Abstract
For advanced epithelial ovarian cancer (EOC), time to recurrence (TTR) is an important indicator to gauge the therapeutic efficacy of postoperative adjuvant chemotherapy. Our objective was to determine the genes that could potentially distinguish patients with short versus long TTR after initial administration of platinum-paclitaxel combination chemotherapy in advanced EOC. Tumor samples of 159 patients were obtained during the primary cytoreduction. Array comparative genomic hybridization (CGH) was carried with genomic DNA from 17 EOC samples (8 with TTR > 15 months and 9 with TTR ≤ 6 months) to screen candidate gene set, copy-number changes (CNC) of which were significantly different between early and late relapse cases. Seventeen candidate genes were identified by array CGH. The analysis of consistency between real-time PCR and array CGH revealed that 4 genes displayed consistent results, namely GSTT1, ISG20L1, STARD5 and FREM1. In a 142-case validation set, CNC of 4 candidate genes was evaluated and verified by real-time PCR. Sixty five point five percent of the patients were correctly divided into early (TTR ≤ 10 months) and late (TTR > 10 months) recurrent group by CNC of the 4 genes using discriminant analysis. The results showed that CNC of 4-gene set could potentially determine early (TTR ≤ 10 months) or late relapse (TTR > 10 months) after initial platinum-paclitaxel combination chemotherapy in advanced EOC.
Keywords: Epithelial ovarian cancer, paclitaxel, platinum, recurrence, array comparative genomic hybridization
Introduction
Epithelial ovarian cancer (EOC) is one of the leading causes of death among gynecologic malignancies. Currently, the standard treatment for advanced ovarian cancer is cytoreductive surgery combined with adjuvant paclitaxel/platinum chemotherapy. Approximately 60-70% of patients achieve complete clinical response, whereas a majority suffers recurrence within 2 years, receiving a poor prognosis. Moreover, even among patients with similar tumor stage, differentiation and residual disease, the chemotherapy generates tremendously different outcomes. With the clinical indicators currently used, it is difficult to determine which of the patients will have a poor response to chemotherapy and experience recurrence in the near future.
The high-throughput screening techniques, microarray techniques, which make it easy to indentify target genes throughout the genome, have been widely used in molecular biology since last decade. In the present study, array-comparative genomic hybridization (aCGH) was used to identify the genes with different copy numbers changes (CNC) between early and late-recurrent advanced EOC patients previously treated with initial debulking surgery and adjuvant paclitaxel/platinum chemotherapy. Subsequently, we used real-time quantitative polymerase chain reaction (qPCR) to verify the correlation between CNC in these genes and time to recurrence (TTR) in the validation set.
Material and methods
Tissue specimens
The present study examined EOC patients administered and treated in Cancer Hospital, Chinese Academy of Medical Sciences between January 2004 and October 2010. All pathological sections were reviewed by a pathologist in our hospital. A total of 159 patients were included in this study, and all the samples were obtained from the tumor tissues of the patient’s first surgery and were immediately frozen in liquid nitrogen and stored at -80°C until use. Prior to the experiment, each specimen was split into two parts: one part was fixed with formalin, paraffinembedded, sectioned and stained with hematoxylin and eosin (H&E), whereas the other part was used to extract genomic DNA (using the Qiagen® DNeasy Blood & Tissue Kit, Hilden, Germany). This study was approved by the Cancer Hospital, Chinese Academy of Medical Scien-ces Institutional Review Broad. Informed consent was obtained from all the patients.
Array CGH
Genomic DNA was digested with restriction enzymes and used as a template. In a reaction catalyzed by Exo-Klenow polymerase, Cy3-dUTP and Cy5-dUTP were used to label the PCR products of the control sample (commercially available genomic DNA extracted from women’s peripheral blood) as well as test specimens (genomic DNA of ovarian cancer tissues). Concentrations and incorporation rates were determined for the labeled DNA samples after they were purified. The fluorescence intensity values of Cy3 and Cy5 were calculated and required to be comparable, with the difference below 1.5 fold.
The labeled genomic DNA samples were hybridized with the microarray Human Genome CGH 4*44K (Agilent Oligo aCGH Hybridization Kit, Agilent Technologies, Santa Clara, CA). The hybridized chips were then scanned to generate graphs, which were used to reveal the signal intensity for each probe with Feature Extraction 9.5.3.1 software using the quality-control microarray. All the procedures, such as hybridization, washing, scanning and data extraction were preceded following standard protocols.
