantly upregulated in the cells after E2 treatment. Moreover, phosphorylation of cytoplasmic IκBα/P65 and expression of nuclear P65 were enhanced in a time‐dependent manner following E2 treatment, suggesting activation of NF‐κB signaling. Conversely, inhibition of the NF‐κB pathway suppressed E2‐mediated upregulation of odonto/osteogenic markers, indicating that the NF‐κB pathway was pivotal for E2‐mediated differentiation.
Conclusion
These findings provide evidence that 10−7 m 17β‐oestradiol promoted odonto/osteogenic differentiation of human DPSCs via activation of the NF‐κB signaling pathway.
Introduction
Dental pulp stem cells (DPSCs) have multiple differentiation capacity and represent a cell source for regenerative medicine and tissue engineering, specially for tooth and pulp regeneration. However, both limited number of available DPSCs in vital pulp and gradual loss of their differentiation capacity following in vitro expansion, significantly restrict their use in clinical applications 1. Effective improvement of their odonto/osteogenic capacity is necessary for upcoming applications of DPSC‐based tissue engineering. To date, there are many factors including pro‐inflammatory cytokines 2, growth factors 3, mechanical stretch 4 and donor age 5, which have been implicated in regulating proliferation and odonto/osteogenic capacity of DPSCs. These results offer an opportunity to reconstruct the extrinsic microenvironment necessary for efficient differentiation of DPSCs, and eventually facilitate their application in tissue regeneration.
A natural steroid hormone, oestrogen has profound impact on the skeletal system. However, direct effects of it on dental tissues/cells, have up to now, remained unclear. Consistent with the fact that composition and calcification of dentine are similar in many ways to intramembranous bone formation, earlier studies have indicated that oestrogen causes similar changes in ground substance of dentine as it does for bone. Moreover, exogenous oestrogen can enhance osteogenic capacity of human bone marrow stromal cells and adipose‐derived stromal cells 6, 7, 8, 9, whereas oestrogen deficiency leads to impaired osteoblastic differentiation of bone marrow stromal cells and periodontal ligament stem cells 10, 11. Previous study has revealed that oestrogen deficiency in vivo can bring about downregulation of committed differentiation of rat DPSCs 12; however, little is known about direct impact of oestrogen on biological features of human DPSCs.
In this study, we investigated effects of oestrogen on odonto/osteogenic differentiation of human DPSCs in vitro. Human DPSCs were isolated from premolars and treated with exogenous 17β‐oestradiol (E2 – oestradiol), the most important form of oestrogen in the body. Differentiation and involvement of the NF‐κB pathway in E2‐treated DPSCs were evaluated in vitro. Our findings demonstrated that 10−7 m 17β‐oestradiol enhanced odonto/osteogenic differentiation of DPSCs by activation of the NF‐κB pathway.
Materials and methods
Cell isolation and identification
Non‐carious human premolars (n = 12) were freshly extracted from six young female patients requiring orthodontic treatment, at the age of 12/13, in the Oral Surgery Department of Jiangsu Provincial Stomatological Hospital, after informed consents were obtained. Pulps were carefully separated, minced into 1 mm3 and treated with a solution containing 3 mg/ml collagenase type I (Sigma, St. Louis, MO, USA) and 4 mg/ml dispase (Sigma), for 60 min at 37 °C. Single cell suspensions were obtained and cultured in phenol red free, low‐glucose Dulbecco's minimum essential medium (phenol red free L‐DMEM; Gibco, Life Technologies, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in 5% CO2. Multi‐colony‐derived stem cells were isolated as previously described 13. To determine their origin from mesenchymal stem cells, DPSCs were immunostained with antibody against STRO‐1 (1:200, Novus Biologicals, Littleton, CO, USA). Stem cells from different patients were mixed, subcultured and then utilized for subsequent experiments. 17β‐oestradiol (E2, Ehrenstorfer Gmbh, Augsburg, Germany) was dissolved in absolute ethyl alcohol at 10−3 m, and stored at −20 °C in the dark. Cells at passages 2–4 were incubated in phenol red free L‐DMEM containing 17β‐oestradiol (E2 group) or 0.01% (v/v) ethyl alcohol as control (control group). Culture media of the E2 group were replaced every other day to maintain constant E2 concentration.
MTT assay
Dental pulp stem cells were seeded at 2 × 103 cells/well into 96‐well plates (Nunc, Roskilde, Denmark). After 24‐h serum starvation in serum‐free media, these stem cells were cultured in complete media supplemented with 10−7 m 17β‐oestradiol. For 11 consecutive days, MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐2,5‐tetrazoliumbromide) assay was performed as previously reported 12. OD values at 490 nm were detected using a microtitre plate reader (Titertek, Helsinki, Finland). Experiments were performed in triplicate and data are presented as mean ± SD.
