Abstract
Ethanol and its metabolite, acetaldehyde, are the definite carcinogens for esophageal squamous cell carcinoma (ESCC), and reduced catalytic activity of aldehyde dehydrogenase 2 (ALDH2), which detoxifies acetaldehyde, increases the risk for ESCC. However, it remains unknown whether the ALDH2 genotype influences the level of acetaldehyde-derived DNA damage in the esophagus after ethanol ingestion. In the present study, we administered ethanol orally or intraperitoneally to Aldh2-knockout and control mice, and we quantified the level of acetaldehyde-derived DNA damage, especially N2-ethylidene-2’-deoxyguanosine (N2-ethylidene-dG), in the esophagus. In the model of oral ethanol administration, the esophageal N2-ethylidene-dG level was significantly higher in Aldh2-knockout mice compared with control mice. Similarly, in the model of intraperitoneal ethanol administration, in which the esophagus is not exposed directly to the alcohol solution, the esophageal N2-ethylidene-dG level was also elevated in Aldh2-knockout mice. This result indicates that circulating ethanol-derived acetaldehyde causes esophageal DNA damage, and that the extent of damage is influenced by knockout of Aldh2. Taken together, our findings strongly suggest the importance of acetaldehyde-derived DNA damage which is induced in the esophagus of individuals with ALDH2 gene impairment. This provides a physiological basis for understanding alcohol-related esophageal carcinogenesis.
Keywords: Carcinogenesis, esophageal squamous cell carcinoma, acetaldehyde, acetaldehyde-derived DNA damage, DNA adduct
Introduction
Squamous cell carcinoma (SCC) is the predominant histological type of esophageal cancer worldwide, particularly in east Asian countries [1]. Epidemiological studies have clearly shown that chronic ethanol consumption and acetaldehyde produced from ethanol contained in alcoholic beverages increase the risk of cancers including esophageal SCC (ESCC) [2]. The International Agency for Research on Cancer certified acetaldehyde from consuming alcoholic beverages as ‘the group I carcinogens’ [3]. Ethanol is absorbed mainly from the duodenum and jejunum, and is transported to the liver, where it is metabolized to acetaldehyde by alcohol dehydrogenase, and acetaldehyde is subsequently detoxified to acetic acid by aldehyde dehydrogenase 2 (ALDH2) [4]. Heavy alcohol consumers with the inactive ALDH2 genotype are reported to have a greater risk for ESCC [5-7]. Thus, reduced catalytic activity of ALDH2 is considered to play crucial roles in the development of ESCC [1,5,8].
Acetaldehyde is a highly reactive compound that can interact with DNA to form DNA adducts, which induce DNA mutations [9-13]. Previous reports have shown that there are several kinds of acetaldehyde-derived DNA adducts including N2-ethylidene-2’-deoxyguanosine (N2-ethylidene-dG), N2-ethyl-2’-deoxyguanosine (N2-Et-dG), and 1’,N2-propano-2’-deoxyguanosine [14-16]. Among them, N2-ethylidene-dG is the most abundant DNA adduct derived from acetaldehyde [15,17]. Matsuda et al. reported that the N2-ethylidene-dG level in the liver or stomach is elevated by ethanol consumption in experimental mouse models [16,17]. Thus, quantification of acetaldehyde-derived DNA adducts provide an index of direct DNA damage caused by acetaldehyde [15-18].
Since the report by Slaughter et al. in 1953, the multicentric development of SCC has been recognized in the squamous epithelium of the esophagus as well as in the head and neck region. Such development is termed ‘field cancerization’ [19], and its occurrence is closely associated with alcohol consumption and ALDH2 gene polymorphism [20-22]. It has been suggested that genetic damage induced by acetaldehyde accumulates in the esophageal mucosa and that this damage is involved in the multicentric development of SCC. However, it remains unclear how alcohol consumption and impairment of ALDH2 promote ESCC development.
To examine whether the ALDH2 genotype determines the level of acetaldehyde-derived DNA damage in the esophagus associated with ethanol consumption, we administered ethanol orally and intraperitoneally in Aldh2-knockout (Aldh2–/–) mice and quantified the N2-ethylidene-dG levels in the esophagus.
Materials and methods
Aldh2-knockout mice
Ten-week-old male Aldh2–/– mice [23], which had been backcrossed with C57BL/6, were obtained from the Department of Environmental Health, University of Occupational and Environmental Health (Fukuoka, Japan). Control C57BL/6 mice (Aldh2+/+) were purchased from Charles River Japan (Yokohama, Japan). The genotype of Aldh2 was determined by polymerase chain reaction as described previously [23,24].