CGH Analytics 3.4 (Agilent) and GeneSpring GX 7.3.1 (Agilent) software were used to perform data analysis. DNA analytics was used to calculate log2 ratio for every probe and identified amplified and deleted probes.
Real-time quantitative PCR
First, 200 ng of genomic DNA, purchased from Promega, was used to prepare six solutions of 10-fold serial dilutions, which were used as the templates. Following the instructions for the Takara real-time quantitative PCR kit, a standard curve was generated. The assay was repeated three times for each test sample. Using GAPDH as the internal control and purchased genomic DNA as the control sample, Ct values of 9 fragments of each sample were determined (Ct value stands for the cycle number needed for the fluorescence intensity to reach the threshold in a qPCR reaction). Finally, the relative content of a test sample to the control sample was calculated using the following equations:
Test samples (Test, T): ΔCtT = CtT (target fragment) - CtT (control fragment)
Control sample (Control, C): ΔCtC = CtC (target fragment) - CtC (control fragment)
ΔΔCt = ΔCtT - ΔCtC
Relative content = 2-ΔΔCt
2-ΔΔCt > 1.5 was set for a target gain and 2-ΔΔCt < 0.5 was set for a target loss. The assay used the SYBR® Premix Ex TaqTM kit (Perfect Real Time, Takara, Japan). The reactions were performed using an Mx3000P Real-Time PCR System. The primers for the PCR reactions are listed in Table 1.
Table 1.
Primer Sequences (designed using Primer Express 5.0 software; primer specificity was validated using a BLAST search)
| Gene | Upstream primer | Downstream primer | Tm°C |
|---|---|---|---|
| GSTT1 | 5’CCAAGCTGCACGATAGGTCAC | 5’GGTATGCTACACACAGCTCCAC | 59 |
| ADAMTS17 | 5’CGCTGTGGGTCCAGATGA | 5’AGATTCGGCATTCCCACG | 59 |
| EFTUD1 | 5’CAGCAGGTCTGGGTGTTAGG | 5’AGGGGTTGCAAGCACGTAG | 60 |
| FES | 5’CTGTCACTGAAGAAGGGATTTG | 5’TGTCATGGATGGGACTTTAGAG | 58 |
| STARD5 | 5’CGGCACCTCTGGTAATCTTTG | 5’TAAGCCAGGTGACCAGAGCC | 61 |
| ABHD2 | 5’TTTGTTAACATTCTGGCCTTATC | 5’TTGGAGCTTCTCTACAGTGGTC | 58 |
| ISG20L1 | 5’TTGTGGCTTGAGGGTAGGG | 5’ACCGAGGGCTGTGAATTAGA | 59 |
| ZDHHC11 | 5’CAGTAGAAGTGACCGTGTTGC | 5’GCAGTTTTGATTTAGAAGTCTCCT | 58 |
| CPEB1 | 5’ATGAATTCCTCGGTTTATAGGTG | 5’GATTGCTGGGACAACCAGG | 60 |
| IQGAP1 | 5’AGCGGACCCAATCATGAC | 5’TGAATCTGATCTACCTTCGACC | 58 |
| KIAA1794 | 5’GCCTTGGAGCAGGTTTATCAC | 5’GGCATTGTTGCTTGTTGGG | 61 |
| ECHDC1 | 5’TCCTCAGAGTAACTTCAAATCATC | 5’CAGCTAAGACTAATGGGAAGAAC | 57 |
| SEC23A | 5’ATTTGGTTTAGTAGTTACAACCGTC | 5’CCAACTTATGTGACTAAACGCAG | 59 |
| FREM1 | 5’AACAGGTTCTTCCCACACTCTC | 5’CAGCAGGATGTGGACAGCAA | 60 |
| ZNF354C | 5’TGAGACTATGAAGAGCACTGACTTG | 5’GGGAATATGGTCTTATGAGAGAAAC | 60 |
| NTRK3 | 5’AAGAAAGCCTGATGAAGTCC | 5’GGGCGACGAGGTCACTAAG | 60 |
| AKAP13 | 5’TGTAAGGGATACCCAGGAACG | 5’CCTGGTCTGGCACCAGTTC | 59 |
Statistical methods
The SPSS 18.0 software was used to perform statistical analyses. The Spearman rank correlation coefficient was used to assess the correlation between the real-time PCR results and the aCGH data. The log-rank test was used to examine the correlation between the CNC and TTR. Discriminant analysis was employed to evaluate the predictive accuracy using the CNC. P < 0.05 indicates statistically significant differences.