Alkaline phosphatase activity assay and alizarin red staining
Dental pulp stem cells in different groups were respectively seeded into 96‐well plates at 2 × 103 cells/well, and 24‐well plates at 1 × 104 cells/well, and then cultured in complete media with or without 10−7 m 17β‐oestradiol. Alkaline phosphatase (ALP) activity assay at days 3/5 and alizarin red staining at day 14 were performed as previously described 1, 14. Nodule staining was then eluted by 10% cetylpyridinium chloride (pH 7.0) and calcium concentrations were determined by absorbance measurement at 526 nm, using a universal microplate reader (BioTek Instruments Inc., Winooski, VT, USA) 14. Data are presented as mean ± SD of three independent experiments.
Real‐time reverse transcription polymerase chain reaction
Total cell RNA in different groups was isolated using TRIzol reagent (Invitrogen, New York, NY, USA) according to the manufacturer's protocols. Then, cDNA was produced using a reverse transcription kit (TaKaRa Biotechnology, Dalian, China). Real‐time reverse transcription polymerase chain reaction (RT‐PCR) was performed in a single tube using SYBR® Premix Ex Taq™ kit (TaKaRa Biotechnology) and ABI 7300 real‐time PCR system. Primers used in the experiment were as follows: OSX, 5′‐ CCTCCTCAGCTCACCTTCTC ‐3′ (forward) and 5′‐ GTTGGGAGCCCAAATAGAAA ‐3′ (reverse); OCN, 5′‐ AGCAAAGGTGCAGCCTTTGT ‐3′ (forward) and 5′‐ GCGCCTGGGTCTCTTCACT ‐3′ (reverse); RUNX2, 5′‐ TCTTAGAACAAATTCTGCCCTTT ‐3′ (forward) and 5′‐ TGCTTTGGTCTTGAAATCACA ‐3′ (reverse); ALP, 5′‐ GACCTCCTCGGAAGACACTC ‐3′ (forward) and 5′‐ TGAAGGGCTTCTTGTCTGTG ‐3′ (reverse); DSPP, 5′‐ ATATTGAGGGCTGGAATGGGGA ‐3′ (forward) and 5′‐ TTTGTGGCTCCAGCATTGTCA ‐3′ (reverse); GAPDH, 5′‐ GAAGGTGAAGGTCGGAGTC ‐3′ (forward) and 5′‐ GAGATGGTGATGGGATTTC ‐3′ (reverse). GAPDH served as the internal control. Data processing was performed by the method of 2−∆∆Ct as previously reported 15. The experiment was repeated three times and data were described as mean ± SD.
Western blot analysis
To explore effects of 17β‐oestradiol on odonto/osteogenic differentiation and expression of NF‐κB pathway proteins, DPSCs in different groups were collected, washed twice in 0.01 m PBS and lysed in radioimmunoprecipitation assay lysis buffer (Beyotime, Nanjing, China) containing 1 mm phenylmethanesulfonyl fluoride. Western blot assay was performed as previously reported using 30 μg protein per lane 12, 14. Primary antibodies used were to proteins listed below: RUNX2, 1:1000, Abcam, Hong Kong, China; OSX, 1:1000, Abcam; OCN, 1:1000, Abcam; DSP, 1:500, Santa Cruz, Dallas, TX, USA; phosphor‐P65, 1:1000, Cell Signaling; P65, 1:1000, Cell Signaling Boston, MA, USA; phosphor‐IκBα, 1:1000, Cell Signaling; IκBα, 1:1000, Cell Signaling; β‐ACTIN, 1:1000, Bioworld, Minneapolis, MN, USA; H1, 1:1000, Cell Signaling. Semi‐quantitative analysis of western blotting was performed with Image‐Proplus 5.0 software (Media Cybernetics Inc., Silver Spring, MD, USA). This experiment was repeated three times.
Statistics
Two‐sample t‐testing was performed to compare means of two independent samples. For multiple comparisons between experimental groups and control groups, Dunnett's test was used to check significant differences. Two‐tailed P‐values less than 0.05 were considered statistically significant. All statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effects of 17β‐oestradiol on proliferation of DPSCs
The cells had typical fibroblast‐ or spindle‐like morphology and stained positively for putative mesenchymal stem‐cell marker STRO‐1 (Fig. 1a). Physiologically relevant concentrations of 17β‐oestradiol (10−9 and 10−7 m; Fig. 1c,d) exerted no effect on cell proliferation, while 10−5 m 17β‐oestradiol significantly inhibited the DPSCs' proliferation (Fig. 1c; P < 0.01).