Free ethanol-drinking model in mice
Aldh2–/– mice and control mice were allowed to drink 5% ethanol or water for 8 weeks (n = 5 per group). The mice were sacrificed, and esophageal tissue specimens were collected, frozen in liquid nitrogen, and stored at -80°C until analyzed. The mice were used in conformity with the regulations of the committee on animal experiments of Kyoto University.
Intraperitoneal ethanol injection model in mice
To examine whether circulating ethanol contributes to induction of DNA damage in the esophagus, we developed an animal model in which Aldh2–/– and control mice were injected with 1 mL of 5% ethanol intraperitoneally. The mice were sacrificed at 1, 4, or 24 h after the injection of ethanol (n = 3, at each time point). Esophageal tissues were collected and stored at -80°C. This experiment conformed to the regulations of the committee on animal experiments of the National Cancer Center Hospital East (Kashiwa, Japan).
DNA isolation, digestion, and quantification of N2-ethylidene-dG
DNA was isolated from tissue specimens using a Gentra Puregene Tissue Kit (Qiagen Inc., Valencia, CA), according to the manufacturer’s instructions. We quantified the N2-ethylidene-dG levels in the esophagus of Aldh2–/– and control mice. As shown in Figure 1, N2-ethylidene-dG is the direct DNA adduct derived from acetaldehyde, and N2-Et-dG is the DNA adduct generated by reduction of N2-ethylidene-dG. To quantify the N2 ethylidene-dG level, we used the method of Wang et al. [25]. Briefly, we added reducing agent, NaBH3CN (final concentration: 100 mM), to the isolated DNA samples. During this procedure, N2-ethylidene-dG is converted to stable N2-Et-dG. Because the endogenous N2-Et-dG level is extremely low, the amount of N2-Et-dG converted from N2-ethylidene-dG can be used to estimate the endogenous N2-ethylidene-dG level [15,25]. The DNA adduct standard, N2-Et-dG, and its stable isotope, [U-15N5]-labeled N2-Et-dG, were synthesized as described previously [16,26]. Twenty micrograms of DNA sample was digested as described previously [17] and then subjected to liquid chromatography tandem mass spectrometry (LC/MS/MS). LC/MS/MS analyses were performed using a Shimadzu LC system (Shimadzu Corporation, Kyoto, Japan) interfaced with a Quattro Ultimo triple-stage quadruple MS (Waters/Micromass UK Ltd, Manchester, UK) according to the methods as described previously [17].
Figure 1.
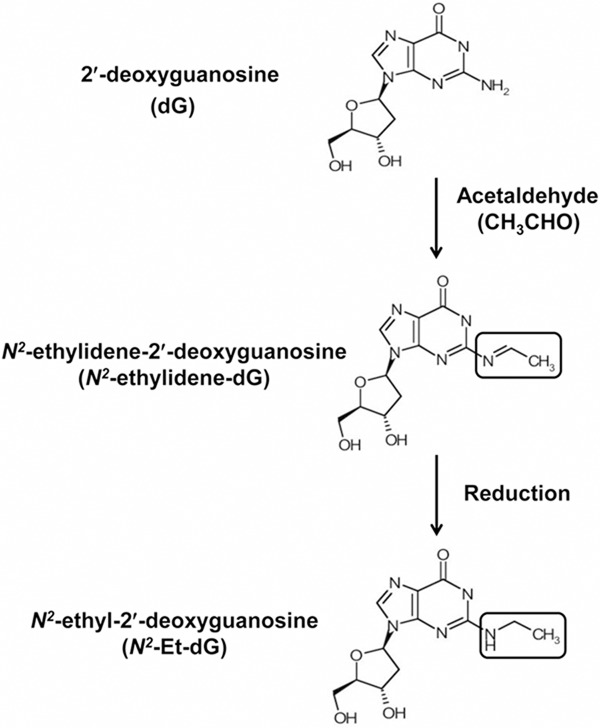
Scheme of the formation of acetaldehyde-derived DNA adducts. Acetaldehyde binds to 2’-deoxyguanosine (dG) and forms N2-ethylidene-2’-deoxyguanosine (N2-ethylidene-dG). N2-ethyl-2’-deoxyguanosine (N2-Et-dG) is generated by reduction of N2-ethylidene-dG.