Results
Array CGH
Patients were classified according to the TTR intervals (from the end of the paclitaxel/platinum chemotherapy to the onset of relapse). The tumor tissues of 8 late-recurrent (TTR > 15 months) and 9 early-recurrent patients (TTR < 6 months) were used for the aCGH analysis. The detailed inclusion criteria were as follows: 1) Histological type was high-grade epithelial ovarian cancer; 2) The patients had not received neoadjuvant chemotherapy; 3) Surgical-pathological stages were III-IV (International Federation of Gynecology and Obstetrics, FIGO staging system); 4) Tumor contents were greater than 70%, as revealed by H&E staining; 5) The patients received primary cytoreductive surgery followed by 6-8 cycles of paclitaxel/platinum chemotherapy before recurrence. The clinical features of the 17 patients, including their ages at diagnosis, residual disease and median TTR were summarized in Table 2.
Table 2.
The clinical features of the 17 patients in the aCGH experiment
| Early recurrence (TTR < 6 months) | Late recurrence (TTR > 15 months) | |
|---|---|---|
| Number of cases | 9 | 8 |
| Median age (years) | 52 | 53 |
| Residual disease | ||
| < 1 cm | 3 | 4 |
| ≥ 1 cm | 6 | 4 |
| Median TTR (months) | 3 | 35 |
| Median OS (months) | 23 | 60 |
OS: overall survival; TTR: time to recurrence.
The CGH Analytics 3.4 software was first used to normalize the fluorescence intensity values of probes within each microarray and between individual microarrays. Subsequently, the GeneSpring 7.3.1 was used to compare the data of early- and late-recurrent patients (adopting ADM-2 as the calculation algorism and 6.0 as the threshold). The results revealed that the two groups displayed apparent differences in CNC. There were 22 probes exhibiting fold changes above 1.50 (gain) or below 0.7 (loss) with P < 0.05. Their chromosomal locations and their residing genes are listed Table 3.
Table 3.
The 22 probes exhibiting significant copy number differences (a fold change > 1.5 or < 0.7 with P < 0.05) between the early- and late-recurrent cases, as revealed by aCGH
| Probe | Fold Change | P value | Location | Gene Discription |
|---|---|---|---|---|
| A_14_P111340 | 2.426 | 0.026 | 22q11.23 | Homo sapiens glutathione S-transferase theta 1 (GSTT1), mRNA. |
| A_14_P137469 | 1.608 | 0.040 | 15q26.3 | Homo sapiens ADAM metallopeptidase with thrombospondin type 1 motif, 17 (ADAMTS17), mRNA. |
| A_14_P119952 | 1.571 | 0.012 | 15q25.3 | Homo sapiens neurotrophic tyrosine kinase, receptor, type 3 (NTRK3), transcript variant 1, mRNA. |
| A_14_P102170 | 1.557 | 0.037 | 15q25.3 | Unknown |
| A_14_P131652 | 1.555 | 0.024 | 15q26.3 | chr15:099528202-099528261 |
| A_14_P110170 | 1.55 | 0.026 | 15q25.2 | Homo sapiens elongation factor Tu GTP binding domain containing 1 (EFTUD1), transcript variant 2, mRNA. |
| A_14_P105951 | 1.534 | 0.048 | 15q26.1 | Homo sapiens feline sarcoma oncogene (FES), mRNA. |
