Abstract
Background
Aortic stenosis is a frequent valvular disease especially in elderly patients. Catheter-based valve implantation has emerged as a valuable treatment approach for these patients being either at very high risk for conventional surgery or even deemed inoperable. The German Aortic Valve Registry (GARY) provides data on conventional and catheter-based aortic procedures on an all-comers basis.
Methods and results
A total of 13 860 consecutive patients undergoing repair for aortic valve disease [conventional surgery and transvascular (TV) or transapical (TA) catheter-based techniques] have been enrolled in this registry during 2011 and baseline, procedural, and outcome data have been acquired. The registry summarizes the results of 6523 conventional aortic valve replacements without (AVR) and 3464 with concomitant coronary bypass surgery (AVR + CABG) as well as 2695 TV AVI and 1181 TA interventions (TA AVI). Patients undergoing catheter-based techniques were significantly older and had higher risk profiles. The stroke rate was low in all groups with 1.3% (AVR), 1.9% (AVR + CABG), 1.7% (TV AVI), and 2.3% (TA AVI). The in-hospital mortality was 2.1% (AVR) and 4.5% (AVR + CABG) for patients undergoing conventional surgery, and 5.1% (TV AVI) and AVI 7.7% (TA AVI).
Conclusion
The in-hospital outcome results of this registry show that conventional surgery yields excellent results in all risk groups and that catheter-based aortic valve replacements is an alternative to conventional surgery in high risk and elderly patients.
Keywords: Aortic stenosis, Surgery, Catheter-based valve replacement, GARY
Introduction
Aortic stenosis is the most frequent type of valvular heart disease in the Western Countries and presents mostly in an advanced age as a calcific form. The prognosis is poor once the patient becomes symptomatic. Surgical valve replacement is the established standard management, which alleviates symptoms and improves survival.1 Valvuloplasty of the stenosed valve has been over many years a palliative option for the short term for highly selected, inoperable patients. Recently, catheter-based valve implantations have become an alternative for selected, particularly elderly patients.2–4 Smaller, randomized studies confirmed acceptable outcomes in high risk and inoperable patients5,6 for the transvascular (TV) as well as the transapical (TA) approach when compared with conservative or surgical management. This was confirmed in larger registries with longer term follow-up7–14. There are, however, no larger series available comparing conventional surgery with the novel techniques.
In Germany, catheter-based aortic valve implantations have rapidly been accepted at many centres and are performed at increasing numbers. The German Aortic Valve Registry (GARY) is a joint project of the German Cardiac Society (DGK) and the German Society for Thoracic and Cardiovascular Surgery (DGTHG) with the aim to collect complete data on aortic valve interventions for aortic stenosis across Germany. This is the first report on the acute in-hospital outcome in patients recruited in 2011.
Methods
Patients undergoing invasive treatment for acquired aortic valve stenosis [aortic valve surgery as conventional aortic valve replacement (AVR) or Ross and David procedures, trancatheter aortic valve intervention (AVI), or aortic valvuloplasty] in participating centres have consecutively been enrolled in the registry. Transcatheter AVI was divided in the retrograde TV techniques (transfemoral, direct aortic, and transsubclavian access) and the antegrade transapical access (TA). The only exclusion criterion is missing agreement for participation by the patient. The study period started on 1 July 2010 and patient recruitment is scheduled up to the end of 2015. The protocol requests a follow-up at 30 days, 1, 3, and 5 years after the index aortic valve procedure. The protocol of the registry has been previously described in detail.15 Briefly, data from different data sets (baseline, procedural, and outcome data) have been combined in an electronic case report form. Data completeness has been verified by an electronic tool on the basis of the hospitals' reimbursement requests while data accuracy has been monitored by a multistage plausibility check combined with an on-site data verification on a randomly selected 5% of the participating hospitals (audit). Data management and statistical analysis has been performed by the BQS Institute for Quality and Patient safety (www.bqs-institut.de).
In Germany, it is mandatory for reimbursement to deliver a data set of specific cardiac interventions to the independent institute for Quality Assurance (AQUA, Institut für Angewandte Qualitätsförderung und Forschung im Gesundheitswesen, Göttingen, Germany). The data and results are published annually in a quality report and allow the evaluation of the enrolment completeness of GARY. In addition, all sites were asked to confirm in writing that the reported data are consistent with the patients reported to AQUA excluding patients refusing to give informed consent for the registry (∼3–5%).
The present analysis comprises the in-hospital outcome of all patients who underwent conventional aortic valve replacement (AVR) without (n = 6523) or with (n = 3462) concomitant coronary bypass surgery and patients who were treated with TV AVI (n = 2694) or TA AVI (n = 1181) in the year 2011. Patients treated with other surgical procedures of the aortic valve (e.g. Ross, David; n = 354) or balloon valvuloplasty alone (n = 48) are not included in this analysis.