Figure 1.
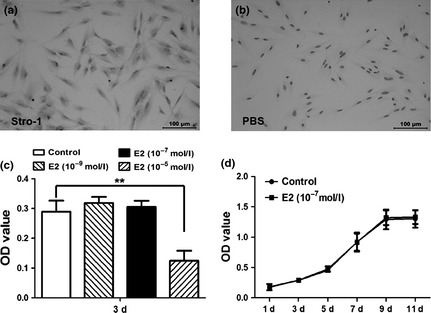
Proliferation features of dental pulp stem cells ( DPSC s) treated by 17β‐oestradiol. (a) Isolated DPSCs were spindle‐like cells with a positive staining for STRO‐1 by immunocytochemistry. (b) PBS served as a negative control. (c) High concentration (10−5 m) of 17β‐oestradiol (E2) had negative effects on the proliferation of DPSCs, while the physiological concentrations (10−7 and 10−9 m) of E2 did not affect the cell growth. (d) MTT assay showed that 10−7 m E2 had no effects on the proliferation of DPSCs in vitro.
Effects of 17β‐oestradiol on odonto/osteogenic differentiation of DPSCs
10−9 m 17β‐oestradiol did not change ALP activity in DPSCs (Fig. 2a; P > 0.05), while 10−7 m 17β‐oestradiol significantly upregulated ALP level respectively at day 3 (P < 0.05) and day 5 (P < 0.01), whereas 10−5 m 17β‐oestradiol noticeably downregulated ALP activity (Fig. 2a; P < 0.01) mostly due to inhibition of cell proliferation (Fig. 1c). Thus, 10−7 m 17β‐oestradiol was selected to be the optimal concentration and was used for the following experiments. Alizarin red staining assay revealed that cells in E2 group produced more calcified nodules than those in control groups (Fig. 2b). Cetylpyridinium chloride quantitative calcium assay demonstrated higher calcium deposition in E2 group than in control groups (Fig. 2c; P < 0.01), indicating that 17β‐oestradiol enhanced mineralization capacity of human DPSCs.
Figure 2.
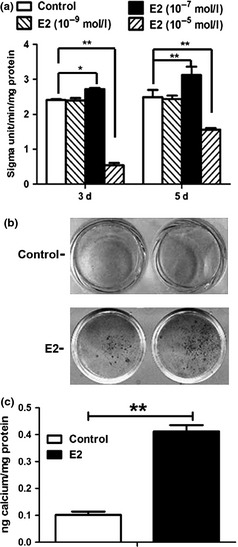
Alkaline phosphatase ( ALP ) activity and calcium deposition in E2‐treated dental pulp stem cells ( DPSC s). (a) 10−9 m E2 did not affect the ALP activity of DPSCs (P > 0.05) and 10−7 m E2 significantly upregulated the ALP levels of DPSCs at day 3 and day 5 (P < 0.05 or P < 0.01), while 10−5 m E2 significantly downregulated the ALP activity (P < 0.01), as compared with control group. Data were described as mean ± SD, n = 6. *P < 0.05, **P < 0.01. (b) Alizarin red staining assay showed that DPSCs in E2 group produced more calcified nodules than those in control group. (c) Quantitative calcium assay demonstrated the stronger calcium deposition in E2 group as compared with control group. Values were presented as mean ± SD, n = 3. **P < 0.01.
Real‐time RT‐PCR showed that expressions of odonto/osteogenic genes (ALP, RUNX2, OSX, OCN, and DSPP) were remarkably enhanced in DPSCs (Fig. 3a) following E2 treatment. In particular, expressions of ALP, RUNX2, OCN and OSX were significantly higher on day 3, while expressions of RUNX2, OCN and DSPP were upregulated by day 7. Western blot findings further confirmed upregulation of odonto/osteogenic proteins (RUNX2, OSX, OCN, and DSP) following E2 treatment (Fig. 3b,c; P < 0.01).
Figure 3.
