Abstract
Inhibitory interactions between neurons of the respiratory network are involved in rhythm generation and pattern formation. Using a computational model of brainstem respiratory networks, we investigated the possible effects of suppressing glycinergic inhibition on the activity of different respiratory neuron types. Our study revealed that progressive suppression of glycinergic inhibition affected all neurons of the network and disturbed neural circuits involved in termination of inspiration. Causal was a dysfunction of postinspiratory inhibition targeting inspiratory neurons, which often led to irregular preterm reactivation of these neurons, producing double or multiple short-duration inspiratory bursts. An increasing blockade of glycinergic inhibition led to apneustic inspiratory activity. Similar disturbances of glycinergic inhibition also occur during hypoxia. A clear difference in prolonged hypoxia, however, is that the rhythm terminates in expiratory apnea. The critical function of glycinergic inhibition for normal respiratory rhythm generation and the consequences of its reduction, including in pathological conditions, are discussed.
Keywords: computational modeling, respiratory rhythm, pre-Bötzinger complex, glycinergic inhibition, apneusis, apnea, hypoxia, translational medicine
1 INTRODUCTION
Inhibitory interactions between neurons of the brainstem respiratory network have been proposed to play a critical role in respiratory rhythm generation and the adjustment of normal breathing movements in vivo (Richter et al., 1986, 1992). Disturbances of these synaptic interactions may become dangerously life threatening as they can lead to an abnormally prolonged inspiration (apneusis) producing breath holdings or to complete cessation of breathing (apnea). There are reports of several diseases in which such disturbances of breathing originate from a failure of glycinergic inhibition that may even cause sudden death, for example, in Rett Syndrome (Stettner et al., 2007), hyperekplexia (Büsselberg et al., 2001a; Harvey et al., 2008; Markstahler et al., 2002), developmental disorders, such as olivo-ponto-cerebellar atrophy (OPCA) (Richter et al., 2003), and also brainstem infarction (El-Khatib et al., 2003).
The circuit mechanisms underlying inhibitory regulation of respiratory network activity and the associated emergent pathogenic processes contributing to network dysfunctions are difficult to analyze in a complex system such as the brainstem respiratory network. Specifically, there is incomplete understanding about the effects of such disturbances on the activity of different respiratory neuron types. In this study, we tried to fill this gap using computational modeling of the effects of progressive depression of glycinergic inhibition on the activity of various types of respiratory neurons. We believe that such an approach is promising as it not only predicts network behavior but allows theoretical and experimental testing of therapeutic strategies to recover breathing as was successfully performed for opioid-induced apnea by potentiating glycinergic synaptic transmission (Shevtsova et al., 2011).
As synaptic transmission of inhibitory neurons is also very sensitive to hypoxia and fades quickly during reduced levels of brain oxygen (Congar et al., 1995), we used our simulations to investigate the possible effects of progressive suppression of glycinergic inhibition to gain insights into basic mechanisms of respiratory rhythm generation and pattern formation and to explore potential inhibitory mechanisms involved in hypoxia-related disturbances of respiratory network activity. Based on our simulations and their comparisons to experimental data, we were able to identify and interpret some of the stages of hypoxic perturbations of neural activity at both the neuronal and the network levels. We suggest that identifying different states of these disturbances can be used to diagnose the degree of severity of disruptions of network inhibitory processes, which might be beneficial for protective medicine.
2 MATERIALS AND METHODS
2.1 Modeling Methods
The computational model of the brainstem respiratory network used in this study had been developed and described in detail by Shevtsova et al. (2011). All neurons were modeled in the Hodgkin–Huxley style (single-compartment models) and incorporated known biophysical properties and available information on channel kinetics as previously characterized in respiratory neurons in vitro. In the model, each population contained 20–50 neurons. Heterogeneity of neurons within each population was set by a random distribution of neuronal parameters and initial conditions to produce physiological variations of baseline membrane potential levels, calcium concentrations, and channel conductances. Each neural population received an additional tonic excitatory drive. To simulate a progressive reduction of the strength of glycinergic inhibition in the network, all weights of glycinergic synapses in the network were equally reduced by 5% steps from their initial default values to zero (100% suppression).