Statistics
The descriptive analysis includes surgical AVR without coronary artery bypass grafting (CABG), surgical AVR with CABG, TV AVI, and TA AVI. Continuous scaled variables were expressed as means ± standard deviation. Statistical significance was tested two-sided with the alpha level of 5%. Comparison of baseline between the four procedures was done by the Kruskal–Wallis rest (any differences) or Mann–Whitney U test (pairwise) for continuous variables. Categorical variables were analysed by Pearson χ2 test, respectively. Fisher's exact test where applicable. Pairwise results were corrected with the Bonferroni–Holm–Shaffer procedure for multiple comparison. Expected values of the EuroSCORE and the German AV-Score were used to analyse risk-stratified observed in-hospital mortality. It is well known that the EUROScore overestimates the operative risk, but it is still widely used. The recently published German AV-Score focuses specifically on aortic valves and has been developed to better reflect the real-world care. It is calculated from 15 variables including age (five risk classes), gender, body mass index (two risk classes), New York Heart Association class (NYHA), myocardial infarction within 3 weeks, critical pre-operative status, pulmonary hypertension, rhythm, left ventricular ejection fraction (two risk classes), redo procedure, endocarditis, peripheral arterial disease, chronic obstructive lung disease (two risk classes), renal failure, and emergency procedure.16
Responsibility, independency, and funding of the registry
The responsible body of the registry is a non-profit organization named Deutsches Aortenklappenregister gGmbH founded by the DGK and DGTHG.
The responsible societies and the BQS Institute are by the virtue of their constitutions independent organizations in both—legal and scientific—views. The registry receives financial support in the format of unrestricted grants by medical device companies (Edwards Lifesciences, JenaValve Technology, Medtronic, Sorin, St Jude Medical, Symetis SA), which however have neither access to data nor influence on their publication.
Results
Centre participation and implantation numbers
The following analysis is based on 13 860 consecutive patients who underwent aortic valve interventions from January 1 to 31 December 2011 covering ∼55% of all aortic interventions in Germany during this period. The data are derived from 78 sites (out of 96 in Germany), of which 62 sites contributed data from the beginning of the year. Since several centres joined only in the course of the year 2011, the number of patients analysed in this manuscript does not match with the number of all aortic procedures performed in Germany. Here, we summarize the results of 6537 conventional aortic valve replacements without and 3464 with concomitant coronary bypass surgery. The transcatheter data set includes 2695 TV and 1181 TA interventions.
Patient selection
The decision for a transcatheter AVI was made in most cases by a Heart Team (TV 86.1, TA 90.4%), but in some patients either a cardiologist (TV 8.7. TA 0.7%) or a cardiac surgeon (TV 5.2. TA 8.9%) decided alone.
Reasons for choosing a transcatheter valve implantation over a conventional AVR were high patient age (TV 69, TA 72%), frailty (TV 44, TA 48%), overall high-operative risk defined as log. EuroScore > 20% or STS-score > 10% (TV 64, TA 65%), porcelain aorta (TV 5, TA 11%), or concomitant diseases limiting life expectancy (TV 8, TA 11%). The patients' wish played a minor role as an additional factor (TV 29, TA 15%).
Patient characteristics and risk profile
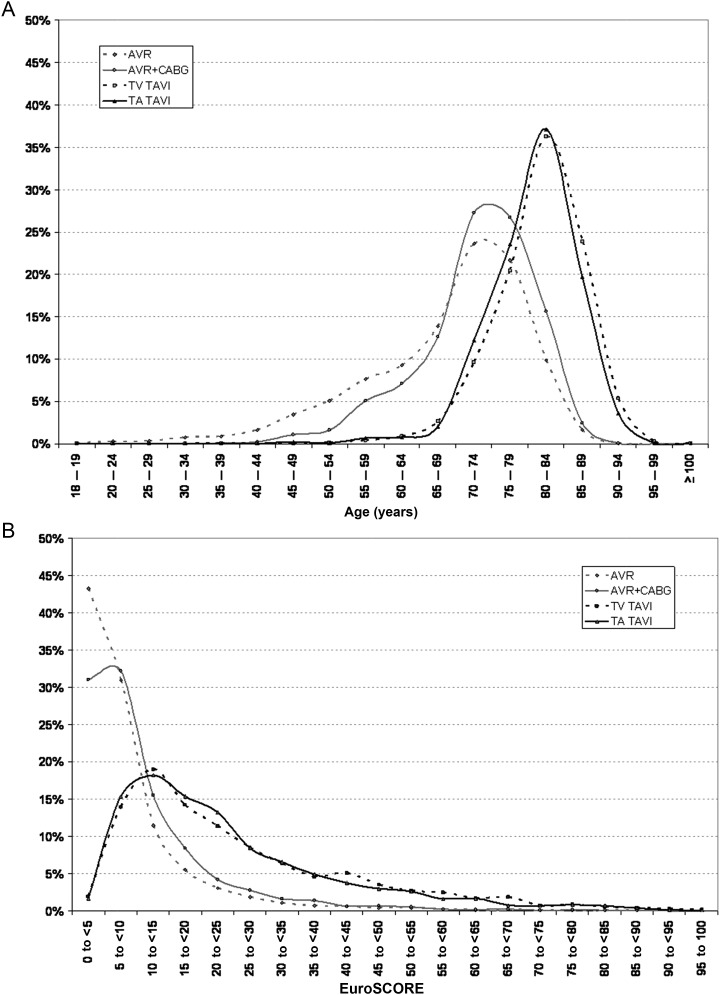
Patients undergoing either conventional AVR or transcatheter AVI differed markedly in baseline characteristics and risk profile. Most transcatheter aortic valve intervention (TAVI) patients (TV 86.3, TA 84.0%) were older than 75 years, whereas this was only the case for a minority of patients undergoing conventional AVR (33.3% without CABG/44.9% with CABG, Figure 1). The mean logistic EUROScore was significantly higher in the TAVI groups (TV 25.9; TA 24.5%) in comparison with patients who underwent conventional AVR without (8.8%) or with concomitant CABG (11.0%). This is concordant with the fact that the TAVI cohort had more cardiovascular risk factors (Table 1).
Figure 1.
Age (A) and risk (B) distribution.
Table 1.