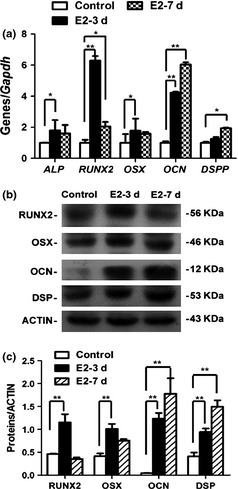
Gene and protein expression in E2‐treated dental pulp stem cells ( DPSC s). (a) Real‐time RT‐PCR demonstrated that the expressions of ALP, RUNX2, OSX, OCN, and DSPP were significantly enhanced in E2‐treated DPSCs at day 3 or day 7 in comparison with control group. Values were described as mean ± SD. **2−ΔΔCt > 2, P < 0.01; *1 < 2−ΔΔCt < 2, P < 0.01; n = 3. (b) Western blot results revealed that the odonto/osteogenic proteins (RUNX2, OSX, OCN, and DSP) were significantly upregulated after 3‐day or 7‐day E2 stimulation, as compared with control DPSCs. (c) Semi‐quantitative analysis confirmed that the expressions of RUNX2, OSX, OCN, and DSP were stronger in E2‐treated DPSCs than those in control group.
NF‐κB pathway involvement in E2‐mediated odonto/osteogenic differentiation of DPSCs
To determine potential involvement of the NF‐κB pathway in E2‐mediated differentiation of DPSCs, we investigated phosphorylation and expression of IκBα and P65 by western blot analysis. Phosphorylation of cytoplasmic IκBα was significantly higher by minute 15 then gradually decreased between 30 and 60 min (Fig. 4a,b), while phosphorylation of cytoplasmic P65 gradually upregulated between 15 and 30 min then downregulated by 60 min following E2 treatment. In addition, expression of nuclear P65 was enhanced by 30 min and then fell by 60 min after E2 treatment.
Figure 4.
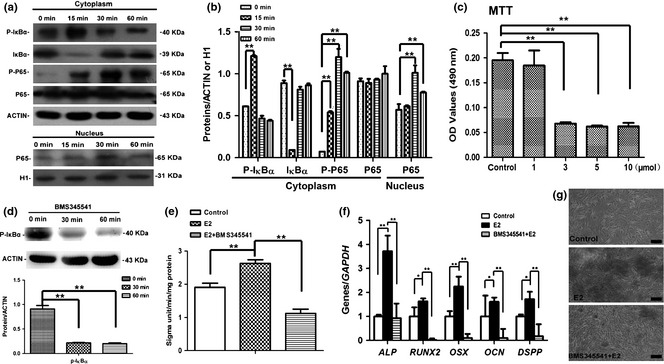
NF‐κB pathway involvement in E2‐mediated odonto/osteogenic differentiation of dental pulp stem cells ( DPSC s). (a) The expression of P‐IκBα increased at 0–15 min and gradually decreased at minutes 30/60 in E2‐treated DPSCs, whereas the expression of P‐P65 gradually increased at 0–30 min after E2 treatment and decreased at minutes 60. Moreover, the expression of nuclear P65 was upregulated at 0–30 min and dowregulated at minutes 60. β‐ACTIN and Histone 1 (H1) served as internal controls respectively for cytoplasmic and nuclear proteins. (b) Semi‐quantitative analysis confirmed the upregulated expression of P‐IκBα and P‐P65 after E2 treatment. **P < 0.01. (c) BMS345541 at high concentrations (3, 5 and 10 m) significantly reduced the proliferative activity, while 1 m BMS345541 did not affect the viability of human DPSCs. **P < 0.01, n = 6. (d) Western blot results for P‐IκBα expression in BMS345541‐treated DPSCs at different time points. 1 m BMS345541 significantly inhibited the expression of P‐IκBα. (e) Alkaline phosphatase (ALP) activity in E2‐treated DPSCs after inhibition of NF‐κB signaling with BMS345541. **P < 0.01. (f) Real‐time RT‐PCR results revealed that the odonto/osteogenic markers (ALP, RUNX2, OSX, OCN, and DSPP) in DPSCs were significantly downregulated in BMS345541+E2 group, as compared with E2 group. **2−ΔΔCt > 2, P < 0.01; *1 < 2−ΔΔCt < 2, P < 0.01; n = 3. (g) Alizarin red staining in E2‐treated DPSCs after inhibiting the NF‐κB pathway with BMS345541. Calcified nodules in BMS345541+E2 group were less than those in E2‐treated DPSCs. Scale bars = 100 μm.
To further determine the role of the NF‐κB pathway in E2‐mediated differentiation of DPSCs, BMS345541 (a specific IKK inhibitor) was used to suppress activity of NF‐κB signaling for 30 min prior to 17β‐oestradiol treatment 16, 17, 18. BMS345541 at higher concentrations (3, 5 and 10 μm) significantly reduced proliferative activity, whereas 1 μm BMS345541 did not affect viability of DPSCs (Fig. 4c; P < 0.01). Moreover, 1 μm BMS345541 significantly inhibited expression of P‐IκBα and was thus used as the optimal dosage in the following experiments (Fig. 4d; P < 0.01). Real‐time RT‐PCR results demonstrated that odonto/osteogenic markers (ALP/ALP, RUNX2, OSX, OCN and DSPP) were significantly downregulated in the E2+BMS345541 group in comparison to the E2 group (Fig. 4e,f). Furthermore, BMS345541 clearly inhibited E2‐mediated mineralization in DPSCs (Fig. 4g).