Statistical analyses
Statistical analyses were performed using SPSS statistics software (version 17; SPSS Inc., Chicago, IL). Data are presented as mean ± standard deviation (SD). The data were analyzed using two-tailed paired t test. P values < 0.05 were considered significant.
Results
N2-ethylidene-dG level in the esophagus after ethanol consumption in mice
Among the water-drinking groups, the average N2-ethylidene-dG level in the esophagus was 1.61 ± 0.63 adducts/107 bases in control mice and 0.80 ± 0.22 adducts/107 bases in Aldh2–/– mice. Among the ethanol-drinking groups, the level of N2-ethylidene-dG was significantly elevated in Aldh2–/– mice (9.73 ± 2.33 adducts/107 bases) but did not increase in control mice (1.62 ± 0.30 adducts/107 bases) (P < 0.001 vs. control mice, n = 5) (Figure 2). Ethanol drinking did not induce obvious histopathological changes in the esophagus of these mice (data not shown).
Figure 2.
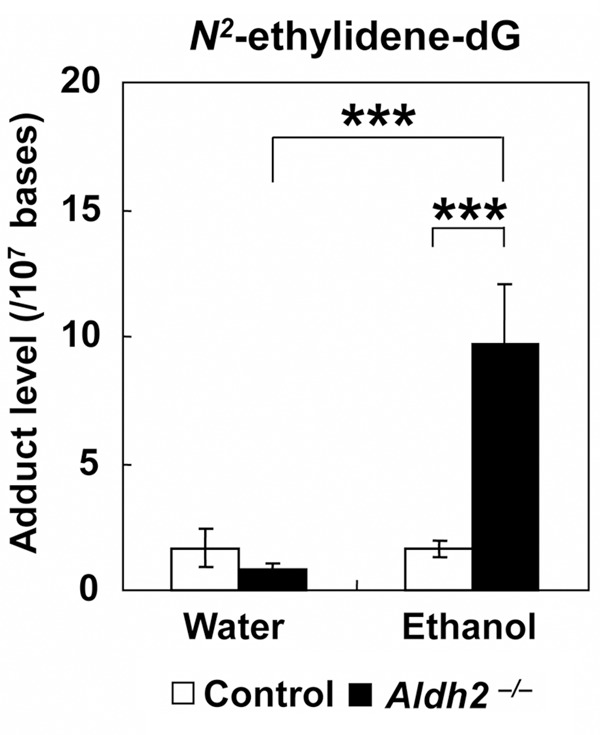
N2-ethylidene-dG levels after ethanol consumption in Aldh2–/– mice. Aldh2–/– and control mice were either allowed to drink 5% ethanol or were given water for 8 weeks, and the esophageal N2-ethylidene-dG level was quantified. The N2-ethylidene-dG level was significantly higher in the esophagus of ethanol-drinking Aldh2–/– mice compared with ethanol-drinking control mice (***P < 0.001) and with water-drinking Aldh2–/– mice (***P < 0.001) (n = 5 in each group).
N2-ethylidene-dG levels in the esophagus after intraperitoneal injection of ethanol in mice
Intraperitoneal injection of ethanol provides a unique experimental model of alcohol-induced damage because the esophagus is not exposed directly to ethanol. Here, we measured the N2-ethylidene-dG levels in the esophagus of Aldh2–/– and control mice after the intraperitoneal injection of ethanol, and we determined how the Aldh2 gene influences the induction of DNA damage caused by acetaldehyde derived from circulating ethanol. The N2-ethylidene-dG levels in the esophagus of control mice at 1, 4, and 24 h after intraperitoneal ethanol injection were 0.71 ± 0.02, 0.79 ± 0.08, and 1.56 ± 0.52 adducts/107 bases, respectively. The N2-ethylidene-dG levels mice were significantly higher in Aldh2–/– mice than in control mice at the same time points; the levels in Aldh2–/– mice were 2.61 ± 1.05 (P = 0.044), 3.76 ± 1.26 (P = 0.028), 2.93 ± 0.47 (P = 0.014) adducts/107 bases (n = 3 at each time point) (Figure 3).
Figure 3.