Baseline characteristics
| Conventional AVR |
TAVI |
P-value of global hypothesis |
P-values of pairwise comparisons [alpha level has to be corrected by the Bonferoni–Holm–Shaffer method for multiple comparisons (6-3-3-3-2-1-rule)] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without CABG | With CABG | Transvascular | Transapical | H0: no differences between any procedures | H0: AVR = AVR + CABG | H0: AVR = TV TAVI | H0: AVR = TA TAVI | H0: AVR + CABG = TV TAVI | H0: AVR + CABG = TA TAVI | H0: TV TAVI = TA TAVI | |
| n | 6523 | 3462 | 2694 | 1181 | |||||||
| Mean age (years) | 68.3 ± 11.3 | 72.5 ± 8.0 | 81.1 ± 6.2 | 80.3 ± 6.1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Female | 2543 (39.0%) | 984 (28.4%) | 1583 (58.8%) | 588 (49.8%) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Mean BMI (kg/m2) | 28.1 ± 4.9 | 28.2 ± 4.4 | 27.0 ± 4.9 | 27.2 ± 5.0 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 | <0.001 | 0.123 |
| New York Heart Association (%) | |||||||||||
| Class I | 489 (7.5) | 197 (5.7) | 92 (3.4) | 40 (3.4) | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 1.000 |
| Class II | 1977 (30.3) | 874 (25.2) | 297 (11.0) | 129 (10.9) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.956 |
| Class III | 3726 (57.1) | 2168 (62.6) | 1968 (73.1) | 883 (74.8) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.268 |
| Class IV | 331 (5.1) | 223 (6.4) | 337 (12.5) | 129 (10.9) | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | 0.180 |
| CAD | 1213 (18.6) | 3362 (97.1) | 1444 (53.6) | 663 (56.1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.151 |
| Myocardial infarction (%) | |||||||||||
| Within 21 days | 49 (0.8) | 171 (4.9) | 91 (3.4) | 22 (1.9) | <0.001 | <0.001 | <0.001 | 0.001 | 0.003 | <0.001 | 0.009 |
| 22–90 days | 46 (0.7) | 89 (2.6) | 74 (2.7) | 38 (3.2) | <0.001 | <0.001 | <0.001 | <0.001 | 0.690 | 0.256 | 0.407 |
| More than 90 days | 224 (3.4) | 290 (8.4) | 266 (9.9) | 153 (13.0) | <0.001 | <0.001 | <0.001 | <0.001 | 0.044 | <0.001 | 0.005 |
| Previous PCI (%) | 522 (8.0) | 599 (17.3) | 780 (29.0) | 348 (29.5) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.759 |
| Previous cardiac surgery (%) | 612 (9.4) | 220 (6.4) | 475 (17.7) | 348 (29.6) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Pulmonary hypertension (%) | 697 (10.8) | 380 (11.1) | 1063 (39.8) | 273 (23.4) | <0.001 | 0.635 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Insulin-dependent DM (%) | 532 (8.2) | 448 (12.9) | 359 (13.3) | 206 (17.5) | <0.001 | <0.001 | <0.001 | <0.001 | 0.675 | <0.001 | 0.001 |
| Arterial hypertension (%) | 5148 (79.5) | 2968 (86.1) | 2321 (86.4) | 1058 (90.0) | <0.001 | <0.001 | <0.001 | <0.001 | 0.737 | 0.001 | 0.002 |
| Atrial fibrillation (%) | 1039 (15.9) | 521 (15.0) | 779 (28.9) | 348 (29.5) | <0.001 | 0.259 | <0.001 | <0.001 | <0.001 | <0.001 | 0.730 |
| Obstructive lung disease (%) | |||||||||||
| On medication | 407 (6.2) | 243 (7.0) | 406 (15.1) | 166 (14.1) | <0.001 | 0.136 | <0.001 | <0.001 | <0.001 | <0.001 | 0.431 |
| Without medication | 248 (3.8) | 179 (5.2) | 126 (4.7) | 75 (6.4) | <0.001 | 0.002 | 0.055 | <0.001 | 0.407 | 0.120 | 0.033 |
| LVEF (%) | |||||||||||
| <30 | 199 (3.1) | 176 (5.1) | 250 (9.3) | 88 (7.5) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | 0.064 |
| 30–50 | 1381 (21.2) | 972 (28.1) | 817 (30.3) | 418 (35.4) | <0.001 | <0.001 | <0.001 | <0.001 | 0.054 | <0.001 | 0.002 |
| Decompensated heart failure (%) | |||||||||||
| Within 12 months | 1047 (16.5) | 711 (21.2) | 1159 (44.9 | 449 (39.5) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| Dialysis (%) | |||||||||||
| Acute | 94 (1.4) | 55 (1.6) | 43 (1.6) | 39 (3.3) | <0.001 | 0.603 | 0.571 | <0.001 | 1.000 | 0.001 | 0.001 |
| Chronic | 79 (1.2) | 52 (1.5) | 83 (3.1) | 54 (4.6) | <0.001 | 0.230 | <0.001 | <0.001 | <0.001 | <0.001 | 0.023 |
| Active endocarditis (%) | 216 (3.3) | 51 (1.5) | 2 (0.1 | 1 (0.1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 |
| Cardiogenic shock (%) | |||||||||||
| Within 48 h | 64 (1.0) | 53 (1.5) | 104 (3.9 | 22 (1.9 | <0.001 | 0.019 | <0.001 | 0.015 | <0.001 | 0.423 | 0.001 |
| Cardiopulmonary resuscitation (%) | |||||||||||
| Within 48 h | 4 (0.1) | 9 (0.3) | 5 (0.2) | 1 (0.1) | 0.050 | 0.016 | 0.134 | 0.564 | 0.601 | 0.468 | 0.674 |
| Patient on mechanical ventilation (%) | 38 (0.6) | 17 (0.5) | 72 (2.7) | 8 (0.7) | <0.001 | 0.670 | <0.001 | 0.681 | <0.001 | 0.490 | <0.001 |
| Neurologicaldysfunction (%) | 489 (7.5) | 304 (8.8) | 352 (13.1) | 174 (14.7) | <0.001 | 0.027 | <0.001 | <0.001 | <0.001 | <0.001 | 0.169 |
| Preop inotropic support (%) | 71 (1.1) | 52 (1.5) | 158 (5.9) | 18 (1.5) | <0.001 | 0.086 | <0.001 | 0.234 | <0.001 | 1.000 | <0.001 |