| A_14_P114159 | 1.527 | 0.031 | 15q25.2 | Unknown |
| A_14_P116733 | 1.525 | 0.033 | 15q25.1 | Homo sapiens START domain containing 5 (STARD5), mRNA. |
| A_14_P112162 | 1.523 | 0.037 | 15q26.1 | Homo sapiens abhydrolase domain containing 2 (ABHD2), transcript variant 1, mRNA. |
| A_14_P111710 | 1.517 | 0.033 | 15q26.1 | Homo sapiens interferon stimulated exonuclease gene 20kDa-like 1 (ISG20L1), mRNA. |
| A_14_P133770 | 1.517 | 0.024 | 5p15.33 | Homo sapiens zinc finger, DHHC-type containing 11 (ZDHHC11), mRNA. |
| A_14_P130346 | 1.511 | 0.030 | 15q25.3 | Homo sapiens A kinase (PRKA) anchor protein 13 (AKAP13), transcript variant 1, mRNA. |
| A_14_P126517 | 1.508 | 0.039 | 15q25.2 | Homo sapiens cytoplasmic polyadenylation element binding protein 1 (CPEB1), transcript variant 1, mRNA. |
| A_14_P132042 | 1.504 | 0.048 | 15q26.1 | Unknown |
| A_14_P130065 | 1.502 | 0.038 | 15q26.1 | Homo sapiens IQ motif containing GTPase activating protein 1 (IQGAP1), mRNA. |
| A_14_P140006 | 1.501 | 0.040 | 15q26.1 | Homo sapiens KIAA1794 (KIAA1794), mRNA. |
| A_14_P127968 | 1.501 | 0.01 | 7q21.11 | chr7:080359807-080359866 |
| A_14_P129666 | 0.664 | 0.029 | 6q22.33 | Homo sapiens enoyl Coenzyme A hydratase domain containing 1 (ECHDC1), mRNA. |
| A_14_P110489 | 0.664 | 0.035 | 14q21.1 | Homo sapiens Sec23 homolog A (S. cerevisiae) (SEC23A), mRNA. |
| A_14_P202219 | 0.656 | 0.022 | 9p22.3 | Homo sapiens FRAS1 related extracellular matrix 1 (FREM1), mRNA. |
| A_14_P133233 | 0.64 | 0.042 | 5q35.3 | Homo sapiens zinc finger protein 354C (ZNF354C), mRNA. |
Based on the log2 ratios for the above 22 probes, the 17 tumor cases were classified using hierarchical cluster analysis. The results indicated that only one late-recurrent patient was mistakenly grouped into the early-recurrent category (Figure 1). The remaining 16 cases were all correctly classified, yielding an accuracy rate of 94.1% (16/17).
Figure 1.
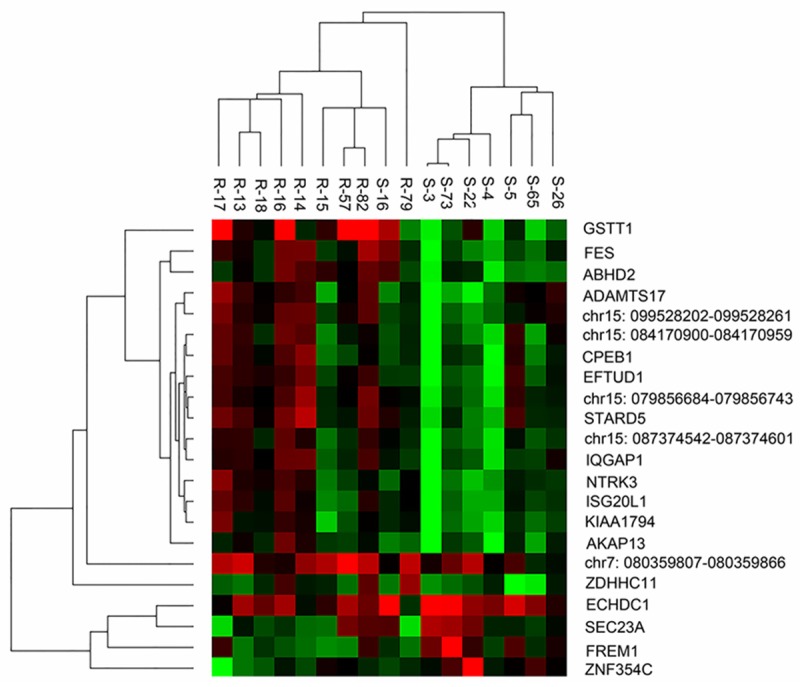
The results of hierarchical cluster analysis on the 17 patients using the results of 22 probes in the array CGH experiment. (R, early recurrence; S, late recurrence).
Validation of the aCGH results
The aCGH experiment had a small sample size that needed to be expanded to validate the candidate genes generated from the screening set, thereby determining the correlation between the CNC of candidate genes and TTR. This was accomplished by real-time qPCR.
The fragments amplified by real-time qPCR completely or partially covered the sequences of the candidate probes. With GAPDH as the internal control, the relative copy number of each gene was calculated. The analysis of consistency between qPCR and aCGH revealed that four genes displayed consistent results, namely GSTT1, ISG20L1, STARD5 and FREM1 (Correlation coefficients were 0.828, 0.657, 0.600, and 0.556, and P values were < 0.001, 0.008, 0.011, 0.030, respectively).