Discussion
Effects of oestrogen on bone tissues/cells have been extensively studied in vitro and in vivo. However, little information has been available concerning its influence on tooth structures/cells. In a previous study our group revealed that DPSCs of an oestrogen‐deficient rat model exhibited reduced differentiation towards the osteo/odontogenic cell lineages 12. To date, effects of exogenous oestrogen at physiological concentrations on proliferation and differentiation of human DPSCs had remained unexplored 12. In the present study, the physiologically minor concentration, 10−5 m 17β‐oestradiol, significantly inhibited proliferation of DPSCs, while physiologically relevant concentrations 10−7 and 10−9 m 17β‐oestradiol, had no effect. 17β‐oestradiol may regulate cell proliferation in a dose‐dependent manner via oestrogen receptor (OR) pathways 19, 20, 21 and its high concentration can significantly inhibit proliferation of OR‐positive cells 22. In particular, high dose 17β‐oestradiol can repress cancer cell proliferation, such as in carcinoma of the breast and colorectal cancer, in post‐menopausal women receiving hormonal replacement therapy 22, 23.
To elucidate impacts of oestrogen on cell differentiation, 17β‐oestradiol should be able to trigger differentiation without inhibiting proliferation of DPSCs. Under such conditions, stem cells can retain stable growth capacity to guarantee their sustainable differentiation. In the current study, we used a physiological concentration of 17β‐oestradiol (10−7 m) 24 to stimulate differentiation of DPSCs in vitro. Clearly, 10−7 m E2 enhanced ALP activity, mineralization capacity and odonto/osteogenic potential of the cells, without affecting their proliferation. Understanding the effects of 17β‐oestradiol on differentiation of DPSCs provides us with the opportunity to test whether 17β‐oestradiol could facilitate the general process of tissue calcification and pulp regeneration.
As odontoblast‐specific markers, DSP protein and DSPP mRNA have been reported to be expressed only in secretory odontoblasts 25, 26. Thus, increased expression of DSPP/DSP in E2‐treated DPSCs indicates that 10−7 m 17β‐oestradiol could promote odontoblastic differentiation. Additionally, RUNX2 and OSX are early‐stage markers of osteoblastic differentiation 27, 28, 29. RUNX2 overexpression can induce mesenchymal stem cells to differentiate into osteoblast lineages, enhance new bone formation and even drive pre‐adipocytes into bone‐forming cells, in vitro 30, 31. OSX is the downstream gene of the BMP‐2/Smad/Runx2 signaling pathway and is highly expressed in functional odonto/osteoblasts 32. OCN mainly appears in late stages of osteoblastic differentiation 15, 28. Thus, upregulation of RUNX2, OSX and OCN after 17β‐oestradiol treatment suggests that 17β‐oestradiol induced biological changes in DPSCs, similar to osteoblastic differentiation and matrix mineralization.
In addition, 17β‐oestradiol treatment has resulted in activation of several signaling pathways including those of JNK, ORK, P38, the OR, PI3K‐AKT‐dependent pathways and NF‐κB 33, 34, 35, 36, 37, 38. The NF‐κB pathway has been reported to be extensively involved in E2‐mediated osteoblastic differentiation and mineralization 12, 24, 39, 40 and canonical NF‐κB pathway is regulated by inhibition of the κB kinase complex (IKK‐α, IKK‐β and IKK‐γ) 41. The IKK complex phosphorylates/degrades IκB and releases NF‐κB subunits, mainly p65 and p50, for nuclear translocation and DNA binding, which subsequently regulate diverse biological processes including apoptosis, cell survival, cell division, cell differentiation and innate immunity as well as cell responses to stimuli 42. In this study, cytoplasmic P‐IκBα/P‐P65 and nuclear P65 were upregulated in a time‐dependent manner following 17β‐oestradiol treatment, suggesting that the NF‐κB pathway was activated in E2‐treated DPSCs. Activation of NF‐κB can promote the odontoblastic phenotype and stimulate odonto/osteogenic differentiation of dental pulp‐derived stem cells 2, 43, 44. Furthermore, inhibition of the NF‐κB pathway dramatically suppressed odonto/osteogenic differentiation of E2‐treated DPSCs, as indicated by downregulation of several odonto/osteogenic markers and reduced mineralization capacity. Based on the present findings, it can be inferred that the NF‐κB pathway plays a pivotal role during committed differentiation of E2‐treated DPSCs.