| Preop IABP (%) | 11 (0.2) | 17 (0.5) | 2 (0.1) | 0 (0.0) | 0.001 | 0.005 | 0.369 | 0.391 | 0.004 | 0.010 | 1.000 |
| Atherosclerotic disease (%) | 1310 (20.1) | 951 (27.5) | 735 (27.3) | 505 (42.8) | <0.001 | <0.001 | <0.001 | <0.001 | 0.863 | <0.001 | <0.001 |
| Peripheral (extremities) | 298 (4.6) | 402 (11.6) | 442 (16.4) | 335 (28.4) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Carotid disease | 358 (5.5) | 457 (13.2) | 302 (11.2) | 209 (17.7) | <0.001 | <0.001 | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 |
| Aortic aneurism | 636 (9.8) | 225 (6.5) | 90 (3.3) | 67 (5.7) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.332 | 0.001 |
| Other | 216 (3.3) | 133 (3.9) | 132 (4.9) | 101 (8.6) | <0.001 | 0.169 | <0.001 | <0.001 | 0.050 | <0.001 | <0.001 |
| Intervention (%) | |||||||||||
| Elective | 5554 (85.1) | 2750 (79.4) | 2088 (77.5) | 1009 (85.4) | <0.001 | <0.001 | <0.001 | 0.824 | 0.069 | <0.001 | <0.001 |
| Urgent | 891 (13.7) | 643 (18.6) | 567 (21.0) | 167 (14.1) | <0.001 | <0.001 | <0.001 | 0.646 | 0.017 | <0.001 | <0.001 |
| Emergent | 78 (1.2) | 69 (2.0) | 39 (1.4 | 5 (0.4) | <0.001 | 0.002 | 0.357 | 0.014 | 0.117 | <0.001 | 0.005 |
BMI, body mass index; CAD, coronary artery disease; PCI, percutaneous coronary intervention; DM, diabetes mellitus; IABP, intra-aortic balloon pump.
The comparison between the TV and TA TAVI patients revealed a significantly higher rate of pulmonary hypertension in the TV group and more patients with pre-operative acute and chronic renal failure with dialysis. The TA patients more often suffered from both peripheral artery disease (28.4 vs. 16.4%) and carotid disease (17.7 vs. 11.2%). In the TV group a considerable number of patients was treated <48 h after or still in cardiogenic shock (3.9 vs. 1.9% TA-AVI) and/or still under inotropic support (5.9 vs. 1.5% TA-AVI).
No difference among all groups was found regarding mean and maximum aortic valve gradients. However, the valve area was somewhat lower in both TAVI groups (Table 2). Likewise, the rate of mild-to-moderate concomitant mitral valve regurgitation was higher in the TAVI-treated patients.
Table 2.
Valve-related baseline characteristics
| Conventional AVR |
TAVI |
P-value of global hypothesis |
P-values of pairwise comparisons [alpha level has to be corrected by the Bonferoni–Holm–Shaffer method for multiple comparisons (6-3-3-3-2-1-rule)] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without CABG | With CABG | Transvascular | Transapical | H0: no differences between any procedures | H0: AVR = AVR + CABG | H0: AVR = TV TAVI | H0: AVR = TA TAVI | H0: AVR + CABG = TV TAVI | H0: AVR + CABG = TA TAVI | H0: TV TAVI = TA TAVI | |
| Aortic valve orifice (echo; cm2) | 0.86 ± 0.66 | 0.85 ± 0.50 | 0.68 ± 0.25 | 0.68 ± 0.20 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.416 |
| Delta Pmean (mmHg) | 44.2 ± 19.5 | 41.1 ± 17.6 | 46.1 ± 17.6 | 43.7 ± 16.3 | <0.001 | <0.001 | 0.118 | 0.014 | <0.001 | 0.002 | <0.001 |
| Delta Pmax (mmHg) | 70.1 ± 29.8 | 65.7 ± 26.7 | 73.4 ± 26.7 | 71.4 ± 25.0 | <0.001 | <0.001 | 0.012 | 0.760 | <0.001 | <0.001 | 0.052 |
| Degree of calcification (%) | |||||||||||
| Low | 7.5 | 7.1 | 5.6 | 3.7 | <0.001 | 0.535 | 0.002 | <0.001 | 0.025 | <0.001 | 0.018 |
| Average | 24.1 | 24.9 | 28.8 | 23.5 | <0.001 | 0.422 | <0.001 | 0.668 | 0.001 | 0.366 | 0.001 |
| Heavy | 57.3 | 62.0 | 63.9 | 65.9 | <0.001 | <0.001 | <0.001 | <0.001 | 0.149 | 0.023 | 0.263 |
| Concomitant mitral regurgitation (%) | |||||||||||
| None | 40.0 | 35.9 | 11.6 | 17.4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Grade I | 44.7 | 48.2 | 57.8 | 54.4 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.055 |
| Grade II | 10.9 | 12.9 | 26.6 | 26.0 | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | 0.719 |
| Grade III | 3.7 | 2.7 | 3.9 | 2.0 | 0.001 | 0.009 | 0.715 | 0.003 | 0.012 | 0.193 | 0.002 |
| Grade IV | 0.7 | 0.3 | 0.1 | 0.2 | <0.001 | 0.011 | <0.001 | 0.056 | 0.126 | 0.739 | 0.590 |
Intra-operative parameters
The procedure times were significantly longer for conventional operations (AVR: 183 ± 69 min, AVR + CABG: 242 ± 79 min) as opposed to the TAVI (TV: 92 ± 51 min, TA: 100 ± 65 min). Following the trend of the past years a biological prosthesis was implanted in most patients undergoing conventional valve replacement (83.8% in patients without concomitant CABG, 92.2% in patients with CABG). In the TV group the CoreValve revalving system (Medtronic, Minneapolis, MN, USA) was used in >60% of the cases, whereas the Sapien valve (Edwards Lifesciences, Irvine USA) was almost exclusively used for the TA route.