All simulations were performed using the simulation package NSM 3.0 developed at Drexel University by S. N. Markin, I. A. Rybak, and N. A. Shevtsova. Differential equations were solved using the exponential Euler integration method with a step of 0.1 ms. Additional details of modeling and simulation methods can be found in Shevtsova et al. (2011).
2.2 Experimental Studies
The computational data were compared with experimental investigations in the arterially perfused in situ brainstem-spinal cord preparation of wild-type mice, in which specific blockade of glycine receptors (GlyRs) (Jonas et al., 1998) was achieved by adding strychnine at concentrations as low as 0.07–0.3 μM to the perfusate (Büsselberg et al., 2001b, 2003). In vivo hypoxia data were obtained from anesthetized cats, which as described in the original publications were ventilated with gases of variable O2 and constant CO2 partial pressures (Richter et al., 1991).
3 RESULTS
3.1 Model Description and Operation in Control Conditions
In this study, we used our computational model of the brainstem respiratory network (Shevtsova et al., 2011) that was specially developed to simulate and theoretically analyze the possible neural mechanisms involved in the recovery of breathing after opioid-induced apnea by potentiating glycinergic inhibition via the 5-HT1A receptor agonist 8-OH-DPAT as demonstrated in experimental studies (Manzke et al., 2010). This model was the first computational model of the brainstem respiratory network in which the two different types of synaptic inhibition found in this network, glycinergic and GABAergic, were separated and performed different functions in the network as described by Schmid et al. (1996). The model (Fig. 1) was developed as an extension of the previous model of Smith et al. (2007) that simulated the core excitatory and inhibitory interactions between respiratory neuronal populations within and between the pre-Bötzinger (pre-BötC) and Bötzinger (BötC) complexes and the rostral ventral respiratory group (rVRG). In contrast to that model, however, the model developed by Shevtsova et al. differentiated glycinergic and GABAergic inhibitory populations.
FIGURE 1.
Model schematic. See text for details.
As shown in Fig.1 and described in Shevtsova et al. (2011), the BötC compartment in the model contains two separate glycinergic postinspiratory/decrementing-expiratory populations (referred to as post-I and dec-E) and two populations of augmenting expiratory neurons: one GABAergic (aug-E(1)) and the other glycinergic (aug-E(2)). The pre-BötC compartment has an excitatory (glutamatergic) population of preinspiratory/inspiratory (pre-I/I) neurons some of which have intrinsic pacemaker bursting properties and two populations of early-inspiratory neurons: one GABAergic (early-I (1)) and the other glycinergic (early-I(2)). The rVRG compartment contains an excitatory(output)populationofaugmenting(ramping)ramp-Ineuronsprojectingtophrenic motoneurons (not included in the model) and an inhibitory (glycinergic) early-I(3) population shaping the augmenting pattern of ramp-Ineurons during inspiration. The experimental basis for the neuronal properties and network interconnections incorporated in this model can be found in our previous papers (Rybak et al., 1997, 2007; Shevtsova et al., 2011; Smith et al., 2007).
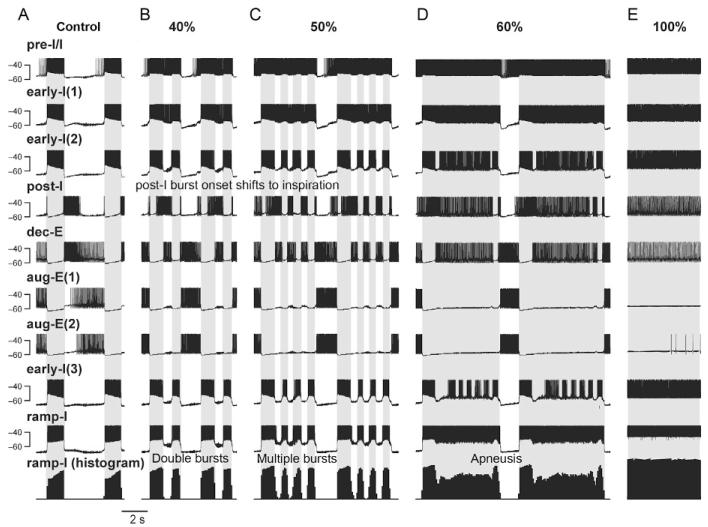
Model performance under control conditions is illustrated in Fig. 2A that shows traces of membrane potentials of one representative neuron from each population and the integrated histogram of activity of the ramp-I population that is considered as a surrogate for the phrenic output defining the phase of inspiration (marked in Fig. 2 by gray background).
