Abstract
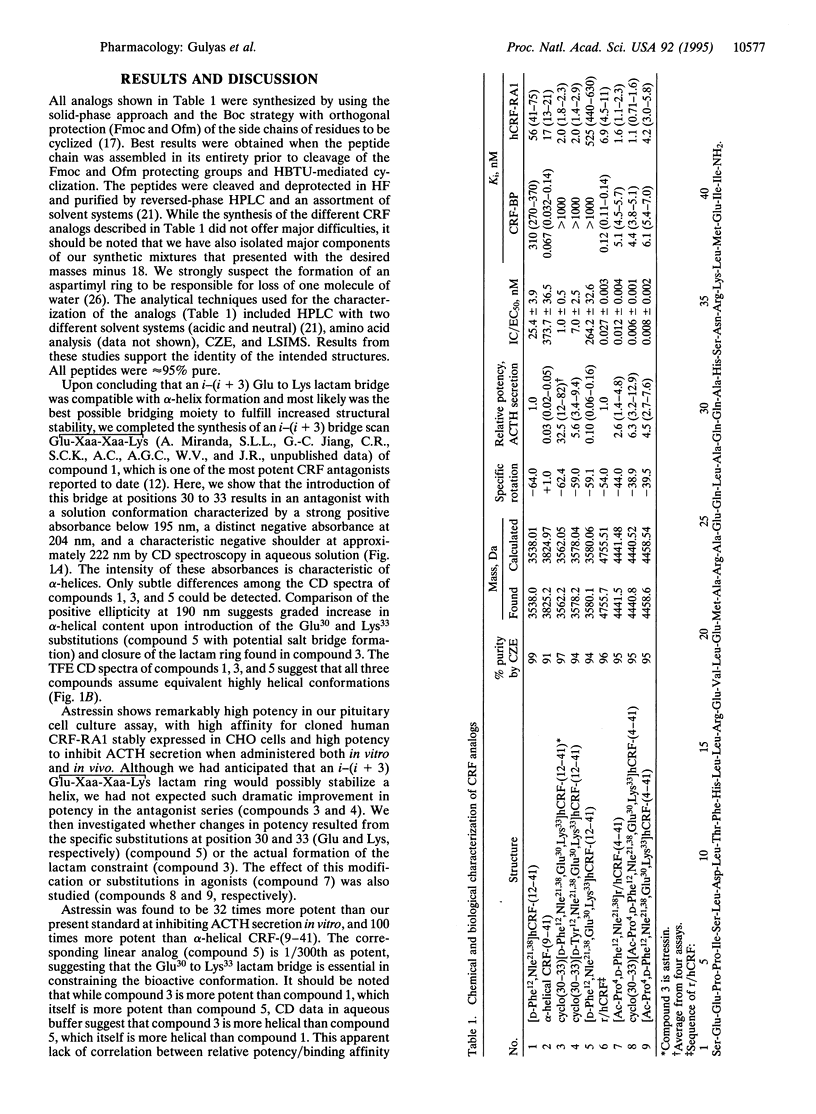
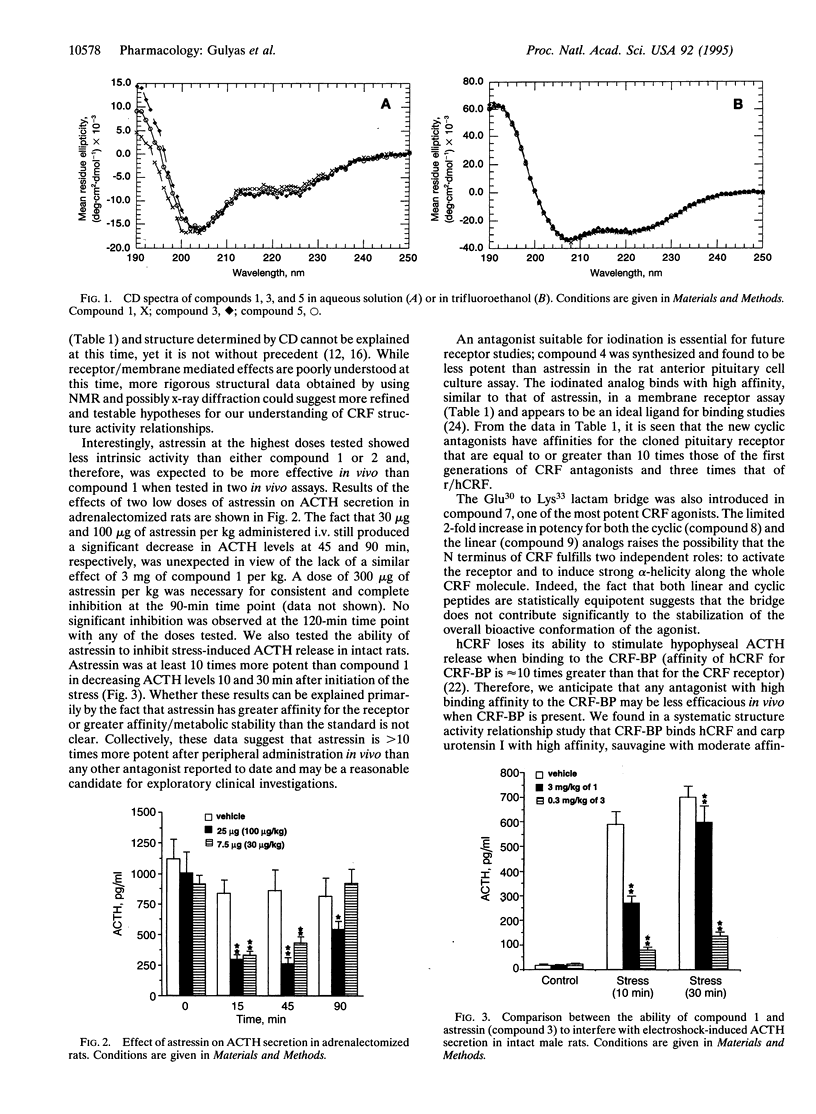
Predictive methods, physicochemical measurements, and structure activity relationship studies suggest that corticotropin-releasing factor (CRF; corticoliberin), its family members, and competitive antagonists (resulting from N-terminal deletions) usually assume an alpha-helical conformation when interacting with the CRF receptor(s). To test this hypothesis further, we have scanned the whole sequence of the CRF antagonist [D-Phe12,Nle21,38]r/hCRF-(12-41) (r/hCRF, rat/human CRF; Nle, norleucine) with an i-(i + 3) bridge consisting of the Glu-Xaa-Xaa-Lys scaffold. We have found astressin [cyclo(30-33)[D-Phe12,Nle21,38,Glu30,Lys33]r/ hCRF(12-41)] to be approximately 30 times more potent than [D-Phe12,Nle21,38]r/hCRF-(12-41), our present standard, and 300 times more potent than the corresponding linear analog in an in vitro pituitary cell culture assay. Astressin has low affinity for the CRF binding protein and high affinity (Ki = 2 nM) for the cloned pituitary receptor. Radioiodinated [D-125I-Tyr12]astressin was found to be a reliable ligand for binding assays. In vivo, astressin is significantly more potent than any previously tested antagonist in reducing hypophyseal corticotropin (ACTH) secretion in stressed or adrenalectomized rats. The cyclo(30-33)[Ac-Pro4,D-Phe12,Nle21,38,Glu30,Lys33++ +]r/hCRF-(4-41) agonist and its linear analog are nearly equipotent, while the antagonist astressin and its linear form vary greatly in their potencies. This suggests that the lactam cyclization reinstates a structural constraint in the antagonists that is normally induced by the N terminus of the agonist.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C. P., Pearse R. V., 2nd, O'Connell S., Rosenfeld M. G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993 Dec;11(6):1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Chen R., Lewis K. A., Perrin M. H., Vale W. W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer T. P., Rose G. D. Side-chain entropy opposes alpha-helix formation but rationalizes experimentally determined helix-forming propensities. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5937–5941. doi: 10.1073/pnas.89.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix A. M., Heimer E. P., Wang C. T., Lambros T. J., Fournier A., Mowles T. F., Maines S., Campbell R. M., Wegrzynski B. B., Toome V. Synthesis, biological activity and conformational analysis of cyclic GRF analogs. Int J Pept Protein Res. 1988 Dec;32(6):441–454. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Gaylinn B. D., Harrison J. K., Zysk J. R., Lyons C. E., Lynch K. R., Thorner M. O. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone. Mol Endocrinol. 1993 Jan;7(1):77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- Hernandez J. F., Kornreich W., Rivier C., Miranda A., Yamamoto G., Andrews J., Taché Y., Vale W., Rivier J. Synthesis and relative potencies of new constrained CRF antagonists. J Med Chem. 1993 Oct 1;36(20):2860–2867. doi: 10.1021/jm00072a004. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Pearse R. V., 2nd, Lin C. R., Rosenfeld M. G. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Lovenberg T. W., Liaw C. W., Grigoriadis D. E., Clevenger W., Chalmers D. T., De Souza E. B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A., Koerber S. C., Gulyas J., Lahrichi S. L., Craig A. G., Corrigan A., Hagler A., Rivier C., Vale W., Rivier J. Conformationally restricted competitive antagonists of human/rat corticotropin-releasing factor. J Med Chem. 1994 May 13;37(10):1450–1459. doi: 10.1021/jm00036a010. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pallai P. V., Mabilia M., Goodman M., Vale W., Rivier J. Structural homology of corticotropin-releasing factor, sauvagine, and urotensin I: circular dichroism and prediction studies. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6770–6774. doi: 10.1073/pnas.80.22.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M. H., Donaldson C. J., Chen R., Lewis K. A., Vale W. W. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993 Dec;133(6):3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Perrin M., Donaldson C., Chen R., Blount A., Berggren T., Bilezikjian L., Sawchenko P., Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E., Behan D. P., Fischer W. H., Linton E. A., Lowry P. J., Vale W. W. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991 Jan 31;349(6308):423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Rivier C., Shen G. H. In the rat, endogenous nitric oxide modulates the response of the hypothalamic-pituitary-adrenal axis to interleukin-1 beta, vasopressin, and oxytocin. J Neurosci. 1994 Apr;14(4):1985–1993. doi: 10.1523/JNEUROSCI.14-04-01985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., Rivier C., Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science. 1984 May 25;224(4651):889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- Romier C., Bernassau J. M., Cambillau C., Darbon H. Solution structure of human corticotropin releasing factor by 1H NMR and distance geometry with restrained molecular dynamics. Protein Eng. 1993 Feb;6(2):149–156. doi: 10.1093/protein/6.2.149. [DOI] [PubMed] [Google Scholar]
- Sutton S. W., Behan D. P., Lahrichi S. L., Kaiser R., Corrigan A., Lowry P., Potter E., Perrin M. H., Rivier J., Vale W. W. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995 Mar;136(3):1097–1102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- Tam J. P., Riemen M. W., Merrifield R. B. Mechanisms of aspartimide formation: the effects of protecting groups, acid, base, temperature and time. Pept Res. 1988 Sep-Oct;1(1):6–18. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Yamamoto G., Bruhn T., Douglas C., Dalton D., Rivier C., Rivier J. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- Vita N., Laurent P., Lefort S., Chalon P., Lelias J. M., Kaghad M., Le Fur G., Caput D., Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993 Nov 29;335(1):1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]