Overall, TAVI patients were treated with prostheses from Edwards Lifesciences (n = 2061) or Medtronic CoreValve (n = 1614). Only a minority was treated with second generation prostheses from Symetis SA (n = 53) or JenaValve (n = 53). The remaining patients (n = 94) received either devices under clinical evaluation or no device due to different reasons.
In the majority of patients, the native aortic valve was treated. Within the TAVI group, however, 81 patients (2.1%) received a valve-in-valve due to deterioration of a previously implanted surgical bioprosthesis.
Most TA patients (95%) were operated under general anaesthesia, whereas this was only the case in 44% of the TV patients. A small proportion of TAVI patients underwent the procedure electively with under support of a heart lung machine (0.5%).
A true ‘Heart team approach’ with at least one cardiologist and one cardiac surgeon performing the procedure together was present in 40.3% of the TV and 69.4% of the TA cases. The mean fluoroscopy time was lower in the TA (9 ± 19 min) than in the TV group (18 ± 11 min).
The intra-operative complication rates were low. Conversion to sternotomy was necessary in 2.0% of the TA and 1.4% of the TV cases. Unplanned cardiopulmonary bypass was necessary in 2.1% of the TA cases and 0.7% of the TV cases.
Vascular complications (intra- and post-operative) were expectedly highest in the TV AVI patients (15.9%). Other complication rates are listed in Table 3.
Table 3.
Procedural data
| Conventional AVR |
TAVI |
|||
|---|---|---|---|---|
| Without CABG | With CABG | Transvascular (%) | Transapical (%) | |
| Heart team approach | 78 (1.3%) | 57 (1.8%) | 1.085 (40.3) | 820 (69.4) |
| General anaesthesia | — | — | 1.179 (43.7) | 1.121 (94.9) |
| Procedure duration (min) | 183 ± 69 | 242 ± 79 | 92 ± 51 | 100 ± 65 |
| Mean radiation time (min) | — | — | 18 ± 11 | 9 ± 19 |
| Balloon predilatation | 2.332 (86.5) | 1.112 (94.2) | ||
| Rapid pacing during implantation | 1.546 (57.4) | 1.046 (88.5) | ||
| Complications (%) | ||||
| Coronary occlusion | 6 (0.1) | 6 (0.2) | 7 (0.3) | 0 (0.0) |
| Pericardial tamponade | 3 (0.05) | 0 (0.0) | 38 (1.4) | 2 (0.2) |
| Device embolization | 0 (0.0) | 0 (0.0) | 16 (0.6) | 4 (0.3) |
| Left ventricular decompensation | 12 (0.2) | 28 (0.8) | 24 (0.9) | 28 (2.4) |
| Vascular complications | 117 (1.8) | 78 (2.3) | 427 (15.9) | 48 (4.1) |
| Conversion to sternotomy | N/A | N/A | 38 (1.4) | 24 (2.0) |
‘LV decompensation’ is defined as (i) need for prolonged (>24 h) high-dose inotropic support and/or (ii) need for implantation of an intra-aortic balloon pump or extracorporeal membrane oxygenation and/or (iii) pulmonary oedema.
Peri-operative outcome
The different pre-operative risk profiles led to differences in the unadjusted post-operative outcome parameters. In-hospital mortality for all patients (including emergent procedures, endocarditis, and similar high-risk patients) was 2.1% in the surgical group for AVR alone and 4.5% for patients with AVR plus CABG. In the TV group, the in-hospital mortality was 5.1% (CoreValve 4.3%, Sapien 5.8%, n.s.) and in-patients who underwent TA AVI 7.7% (Figure 2). The in-hospital stroke rate was low in all groups with 1.3% (AVR), 1.9% (AVR + CABG), 1.7% (TV TAVI), and 2.3% (TA TAVI). More outcome data are given in Table 4.
Figure 2.
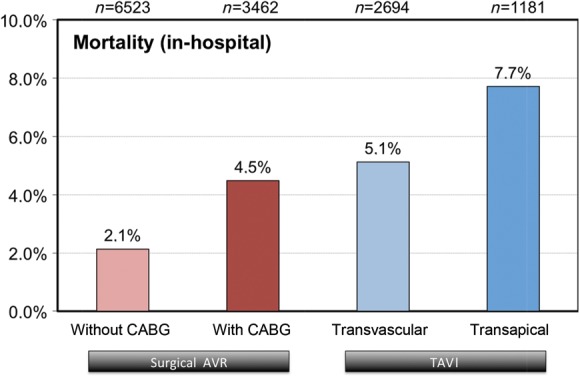
In-hospital outcome.