FIGURE 2.
Simulations of effects of depressing glycinergic inhibition on the activity of different respiratory neuron types and the output motor pattern. Traces of membrane potentials of the representative single neurons from each population in the network (Fig. 1) and the integrated histogram of ramp-I output (bottom trace) under normal conditions (A) and following a graded reduction of glycinergic inhibition in the network (by 40%, 50%, 60%, and 100%, in B–E, respectively). In all diagrams, the inspiratory phase defined by the activity of the ramp-I population is highlighted by gray background.
In control conditions, the model generated a typical three-phase respiratory rhythmic pattern (Richter et al., 1986) similar to that in our previous models (Rybak et al., 1997, 2007; Shevtsova et al., 2011; Smith et al., 2007) and reproduced the typical firing patterns and membrane potential changes of different types of respiratory neurons (Richter, 1982). Specifically (see Fig. 2A), the pre-I/I neurons start firing prior to the onset of inspiration (as defined by the ramp-I histogram at the bottom). All three early-I neuron types (GABAergic, early-I(1), and glycinergic, early-I(2) and early-I(3)) exhibit similar decrementing activity patterns during inspiration. The aug-E neurons, GABAergic aug-E(1) and glycinergic aug-E(2), are both active in late expiration. The glycinergic post-I neuron type is active in the postinspiratory phase, whereas the glycinergic dec-E neurons demonstrate adaptive responses throughout expiration.
Inhibitory interactions define firing behaviors of different neuron populations during the respiratory cycle. Specifically, during expiration, all four expiratory populations of BötC inhibit all inspiratory populations of pre-BötC and rVRG. The glycinergic post-I population of BötC stops firing in the postinspiratory phase because of inhibition provided by both aug-E populations of BötC. Before the end of expiration, the pre-I/I excitatory population of pre-BötC starts firing when released from the decreasing inhibition from the dec-E population, which exhibits spike frequency adaptation in the model and is progressively inhibited by the aug-E(1) population. The pre-I/I neurons then excite both of the pre-BötC early-I populations. These populations fire, inhibit all expiratory populations of BötC, and drive the start of inspiration (the onset of ramp-I population activity in rVRG). During inspiration both of the early-I populations exhibit progressively reduced activity because of their Ca2+-induced spike frequency adaptation and at some moment allow a release of activity in the two glycinergic populations of BötC (post-I and dec-E neurons). Firing of these two populations provides switching to expiration with inhibition of all inspiratory neurons in pre-BötC and rVRG. Then the cycle processes repeat.
3.2 Simulating the Effects of Progressive Reduction of Glycinergic Inhibition in the Network
Figure 2 shows the representative traces of membrane potentials of single neurons from each population in the network and the integrated ramp-I output (bottom trace) under control conditions (panel A) and following a graded reduction of glycinergic inhibition in the network (by 40%, 50%, 60%, and 100%, see panels (B–E), respectively).
After blockade of glycinergic inhibition by 40% (Fig. 2B), post-I neurons escape the glycinergic inhibition by early-I(2) neurons and their activity shifts into the inspiratory phase. At the same time, the reduction of inhibition of the pre-I/I and both early-I populations by the glycinergic post-I and dec-E populations results in a prolonged activity of the pre-I/I and early-I(1) neurons. The dec-E and early-I(2) neurons have mutual inhibiting glycinergic synaptic interactions that are reduced simultaneously, which results in a short postinspiratory phase, leading to secondary inspiratory bursts also seen by the double bursts in the ramp-I output. Progressive increase of the blockage of glycinergic inhibition (e.g., by 50%; Fig. 2C) leads to the generation of a series of multiple inspiratory bursts discharged at short intervals. Further increased blockade of glycinergic inhibition (by 60% in Fig. 2D) results in long-lasting output bursts with inspiratory discharges of steady intensity resembling apneusis. The model revealed that this inspiratory prolongation originates preferentially from a repetitive alternation between pre-I/I and early-I and aug-E neuronal discharges. A full blockade of glycinergic inhibition in the model leads to the full cessation of rhythmic oscillations and continuous (apneustic) inspiratory output activity (Fig. 2E).