The four genes were verified in the validation set with the inclusion criteria as follows: 1) Histological type was grade 2/3 epithelial ovarian cancer; 2) Surgical-pathological stages were III-IV (FIGO staging system); 3) Tumor contents were greater than 30%, as revealed by H&E staining; 4) The patients received cytoreductive surgery followed by 6-8 cycles of paclitaxel/platinum chemotherapy; 5) Follow-up time longer than 18 months.
A total of 142 patients were recruited in the validation set, with a median follow-up time of 30 months (18-87 months). Till the last follow-up, 88 patients died, 122 patients exhibited recurrence, and the remaining 20 patients haven’t recurred with TTR longer than 10 months. Two-year and 5-year survival rates were 81.3% and 50.6%, respectively. The median TTR was 9 months, and the median overall survival was 60 months. There were 70 late-recurrent patients (TTR > 10 months) and 72 early-recurrent patients (TTR ≤ 10 months) (Table 4).
Table 4.
The clinicopathologic characteristics of the validation set patients
| Early recurrence (TTR ≤ 10 months) | Late recurrence (TTR > 10 months) | |
|---|---|---|
| Number of cases | 72 | 70 |
| Median age (years) | 58 | 55 |
| Staging | ||
| Stage III | 63 | 62 |
| Stage IV | 9 | 8 |
| Histological type | ||
| Serous adenocarcinoma | 60 | 51 |
| Mucinous adenocarcinoma | 1 | 1 |
| Clear cell carcinoma | 2 | 4 |
| Other types | 9 | 14 |
| NAC | ||
| Yes | 47 | 21 |
| No | 25 | 49 |
| Residual disease | ||
| 0 | 5 | 19 |
| < 1 cm | 31 | 31 |
| ≥ 1 cm | 36 | 20 |
| Median TTR (months) | 3 | 30 |
NAC: Neoadjuvant chemotherapy; TTR: time to recurrence.
The CNC of the four candidate genes, GSTT1, ISG20L1, STARD5 and FREM1, were detected in the validation set by real-time qPCR. Discriminant analysis revealed that the predication of early and late recurrence based on CNC of the four-gene set yielded an accuracy rate of 65.5% (Table 5). In addition, the median TTR of the late- and early-recurrent tumors, determined by CNC of the four-gene set, were 15 and 6 months, respectively (Figure 2, P = 0.0002).
Table 5.
Accuracy of CNC of the four-gene set by real-time qPCR to predict early and late recurrence
| Prediction based on the four genes | Accuracy | ||
|---|---|---|---|
|
| |||
| Early recurrence | Late recurrence | ||
| Early recurrence (TTR ≤ 10 months) | 54 | 18 | 65.5% (93/142) |
| Late recurrence (TTR > 10 months) | 31 | 39 | |
TTR: time to recurrence.
Figure 2.
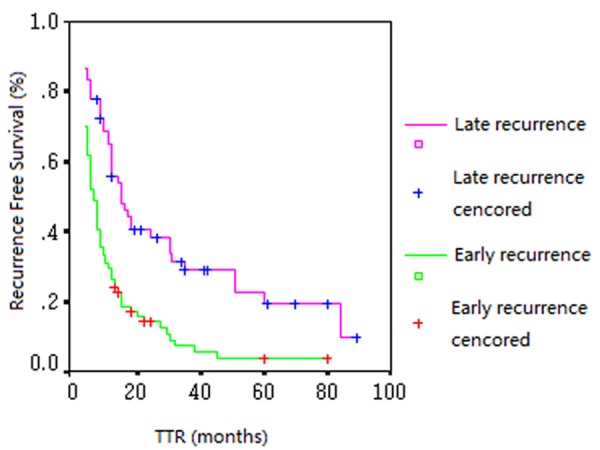
The Validation set: Kaplan-Meier curves of TTR of the early and late recurrent groups determined by CNC of the four-gene set (P = 0.0002).
In the validation set, 74 patients received primary cytoreductive surgery after diagnosis, while the other 68 patients received neo-adjuvant chemotherapy (NAC) followed by debulking surgery. Subgroup analysis showed that the predictive accuracy rates of the four-gene set were 70.33% (52/74) and 60.3% (41/68) in non-NAC and NAC subgroup, respectively (Table 6). Chi square test showed that there was no significant difference between them (P = 0.14).
Table 6.