Table 4.
Unadjusted outcome
| Conventional AVR |
TAVI |
|||
|---|---|---|---|---|
| Without CABG | With CABG | Transvascular | Transapical | |
| n | 6523 | 3462 | 2694 | 1181 |
| In-hospital mortality (%) | 139 (2.1) | 155/(4.5) | 138 (5.1) | 91 (7.7) |
| Cerebrovascular event (%) | 80 (1.3) | 61 (1.9) | 43 (1.7) | 25 (2.2) |
| (redo-)thoracotomy (%) | 453 (6.9) | 262 (7.6) | 25 (0.9) | 52 (4.4) |
| Valve-related redo intervention (%) | 22 (0.3) | 6 (0.2) | 11 (0.4) | 3 (0.3) |
| Duration of mechanical ventilation (h) | 32 ± 111 | 47 ± 156 | 29 ± 108 | 37 ± 124 |
| Post-operative need for haemodialysis (%) | ||||
| Acute | 203 (3.2) | 165 (4.9) | 75 (2.9) | 73 (6.7) |
| Chronic | 16 (0.3) | 10 (0.3) | 2 (0.1) | 8 (0.7) |
| New pacemaker | 236 (4.6) | 108 (3.9) | 390 (23.7) | 71 (9.9) |
| Transfusions (%) | ||||
| 0–2 Units | 4.579 (70.6) | 1.993 (58.0) | 2.336 (88.5) | 875 (74.6 |
| >2 Units | 1.908 (29.4) | 1.445 (42.0) | 305 (11.5) | 298 (25.4) |
| Residual aortic regurgitation (%) | ||||
| None | — | — | 990 (37.2) | 653 (57.3) |
| Grade I | — | — | 1.474 (55.5) | 440 (38.6) |
| Grade II | — | — | 185 (7.0) | 39 (3.4) |
| Grade III/IV | — | — | 9 (0.3) | 7 (0.6) |
‘Redo thoracotomy’ is defined as post-procedural re-exploration for any reason.
The incidence of post-interventional atrioventriclar block requiring pacemaker implantation was highest in TV-treated patients (25.0%) when compared with the TA group (11.3%) and conventionally treated patients (5.2% without; 4.5% with CABG).
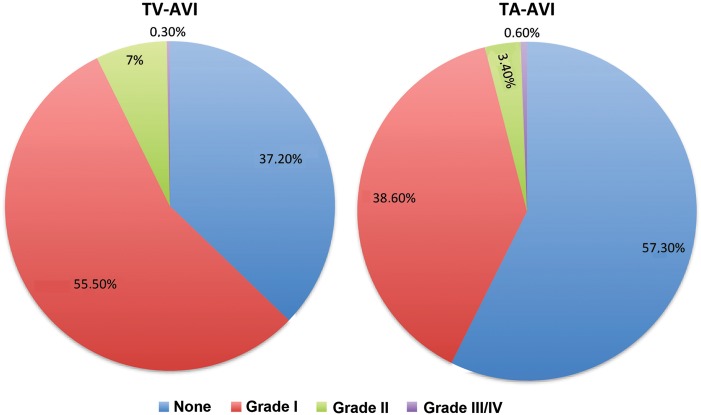
Catheter-based valve implantations may be associated with residual aortic regurgitation. The GARY registry found very good results in both catheter-treated groups with either no regurgitation (TV 37.2, TA 57.3%) or non-valve academic research consortium-relevant regurgitation grade I (TV 55.5, TA 38.6%). Grade II-regurgitation occurred more often in the TV group (7.3%) when compared with the TA group (4.0%; Table 4). The duration of post-intervention mechanical ventilation was shorter in the TV group in comparison with TA (29 ± 108 vs. 37 ± 124 h) (Figure 3).
Figure 3.
Residual aortic regurgitation.
Risk assessment
The EuroScore grossly overestimated the real mortality in the GARY registry for all groups (each EuroScore vs. observed mortality: conventional surgery without CABG 8.8 vs. 2.1%; conventional surgery with CABG 11.0 vs. 4.5%; TV-AVI 25.9 vs. 5.1%; TA-AVI 24.5 vs. 7.7%).
The German AV-Score demonstrated reliable risk discrimination for the low-to-intermediate risk patients in all groups. In the TV-AVI group and in conventionally operated patients without bypass surgery, the observed mortality for high-risk patients was significantly lower than expected from the German AV-Score (Figure 4).
Figure 4.
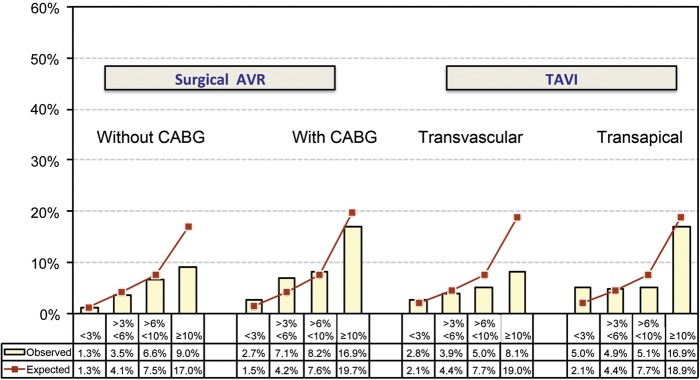
Observed (bars) vs. predicted (lines) in-hospital mortality by the German AV-Score. Low-risk groups showed a good correlation. The risk was lower in the high-risk groups undergoing aortic valve replacement without CABG and transvascular TAVI.