3.3 Comparison of the Simulation Results with Experimental Data
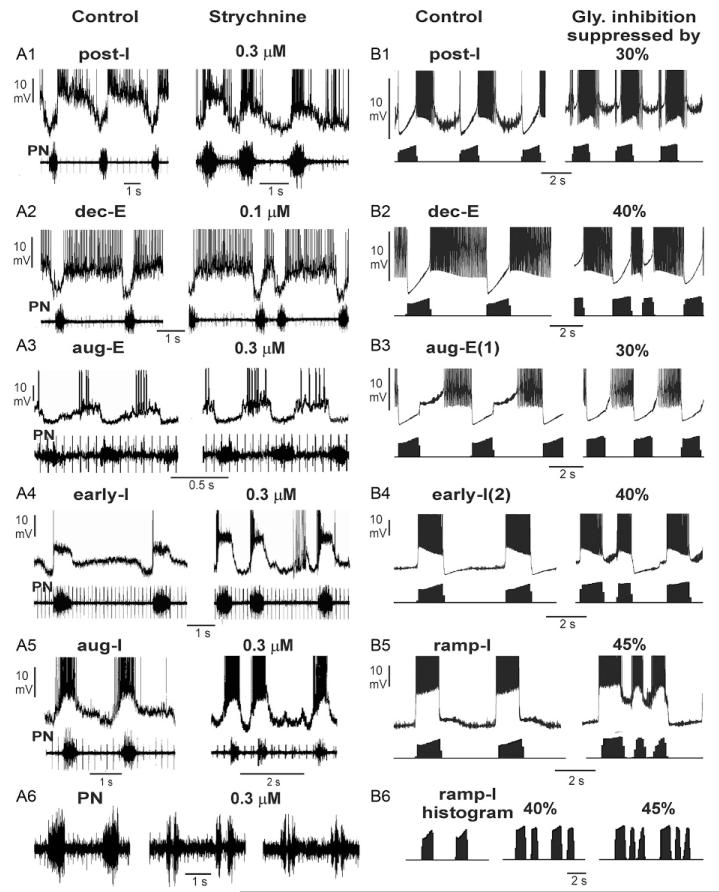
Experimental suppression of glycinergic inhibition by its pharmacological blockers (e.g., strychnine) in small concentrations/doses (to maintain blocker specificity) is never 100% complete. Therefore, we compared the experimental data showing the effect of strychnine with our simulations that predicted the possible changes in the activity of different neuron types and the output motor patterns following partial suppression of glycinergic inhibition. Such comparisons are shown in Fig. 3.
FIGURE 3.
Comparison of simulation results with experimental data on perturbations of the activity of different respiratory neuron types and the output motor pattern by partial suppression of glycinergic inhibition. Experimental data (A1–A6) and the corresponding simulation results (B1–B6) illustrate disturbances of activity of post-I (A1 and B1), dec-E (A2 and B2), aug-E (A3 and B3), early-I (A4 and B4), and aug-I/ramp-I (A5 and B5) neuron types, and output motor activity (PN, phrenic nerve activity; A6 and B6). Original data in A1 (unpublished) and A3 are from the study of Büsselberg et al. (2001b), and for A2, A4, A5, and A6 from Büsselberg et al. (2003). See text for details.
The analysis of cellular data obtained by intracellular recordings in the perfused in situ rat preparation after administration of strychnine revealed several critical changes in respiratory network operation accompanied by, and probably strongly connected with, the observed changes in the activity of postinspiratory neurons (Büsselberg et al., 2001b, 2003). Specifically these experimental studies showed that strychnine application can cause a shift in the onset of post-I neuron discharges into the inspiratory phase (Fig. 3A1). A similar shift in the onset of post-I activity was reproduced in our simulations after 30% suppression of glycinergic inhibition (see Fig. 3B1). This post-I activity shift occurred in the model because the reduced inhibition from the glycinergic early-I neurons allowed the post-I neurons to start firing within inspiration.