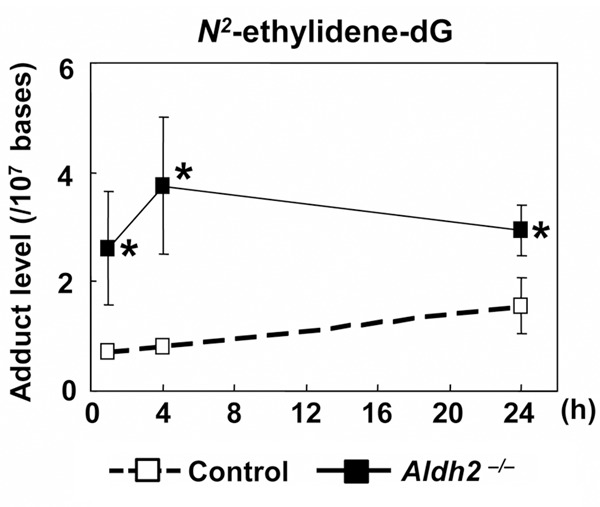
N2-ethylidene-dG levels after intraperitoneal injection of ethanol in Aldh2–/– mice. Aldh2–/– and control mice were injected with 1 mL of 5% ethanol intraperitoneally. N2-ethylidene-dG levels in the esophagus were measured at 1, 4, or 24 h after the injection. The esophageal N2-ethylidene-dG level was significantly higher in Aldh2–/– mice than in control mice at all time points (*P < 0.05 vs. control mice) (n = 3 at each time point).
Discussion
In this study, we found that impairment of the Aldh2 gene and ethanol drinking were closely related to the induction of acetaldehyde-derived DNA damage in the esophagus. In our model of intraperitoneal ethanol administration, in which the esophagus is not exposed directly to ethanol, esophageal DNA damage was related to the circulating ethanol-derived acetaldehyde.
Although epidemiological evidence suggests that acetaldehyde is involved in the carcinogenesis of ESCC [5,20,21,27], it is unknown how acetaldehyde acts on the esophagus. In the present study, acetaldehyde-derived genetic damage was assessed by measuring the N2-ethylidene-dG level in the esophagus. As expected, the esophageal N2-ethylidene-dG level was significantly increased by ethanol consumption in Aldh2–/– mice. This result indicates that the Aldh2 genotype strongly affects accumulation of acetaldehyde-derived DNA damage in the esophagus after ethanol consumption.
One limitation of the experimental approach using oral ethanol consumption is that one cannot determine whether the DNA adduct level is influenced by the direct exposure of the esophagus to the alcohol solution or by acetaldehyde derived from ethanol circulating systematically after having been absorbed from the gastrointestinal tract. Therefore, we established an experimental mouse model in which the esophagus is not exposed directly to the alcohol solution but, instead, the ethanol is injected into the abdominal cavity. In this model, ethanol is absorbed from the peritoneum and is metabolized to acetaldehyde in the liver, and then acetaldehyde circulates and is distributed systematically. Interestingly, even in this experimental model, the acetaldehyde-derived N2-ethylidene-dG level was significantly higher in the esophagus of Aldh2–/– mice than in control mice. As shown in previous clinical reports, acetaldehyde can be detected in the saliva or exhaled breath after alcohol drinking [28,29]. In our in vivo experiments, we cannot exclude the possibility that the esophagus may have been exposed to acetaldehyde derived from these origins and that this might have affected the N2-ethylidene-dG level in the esophagus. Regardless, our data provide important evidence that impairment of ALDH2 is involved in the induction of esophageal DNA adducts caused by acetaldehyde derived from circulating ethanol.
In conclusion, our study strongly suggests the importance of acetaldehyde-derived DNA damage in the alcohol-mediated carcinogenesis of ESCC, especially in individuals with impairment of ALDH2. Understanding the mechanisms responsible for this effect may contribute to the development of ways to prevent ESCC.
Acknowledgements
The authors thank Mari Nakane-Takahashi from the National Cancer Center East for technical assistance. We appreciate the assistance of Hiroshi Nakagawa from the University of Pennsylvania (Division of Gastroenterology, Department of Medicine) for proofreading of the article. This study was supported by the National Cancer Center and Development Fund (36).
Disclosure of conflict of interest
The authors disclose no potential conflicts of interest.