In our previous study, DPSCs from the oestrogen deficiency rat model also exhibited activated NF‐κB pathway, but with reduced odontogenesis/osteogenesis 12. Oestrogen deficiency usually leads to excessive expression of TNF‐α which definitively causes activation of NF‐κB 12, 39, 45, 46. TNF‐α, a potent pro‐inflammatory cytokine, inhibits osteoblastogenesis and stimulates osteoclastogenesis under diverse inflammatory conditions 47, 48, 49, 50. Here, it is possible that 17β‐oestradiol may induce odonto/osteoblastic differentiation of human DPSCs via activation of OR‐α/NF‐κB, but not by the canonical TNF‐α/NF‐κB pathway, and accordingly trigger differentiation‐related gene expression and mineralization 9, 39. Data accumulated here suggest that activation of the NF‐κB pathway in DPSCs from different species may cause distinctive differentiation potency via different upstream and downstream signaling pathways. More intensive studies are required to explore upstream and downstream cell signaling of the NF‐κB pathway during 17β‐oestradiol or oestrogen deficiency‐mediated differentiation of DPSCs.
In conclusion, 17β‐oestradiol promoted odonto/osteogenic differentiation of human DPSCs via activation of the NF‐κB pathway. These findings provide strong clues for therapeutic application of 17β‐oestradiol in reconstruction of the inductive microenvironment necessary for composing of bio‐teeth, bio‐pulp and even bio‐bone. However, further studies are warranted to investigate other potential mechanisms associated with E2‐mediated odonto/osteogenic differentiation of DPSCs.
Acknowledgements
The authors would like to thank Dr. Ruoning Wang for his help in improving scientific English language in this manuscript. This study was supported by the Medical Elitist Project of Jiangsu Province (No. RC2011140), the Key Project of National Natural Science Fund (No. 81230022) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, No. 2011‐137).
Reference
- 1. Yu J, He H, Tang C, Zhang G, Li Y, Wang R et al (2010) Differentiation potential of STRO‐1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 11, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Zhang S, Pang X, Fan M (2012) Pro‐inflammatory cytokines induce odontogenic differentiation of dental pulp‐derived stem cells. J. Cell. Biochem. 113, 669–677. [DOI] [PubMed] [Google Scholar]
- 3. Kim SG, Zhou J, Solomon C, Zheng Y, Suzuki T, Chen M et al (2012) Effects of growth factors on dental stem/progenitor cells. Dent. Clin. North Am. 56, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hata M, Naruse K, Ozawa S, Kobayashi Y, Nakamura N, Kojima N et al (2013) Mechanical stretch increases the proliferation while inhibiting the osteogenic differentiation in dental pulp stem cells. Tissue Eng. Part A 19, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma D, Ma Z, Zhang X, Wang W, Yang Z, Zhang M et al (2009) Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J. Endod. 35, 1546–1553. [DOI] [PubMed] [Google Scholar]
- 6. Hong L, Zhang G, Sultana H, Yu Y, Wei Z (2011) The effects of 17‐beta estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev. 20, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. James AW, Theologis AA, Brugmann SA, Xu Y, Carre AL, Leucht P et al (2009) Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One 4, e7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong L, Colpan A, Peptan IA, Daw J, George A, Evans CA (2007) 17‐Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose‐derived stromal cells. Tissue Eng. 13, 1197–1203. [DOI] [PubMed] [Google Scholar]
- 9. Chen FP, Hu CH, Wang KC (2013) Estrogen modulates osteogenic activity and estrogen receptor mRNA in mesenchymal stem cells of women. Climacteric 16, 154–160. [DOI] [PubMed] [Google Scholar]
- 10. Haque T, Uludag H, Zernicke RF, Winn SR, Sebald W (2005) Bone marrow cells from normal and ovariectomized rats respond differently to basic fibroblast growth factor and bone morphogenetic protein 2 treatment in vitro. Tissue Eng. 11, 634–644. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Li Y, Zhou Q, Ding Y (2011) Estrogen deficiency leads to impaired osteogenic differentiation of periodontal ligament stem cells in rats. Tohoku J. Exp. Med. 223, 177–186. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Yan M, Yu Y, Wu J, Yu J, Fan Z (2013) Estrogen deficiency inhibits the odonto/osteogenic differentiation of dental pulp stem cells via activation of the NF‐kappaB pathway. Cell Tissue Res. 352, 551–559. [DOI] [PubMed] [Google Scholar]