Discussion
The presented data pools for the first time a large number of patients who received either conventional or catheter-based aortic valve replacement. Conventional surgery was associated in all risk groups with an excellent outcome, which is in concordance with or even better than recent publications. Furthermore, the data support the favourable results of catheter-based aortic valve replacements from previous randomized studies and registries in an all comer real-world patient population. The direct comparison with conventional surgical procedures allows to better define the role of this new technique in the future. Overall, we could confirm that the vast majority of patients treated by this new technology were at very high risk or elderly as shown in Figure 1. The mean EuroScore was more than two-fold higher in the TAVI patients, accordingly.
This finding as well as the increasing total number of aortic valve interventions in Germany ( + 7.3%)17 suggests that this new technique was used regularly in patients who previously were not considered for valve surgery. Accordingly, the baseline characteristics in the treatment arms are considerably different, which thereby precludes reasonable risk adjustments. Similarly, the use of the EuroScore as comparator considerably overestimates the true risk in this patient population.18
For a better comparison, the German AV-Score was calculated which is derived from a 2007 data set of patients undergoing only aortic valve surgery.16 This score better represents this patient population, as proven in the low-risk groups, but overestimated the risk in high-risk groups. This may be explained by the fact that TAVI was done only at a very low rate in 2008 and some particular high-risk patients not captured by the AV score have shifted over the time to TAVI.1 This obviously holds especially true for elderly patients, since most patients beyond the age of 85 were treated with a percutaneous approach. It is reassuring that TAVI in this high-risk subset resulted in lower mortality at least as predicted by the score. Vice versa, patients with a high AV-score undergoing conventional surgery also had a lower risk as predicted in the pre-TAVI era. When TAVI was not routinely performed in 2009 the mortality of conventional AVR with concomitant CABG was 36% higher, without concomitant CABG even 45% higher. Accordingly, our registry seems to disclose an important paradigm change in outcome and care.
During most of the observation period in 2011 only the self-expanding Medtronic CoreValve and the balloon-expandable Edwards Sapien were commercially available. Other valves played therefore no role in this report. Accordingly, in TV procedures the Medtronic CoreValve prosthesis was used in 60%, whereas the TA approach was largely dominated by Edwards Sapien valves. There was a trend that TA procedures had a higher mortality when compared with the TV approach. However, we would not overestimate this result because of the great variation observed. Specifically, risk factors were unevenly distributed between both groups. Furthermore, this technique is frequently used as an alternate route of access when the femoral approach is not feasible leading to a certain degree of pre-selection bias. The difference should also not be related to the valve types, since mortalities in the TV groups were the same.
Since residual aortic regurgitation has been described as independent risk factor for mortality, it was reassuring that the success rate seen in our registry was very high (residual aortic regurgitation ≤ grade 1 in 92.7% with TV-AVI and even 96.0% in TA-AVI). The yet unknown durability will stay a matter of debate.
With respect to complications, stroke during valve replacement is the most feared event. However, the rate of strokes was similar in the TV and the TA approach and was in the range of patients undergoing combined valve and bypass surgery. The rate for new pacemakers was several folds higher than in conventional surgery and as expected from previous reports more frequent in the CoreValve patients and therefore more frequent after the TV approach.19 The very low rates of coronary occlusions in all groups is reassuring—especially for the TAVI approach, in which this complication has long been feared. With the exception of vascular complications there was no major difference between the different routes of access.
The current data are reassuring that the results seen in the randomized trials as well as in other registries are reproducible in such a large-scale data collection. Most other studies primarily report the 30 days outcome, which, however, is very close to the in-hospital outcome in our study. The length of hospitalization was rather similar and accordingly no critical factor in comparing the techniques. The experience with the procedure varied considerably as many centres just started the catheter valve programme and were still in the learning curve or just released from proctoring. One aim of this registry is to monitor the quality of indications and procedural outcome, and to provide a benchmark report back to the individual centres. Naturally, registries have limitations and cannot replace randomized evidence. Several centres joined the registry only in the course of 2011 and only 78 out of 96 potential centres contributed to GARY, with the result that only ∼55% of all procedures performed in Germany at that year have been captured. However, through verification of the reported data with the compulsory quality reports ensured a high grade of completeness as far as possible. In addition, registries have the inherent limitation to be not completely controlled with the risk of under-reporting of events or complications.
However, only ‘all-comer’ registers allow to assess the penetration and use of a new technology and become particularly important when a traditional approach like conventional surgery is under debate. Furthermore, it is usually difficult to enter suitable patients in adequate numbers into randomized trials, which leads to selection bias. The high number of patients in registries must therefore, at least partly, be used for further placing new technology in patient care.
Conclusions
The in-hospital outcome results of this registry confirm that conventional surgery yields excellent results in all risk groups and that catheter-based aortic valve replacements are an alternative to conventional surgery in high risk and elderly patients. Although the results are very promising, long-term follow-up needs to confirm this conclusion in terms of mortality and quality of life. The GARY registry will over the next years provide solid data to further define the role of catheter-based aortic valve procedures.
Funding
The responsible body of the registry is a nonprofit organization named Deutsches Aortenklappenregister gGmbH founded by the DGK and DGTHG. The registry receives financial support in the format of unrestricted grants by medical device companies (Edwards Lifesciences, JenaValve Technology, Medtronic, Sorin, St. Jude Medical, Symetis S.A.). Open Access was funded by The Deutsche Aortenklappenreister gGmbH.