References
- 1.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 3.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama A, Muramatsu T, Omori T, Yokoyama T, Matsushita S, Higuchi S, Maruyama K, Ishii H. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in Japanese alcoholics. Carcinogenesis. 2001;22:433–439. doi: 10.1093/carcin/22.3.433. [DOI] [PubMed] [Google Scholar]
- 6.Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, Tsunoda T, Kamatani N, Kubo M, Nakamura Y, Matsuda K. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 7.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama A, Omori T, Yokoyama T. Alcohol and aldehyde dehydrogenase polymorphisms and a new strategy for prevention and screening for cancer in the upper aerodigestive tract in East Asians. Keio J Med. 2010;59:115–130. doi: 10.2302/kjm.59.115. [DOI] [PubMed] [Google Scholar]
- 9.Fang JL, Vaca CE. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997;18:627–632. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS, McIntee EJ, Wang M. New DNA adducts of crotonaldehyde and acetaldehyde. Toxicology. 2001;166:31–36. doi: 10.1016/s0300-483x(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng G, Shi Y, Sturla SJ, Jalas JR, McIntee EJ, Villalta PW, Wang M, Hecht SS. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem Res Toxicol. 2003;16:145–152. doi: 10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 12.Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, Kawamoto T. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact. 2010;188:367–375. doi: 10.1016/j.cbi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- 14.Hori K, Miyamoto S, Yukawa Y, Muto M, Chiba T, Matsuda T. Stability of acetaldehyde-derived DNA adduct in vitro. Biochem Biophys Res Commun. 2012;423:642–646. doi: 10.1016/j.bbrc.2012.05.158. [DOI] [PubMed] [Google Scholar]
- 15.Yukawa Y, Muto M, Hori K, Nagayoshi H, Yokoyama A, Chiba T, Matsuda T. Combination of ADH1B*2/ALDH2*2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of Japanese alcoholics. Cancer Sci. 2012;103:1651–1655. doi: 10.1111/j.1349-7006.2012.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda T, Matsumoto A, Uchida M, Kanaly RA, Misaki K, Shibutani S, Kawamoto T, Kitagawa K, Nakayama KI, Tomokuni K, Ichiba M. Increased formation of hepatic N2-ethylidene-2’-deoxyguanosine DNA adducts in aldehyde dehydrogenase 2-knockout mice treated with ethanol. Carcinogenesis. 2007;28:2363–2366. doi: 10.1093/carcin/bgm057. [DOI] [PubMed] [Google Scholar]
- 17.Nagayoshi H, Matsumoto A, Nishi R, Kawamoto T, Ichiba M, Matsuda T. Increased formation of gastric N(2)-ethylidene-2’-deoxyguanosine DNA adducts in aldehyde dehydrogenase-2 knockout mice treated with ethanol. Mutat Res. 2009;673:74–77. doi: 10.1016/j.mrgentox.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Gromadzinska J, Mistry Y, Cordell R, Juren T, Segerback D, Farmer PB. Detection of acetaldehyde derived N(2)-ethyl-2’-deoxyguanosine in human leukocyte DNA following alcohol consumption. Mutat Res. 2012;737:8–11. doi: 10.1016/j.mrfmmm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Muto M, Hitomi Y, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Association of aldehyde dehydrogenase 2 gene polymorphism with multiple oesophageal dysplasia in head and neck cancer patients. Gut. 2000;47:256–261. doi: 10.1136/gut.47.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muto M, Nakane M, Hitomi Y, Yoshida S, Sasaki S, Ohtsu A, Yoshida S, Ebihara S, Esumi H. Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis. 2002;23:1759–1765. doi: 10.1093/carcin/23.10.1759. [DOI] [PubMed] [Google Scholar]
- 22.Muto M, Takahashi M, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008–1012. doi: 10.1093/carcin/bgi035. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa K, Kawamoto T, Kunugita N, Tsukiyama T, Okamoto K, Yoshida A, Nakayama K, Nakayama K. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476:306–311. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 24.Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin Exp Res. 2005;29:1959–1964. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Yu N, Chen L, Villalta PW, Hochalter JB, Hecht SS. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem Res Toxicol. 2006;19:319–324. doi: 10.1021/tx0502948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillestrom PR, Hoberg AM, Weimann A, Poulsen HE. Quantification of 1,N6-etheno-2’-deoxyadenosine in human urine by column-switching LC/APCI-MS/MS. Free Radic Biol Med. 2004;36:1383–1392. doi: 10.1016/j.freeradbiomed.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama A, Kato H, Yokoyama T, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, Watanabe H, Fukuda H, Yoshimizu H. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1851–1859. doi: 10.1093/carcin/23.11.1851. [DOI] [PubMed] [Google Scholar]
- 28.Vakevainen S, Tillonen J, Agarwal DP, Srivastava N, Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin Exp Res. 2000;24:873–877. [PubMed] [Google Scholar]
- 29.Tardif R, Liu L, Raizenne M. Exhaled ethanol and acetaldehyde in human subjects exposed to low levels of ethanol. Inhal Toxicol. 2004;16:203–207. doi: 10.1080/08958370490277272. [DOI] [PubMed] [Google Scholar]