- 13. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Yan M, Wang Y, Lei G, Yu Y, Zhao C et al (2013) Proliferation and osteo/odontoblastic differentiation of stem cells from dental apical papilla in mineralization‐inducing medium containing additional KH(2)PO(4). Cell Prolif. 46, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang S, Mu J, Fan Z, Yu Y, Yan M, Lei G et al (2012) Insulin‐like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 8, 346–356. [DOI] [PubMed] [Google Scholar]
- 16. Jani TS, DeVecchio J, Mazumdar T, Agyeman A, Houghton JA (2010) Inhibition of NF‐kappaB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) or oxaliplatin. J. Biol. Chem. 285, 19162–19172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A (2006) BMS‐345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin. Cancer Res. 12, 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW et al (2003) BMS‐345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF‐kappa B‐dependent transcription in mice. J. Biol. Chem. 278, 1450–1456. [DOI] [PubMed] [Google Scholar]
- 19. Greenberg JA, Somme S, Russnes HE, Durbin AD, Malkin D (2008) The estrogen receptor pathway in rhabdomyosarcoma: a role for estrogen receptor‐beta in proliferation and response to the antiestrogen 4'OH‐tamoxifen. Cancer Res. 68, 3476–3485. [DOI] [PubMed] [Google Scholar]
- 20. Vanderhorst VG, Gustafsson JA, Ulfhake B (2005) Estrogen receptor‐alpha and ‐beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J. Comp. Neurol. 488, 152–179. [DOI] [PubMed] [Google Scholar]
- 21. Nair HB, Luthra R, Kirma N, Liu YG, Flowers L, Evans D et al (2005) Induction of aromatase expression in cervical carcinomas: effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 65, 11164–11173. [DOI] [PubMed] [Google Scholar]
- 22. Wazer DE, Joyce M, Solares G, Schmidt‐Ullrich R (1991) Proliferative inhibition of human breast carcinoma cells by high concentration estradiol does not alter radiosensitivity. Breast Cancer Res. Treat. 18, 141–148. [DOI] [PubMed] [Google Scholar]
- 23. Motylewska E, Lawnicka H, Melen‐Mucha G (2007) Oestradiol and tamoxifen inhibit murine Colon 38 cancer growth and increase the cytotoxic effect of fluorouracil. Endokrynol. Pol. 58, 426–434. [PubMed] [Google Scholar]
- 24. Yamaguchi M, Weitzmann MN (2009) The estrogen 17beta‐estradiol and phytoestrogen genistein mediate differential effects on osteoblastic NF‐kappaB activity. Int. J. Mol. Med. 23, 297–301. [PubMed] [Google Scholar]
- 25. Iejima D, Sumita Y, Kagami H, Ando Y, Ueda M (2007) Odontoblast marker gene expression is enhanced by a CC‐chemokine family protein MIP‐3alpha in human mesenchymal stem cells. Arch. Oral Biol. 52, 924–931. [DOI] [PubMed] [Google Scholar]
- 26. Chen S, Gluhak‐Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L et al (2009) Runx2, osx, and dspp in tooth development. J. Dent. Res. 88, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Komori T (2010) Regulation of osteoblast differentiation by runx2. Adv. Exp. Med. Biol. 658, 43–49. [DOI] [PubMed] [Google Scholar]
- 28. Ni P, Fu S, Fan M, Guo G, Shi S, Peng J et al (2011) Preparation of poly(ethylene glycol)/polylactide hybrid fibrous scaffolds for bone tissue engineering. Int. J. Nanomedicine 6, 3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wade‐Gueye NM, Boudiffa M, Vanden‐Bossche A, Laroche N, Aubin JE, Vico L et al (2012) Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge. Bone 50, 1064–1073. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi T (2011) Overexpression of Runx2 and MKP‐1 stimulates transdifferentiation of 3T3‐L1 preadipocytes into bone‐forming osteoblasts in vitro. Calcif. Tissue Int. 88, 336–347. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Tu Q, Grosschedl R, Kim MS, Griffin T, Drissi H et al (2011) Roles of SATB2 in osteogenic differentiation and bone regeneration. Tissue Eng. Part A 17, 1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Celil AB, Hollinger JO, Campbell PG (2005) Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J. Cell. Biochem. 95, 518–528. [DOI] [PubMed] [Google Scholar]