Conflict of interest: CWH received speakers honoraria from Medtronic and Edwards and serves in the advisory board of Medtronic. HM received proctor salary and/or speakers honoraria from Abbott, Medtronic, Edwards, St. Jude Medical, and Symetis. HRF is a cofounder of the TAVI company JenaValve and receives consulting fees. RZ received research grants from Medtronic and Edwards. The other authors have no conflicts to report.
References
- 1.Joint Task Force on the Management of Valvular Heart Disease of the European Society of, C., S. European Association for Cardio-Thoracic, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs10.9. [DOI] [Google Scholar]
- 2.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.CIR.0000047200.36165.B.8. [DOI] [PubMed] [Google Scholar]
- 3.Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, Doss M, Borger MA, Schuler G, Glogar D, Fehske W, Wolner E, Mohr FW, Mack M. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation. 2007;116(11 Suppl):I240–I245. doi: 10.1161/CIRCULATIONAHA.106.677237. [DOI] [PubMed] [Google Scholar]
- 4.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.58288.2. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S P.T. Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa100823.2. [DOI] [PubMed] [Google Scholar]
- 6.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ P.T. Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa110351.0. [DOI] [PubMed] [Google Scholar]
- 7.Di Mario C, Eltchaninoff H, Moat NE, Goicolea J, Ussia GP, Kala P, Wenaweser P, Zembala M, Nickenig G, Barrero E, Snow P, Iung B, Zamorano JL, Schuler G, Corti R, Alfieri O, Prendergast B, Ludman P, Windecker S, Sabate M, Gilard M, Witowski A, Danenberg H, Schroeder E, Romeo F, Macaya C, Derumeaux G, Maggioni A, Tavazzi L. The 2001-12 pilot european sentinel registry of transcatheter aortic valve implantation: in-hospital results in 4571 patients. EuroIntervention. 2013;8:1362–1371. doi: 10.4244/EIJV8I12A20.9. [DOI] [PubMed] [Google Scholar]
- 8.Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau-Gouge P, Tribouilloy C, Debrux JL, Pavie A, Gueret P F.R. Investigators. Transcatheter aortic valve implantation: early results of the FRANCE (FRench aortic national coreValve and edwards) registry. Eur Heart J. 2011;32:191–197. doi: 10.1093/eurheartj/ehq26.1. [DOI] [PubMed] [Google Scholar]
- 9.Kempfert J, Rastan A, Holzhey D, Linke A, Schuler G, van Linden A, Blumenstein J, Mohr FW, Walther T. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation. 2011;124(11 Suppl):S124–S129. doi: 10.1161/CIRCULATIONAHA.110.01342.5. [DOI] [PubMed] [Google Scholar]
- 10.Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, Romano M, Serruys P, Wimmer-Greinecker G Group PEI. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq42.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (united kingdom transcatheter aortic valve implantation) registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.05.0. [DOI] [PubMed] [Google Scholar]
- 12.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochelliere R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.01.4. [DOI] [PubMed] [Google Scholar]
- 13.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, Antoniucci D, Napodano M, De Carlo M, Fiorina C, Ussia GP. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.94653.3. [DOI] [PubMed] [Google Scholar]
- 14.Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, Hambrecht R, Sack S, Hauptmann KE, Richardt G, Figulla HR, Senges J, German I. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. doi: 10.1093/eurheartj/ehq33.9. [DOI] [PubMed] [Google Scholar]
- 15.Beckmann A, Hamm C, Figulla HR, Cremer J, Kuck KH, Lange R, Zahn R, Sack S, Schuler GC, Walther T, Beyersdorf F, Bohm M, Heusch G, Funkat AK, Meinertz T, Neumann T, Papoutsis K, Schneider S, Welz A, Mohr FW, Board GE. The german aortic valve registry (GARY): a nationwide registry for patients undergoing invasive therapy for severe aortic valve stenosis. Thorac Cardiovasc Surg. 2012;60:319–325. doi: 10.1055/s-0032-132315.5. [DOI] [PubMed] [Google Scholar]
- 16.Kötting J, Schiller W, Beckmann A, Schäder E, Döbler K, Hamm C, Veit C, Welz A. German aortic valve score: a new scoring system for prediction of mortality related to aortic valve procedures in adults. Eur J Cardiothorac Surg. 2013;43:971–977. doi: 10.1093/ejcts/ezt114. [DOI] [PubMed] [Google Scholar]
- 17.Funkat AK, Beckmann A, Lewandowski J, Frie M, Schiller W, Ernst M, Hekmat K. Cardiac surgery in Germany during 2011: a report on behalf of the German society for thoracic and cardiovascular surgery. Thorac Cardiovasc Surg. 2012;60:371–382. doi: 10.1055/s-0032-132672.4. [DOI] [PubMed] [Google Scholar]
- 18.Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, Kempfert J, Falk V, Mohr FW. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg. 2009;87:1440–1445. doi: 10.1016/j.athoracsur.2009.01.05.7. [DOI] [PubMed] [Google Scholar]
- 19.Erkapic D, Kim WK, Weber M, Mollmann H, Berkowitsch A, Zaltsberg S, Pajitnev DJ, Rixe J, Neumann T, Kuniss M, Sperzel J, Hamm CW, Pitschner HF. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. 2010;12:1188–1190. doi: 10.1093/europace/euq09.4. [DOI] [PubMed] [Google Scholar]