Accuracies of CNC of the four-gene set by real-time qPCR to predict early and late recurrence in non-NAC and NAC subgroups
| Prediction based on the four genes | Accuracy | ||
|---|---|---|---|
|
| |||
| Early recurrence | Late recurrence | ||
| Non-NAC | |||
| Early recurrence (TTR ≤ 10 months) | 22 | 5 | 70.3% (52/74) |
| Late recurrence (TTR > 10 months) | 17 | 30 | |
| NAC | |||
| Early recurrence (TTR ≤ 10 months) | 32 | 13 | 60.3% (41/68) |
| Late recurrence (TTR > 10 months) | 14 | 9 | |
NAC: neoadjuvant chemotherapy; TTR: time to recurrence.
Multivariate analysis with the Cox proportional hazard model was employed to analyze all cases in the validation set and revealed that the residual tumor (P = 0.03), neoadjuvant chemotherapy (P = 0.006) and the CNC of the four-gene set (P = 0.02) were independent predictors for recurrence.
Discussion
For advanced epithelial ovarian cancer, TTR is an important indicator to gauge the therapeutic efficacy of postoperative adjuvant chemotherapy. The longer the TTR, the more effective the chemotherapy is. As a result, patients with long TTR benefit more from adjuvant paclitaxel/platinum chemotherapy. In contrast, patients with short TTR may try other regimens as adjuvant chemotherapy because of less benefit from paclitaxel/platinum chemotherapy.
Unfortunately, there is currently no marker available to reliably predict chemotherapy response. In other words, it is very difficult to discriminate patients who may experience a poor outcome from platinum-paclitaxel chemotherapy. In recent years, efforts have been dedicated to exploring the relationship between changes in expression profile and chemosensitivity [1-4]. However, there has been no common agreement among research groups on which genes have predicative significance. Hence, this study intended to identify genes associated with TTR after initial administration of platinum-paclitaxel chemotherapy in advanced EOC.
In comparison with alterations in expression level, changes at the DNA level may better reveal the connection between the intrinsic properties of tumors and chemotherapy efficacy. As such, this study integrated aCGH with real-time qPCR, which revealed that CNC of the four-gene set were possibly related to chemotherapy efficacy.
Currently, a non-treatment interval of 6 months is accepted as the cut-off value between platinum-sensitive and -resistant patients worldwide. In the discriminant analyses of the validation set, when we considered 6 months, 10 months and 12 months as the cut-off values to define early and late recurrence, the predictive accuracy of CNC of the 4-gene set was 51.3%, 65.5% and 56.2%, respectively. These results showed that 10 months was the cut-off value that corresponded to the best predictive accuracy using CNC of the four-gene set. It suggested that the patients determined by CNC of the four-gene set to be late recurrent are likely to experience relapse at least 10 months after chemotherapy.
Neo-adjuvant chemotherapy followed by debulking surgery is one of the treatment choices for advanced ovarian cancer patients, especially for those with apparently unresectable tumors. In the validation cohort, some tumor samples were collected after NAC, which might have some effect on the results. Our subgroup analysis compared NAC with non-NAC patients in the validation set revealed that the predictive accuracy of 4-gene set was slightly higher in non-NAC group than in NAC group. To avoid the influence of NAC, further research should be carried with the tumor samples collecting before NAC.
Of the 4 genes identified in this study, GSTT1 encodes a θ class isozyme of glutathione S-transferase (GST). GST is the key enzyme that catalyzes glutathione synthesis and is involved in detoxification processes of the body. Many clinically applied antitumor drugs, such as cis-diamminedichloroplatinum (II) (DDP), doxorubicin, carmustine and mitomycin, are substrates of GST, which promotes the release of the drugs from cells. Many studies have shown that GST is highly expressed in drug-resistant cancers (most commonly in EOC resistant to platinum, doxorubicin and alkylating antitumor agents) [5-8]. Hence, high GST expression is considered to be one of the mechanisms resulting in the chemoresistance of EOC.
ISG20L1 is also called apoptosis enhancing nuclease (AEN) in humans. ISG20L1 is a target of the p53 family. In response to toxic stress, the transcription of ISG20L1 is regulated by p53 family proteins (p53, p63 and p73), which results in the elevation of its protein level and subsequently leads to autophagic cell death [9]. However, whether changes in the DNA level of the gene is related to chemotherapy efficacy remains to be further investigated.