- 33. Chen HY, Zhang X, Chen SF, Zhang YX, Liu YH, Ma LL et al (2012) The protective effect of 17beta‐estradiol against hydrogen peroxide‐induced apoptosis on mesenchymal stem cell. Biomed. Pharmacother. 66, 57–63. [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Huang L, Yin Y, Fang Y, Zhao J, Chen J (2008) Estrogen induces endothelial progenitor cells proliferation and migration by estrogen receptors and PI3K‐dependent pathways. Microvasc. Res. 75, 45–52. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M (2010) 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB‐dependent pathway. Mol. Cell. Endocrinol. 317, 31–43. [DOI] [PubMed] [Google Scholar]
- 36. Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ et al (2009) Akt mediates 17beta‐estradiol and/or estrogen receptor‐alpha inhibition of LPS‐induced tumor necresis factor‐alpha expression and myocardial cell apoptosis by suppressing the JNK1/2‐NFkappaB pathway. J. Cell Mol. Med. 13, 3655–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stice JP, Mbai FN, Chen L, Knowlton AA (2012) Rapid activation of nuclear factor kappaB by 17beta‐estradiol and selective estrogen receptor modulators: pathways mediating cellular protection. Shock 38, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mo MS, Li HB, Wang BY, Wang SL, Zhu ZL, Yu XR (2013) PI3K/Akt and NF‐kappaB activation following intravitreal administration of 17beta‐estradiol: neuroprotection of the rat retina from light‐induced apoptosis. Neuroscience 228, 1–12. [DOI] [PubMed] [Google Scholar]
- 39. Liao L, Yang X, Su X, Hu C, Zhu X, Yang N et al (2013) Redundant miR‐3077‐5p and miR‐705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 4, e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim T, Ha H, Shim KS, Cho WK, Ma JY (2013) The anti‐osteoporotic effect of Yijung‐tang in an ovariectomized rat model mediated by inhibition of osteoclast differentiation. J. Ethnopharmacol. 146, 83–89. [DOI] [PubMed] [Google Scholar]
- 41. Soysa NS, Alles N (2009) NF‐kappaB functions in osteoclasts. Biochem. Biophys. Res. Commun. 378, 1–5. [DOI] [PubMed] [Google Scholar]
- 42. Jones WK, Brown M, Ren X, He S, McGuinness M (2003) NF‐kappaB as an integrator of diverse signaling pathways: the heart of myocardial signaling? Cardiovasc. Toxicol. 3, 229–254. [DOI] [PubMed] [Google Scholar]
- 43. Paula‐Silva FW, Ghosh A, Silva LA, Kapila YL (2009) TNF‐alpha promotes an odontoblastic phenotype in dental pulp cells. J. Dent. Res. 88, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang J, Zhang C, Tani‐Ishii N, Shi S, Wang CY (2005) NF‐kappaB activation in human dental pulp stem cells by TNF and LPS. J. Dent. Res. 84, 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsui S, Yasui T, Uemura H, Yamamoto S, Matsuzaki T, Tsuchiya N et al (2011) Induction of circulating monocyte chemoattractant protein‐1 in women with gonadotropin‐releasing hormone agonist. J. Reprod. Immunol. 90, 227–234. [DOI] [PubMed] [Google Scholar]
- 46. D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C et al (2008) Estrogen deficiency increases osteoclastogenesis up‐regulating T cells activity: a key mechanism in osteoporosis. Bone 43, 92–100. [DOI] [PubMed] [Google Scholar]
- 47. Sun S, Guo H, Zhang J, Yu B, Sun K, Jin Q (2013) Adenovirus‐mediated expression of bone morphogenetic protein‐2 activates titanium particle‐induced osteoclastogenesis and this effect occurs in spite of the suppression of TNF‐alpha expression by siRNA. Int. J. Mol. Med. 32, 403–409. [DOI] [PubMed] [Google Scholar]
- 48. Mucci JM, Scian R, De Francesco PN, Garcia FS, Ceci R, Fossati CA et al (2012) Induction of osteoclastogenesis in an in vitro model of Gaucher disease is mediated by T cells via TNF‐alpha. Gene 509, 51–59. [DOI] [PubMed] [Google Scholar]
- 49. Cai WW, Zhang MH, Yu YS, Cai JH (2013) Treatment with hydrogen molecule alleviates TNFalpha‐induced cell injury in osteoblast. Mol. Cell. Biochem. 373, 1–9. [DOI] [PubMed] [Google Scholar]
- 50. Lee HL, Yi T, Woo KM, Ryoo HM, Kim GS, Baek JH (2010) Msx2 mediates the inhibitory action of TNF‐alpha on osteoblast differentiation. Exp. Mol. Med. 42, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]