The protein encoded by STARD5 is a part of the regional STARD protein complex and is a cytoplasmic sterol transporter protein. It regulates cholesterol equilibrium in the body via sterol regulatory element-binding proteins and liver X receptors. A study indicated that mutations in STARD genes may cause autoimmune diseases or cancer [10]. However, the connection of this gene to chemosensitivity has not been reported.
FREM1 is a member of the extracellular matrix (ECM) gene family. ECM proteins, mainly consisting of collagen, elastin, proteoglycans and glycoproteins, are the insoluble structural components that constitute intercellular substances and the epithelial vascular matrix. Studies have indicated that the ECM influences a multitude of cellular processes including differentiation, proliferation, adhesion, morphogenesis and phenotypic expression [11]. The development, chemoresistance, invasion and metastasis of malignant tumors are often accompanied by expression changes in ECM proteins and the cognate surface receptors. It has been widely reported that tumor cells may deliver “survival signals” via intercellular as well as cell-matrix adhesions, which antagonize the pro-apoptotic effects of chemotherapy drugs and cause the tumors to exhibit chemoresistance.
In summary, CNC of the four genes (GSTT1, ISG20L1, STARD5 and FREM1) reported here may be related to paclitaxel-platinum chemosensitivity in EOC. The predictive effects and underlying mechanisms of CNC of these genes need to be further elucidated.
Acknowledgements
We thank Prof Xin-Yu Zhang for his support in the analysis of array CGH results. Grant support: Capital Health Research and Development Foundation (Grant number: 2007-3021).
Disclosure of conflict of interest
None.
References
- 1.Bachvarov D, L’esperance S, Popa I, Bachvarova M, Plante M, Têtu B. Gene expression patterns of chemoresistant and chemosensitive serous epithelial ovarian tumors with possible predictive value in response to initial chemotherapy. Int J Oncol. 2006;29:919–933. [PubMed] [Google Scholar]
- 2.Helleman J, Jansen MP, Span PN, van Staveren IL, Massuger LF, Meijer-van Gelder ME, Sweep FC, Ewing PC, van der Burg ME, Stoter G, Nooter K, Berns EM. Molecular profiling of platinum resistant ovarian cancer. Int J Cancer. 2006;118:1963–1971. doi: 10.1002/ijc.21599. [DOI] [PubMed] [Google Scholar]
- 3.Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, Aprelikova O, Yee CJ, Zorn KK, Birrer MJ, Barrett JC, Boyd J. Gene expression profiles associatedwith response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11:6300–6310. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]
- 4.Sabatier R, Finetti P, Bonensea J, Jacquemier J, Adelaide J, Lambaudie E, Viens P, Birnbaum D, Bertucci F. Br J Cancer. 2011;105:304–311. doi: 10.1038/bjc.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvanayagam ZE, Cheung TH, Wei N, Vittal R, Lo KW, Yeo W, Kita T, Ravatn R, Chung TK, Wong YF, Chin KV. Prediction of chemotherapeutic response in ovarian cancer with DNA microarray expression profiling. Cancer Genet Cytogenet. 2004;154:63–66. doi: 10.1016/j.cancergencyto.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Nagle CM, Chenevix-Trench G, Spurdle AB, Webb PM. The role of glutathione-S-transferase polymorphisms in ovarian cancer survival. Eur J Cancer. 2007;43:283–290. doi: 10.1016/j.ejca.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Kim MK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol. 2009;113:264–269. doi: 10.1016/j.ygyno.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Beeghly A, Katsaros D, Chen H, Fracchioli S, Zhang Y, Massobrio M, Risch H, Jones B, Yu H. Glutathione S-transferase polymorphisms and ovarian cancer treatment and survival. Gynecol Oncol. 2006;100:330–337. doi: 10.1016/j.ygyno.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Eby KG, Rosenbluth JM, Mays DJ, Marshall CB, Barton CE, Sinha S, Johnson KN, Tang L, Pietenpol JA. ISG20L1 is a p53 family target gene that modulates genotoxic stress-induced autophagy. Mol Cancer. 2010;29:95. doi: 10.1186/1476-4598-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 11.Petrou P, Makrygiannis AK, Chalepakis G. The Fras1/Frem family of extracellular matrix proteins: structure, function, and association with Fraser syndrome and the mouse bleb phenotype. Connect Tissue Res. 2008;49:277–282. doi: 10.1080/03008200802148025. [DOI] [PubMed] [Google Scholar]