In contrast to the post-I neurons, the activity of the dec-E neurons, following the reduction of glycinergic inhibition, did not shift to inspiration in both the experimental recordings from such neurons (Büsselberg et al., 2003) and our simulations (Fig. 3B2). Our simulations support the proposal that in contrast to the post-I neurons, the dec-E type of neurons receive mostly GABAergic inhibition during inspiration, which keeps these neurons inhibited during inspiration even when glycinergic inhibition is suppressed. Note also the obvious irregularity in the expiratory duration leading to occasional doubling of the inspiratory bursts, which is seen in both experimental records and our simulations with 40% inhibition suppression.
Figures 3A3 and B3 compare the effect of suppressing glycinergic inhibition on the activity of aug-E neurons, recorded in the intact rat brainstem (Büsselberg et al., 2001b; panel A3), and from our model simulations (panel B3, an aug-E(1) neuron after 30% suppression of glycinergic inhibition). In both experimental and modeling traces, following the suppression of glycinergic inhibition, the aug-E neurons started firing just after termination of phrenic nerve discharges, which can be explained by the shift of post-I activity into inspiration and the reduced expiratory glycinergic inhibition from the post-I neurons.
Figure 3A4 represents intracellular recordings from an early-I neuron (from Büsselberg et al., 2003) in control conditions and after administration of strychnine, and in Fig. 3B4, we show the corresponding traces of an early-I(2) neuron from our simulations under control conditions and following 40% suppression of glycinergic inhibition. The decreased inhibition of inspiratory neurons during the postinspiratory period by the glycinergic post-I and dec-E neurons causes the predicted appearance of the secondary inspiratory bursts seen in both the early-I neuron and phrenic activities (Büsselberg et al., 2003).
Figures 3A5 and B5 compare the effect of glycinergic inhibition blockade on the activity of presumably an aug-I neuron (panel A5, from Büsselberg et al., 2003) and from a ramp-I neuron from our simulations (45% of glycinergic inhibition suppression). As seen in these panels and in the panels A6 and B6 (showing only experimentally recorded phrenic activity and integrated ramp-I population activity from our simulation), the partial blockade of glycinergic inhibition produces double, triple, and sometimes multiple inspiratory discharges as also seen in other panels of Fig. 3. The model showed that the post-I neurons start to fire action potentials shortly after the onset of phrenic nerve bursts (Manzke et al., 2010; Shevtsova et al., 2011) allowing only a short period of undisturbed evolution of inspiratory activity. The post-I neurons continue to fire throughout the plateau of the recorded prolonged (apneustic) phrenic nerve discharge and decline thereafter. This residual post-I discharge following the apneustic bursts, however, seems to be too weak to suppress all pre-I/I discharges, which may trigger very brief discharges (not illustrated) or the secondary bursts of phrenic nerve activity (Büsselberg et al., 2001b).
4 DISCUSSION
Under normal conditions, the three-phase pattern of rhythmic breathing depends on intact excitatory and inhibitory synaptic interactions between populations of respiratory neurons in the complex respiratory network (Richter, 1982; Smith et al., 2007). Inhibitory interactions include glycinergic inhibition involved in termination of respiratory phases and GABAergic inhibition that stabilizes antagonistic inspiratory and expiratory phases of oscillation (Schmid et al., 1996). The specific roles of glycinergic versus GABAergic inhibitory interactions in the respiratory network have not been definitively established, although the results from pharmacological experiments analyzed here suggest that glycinergic inhibition may importantly contribute to normal rhythm generation and pattern formation. Recent studies have demonstrated that more than 50% of respiratory neurons in the pre-BötC are glycinergic (Manzke et al., 2009; Winter et al., 2009) and there is a denser concentration of glycinergic neurons in the BötC (see Manzke et al., 2010). This problem has become even more interesting by the recent findings of Koizumi et al. (2013) that there are substantial subpopulations of inhibitory inspiratory neurons that are cophenotypic glycinergic and GABAergic neurons in the neonatal pre-BötC. Our model, which is based on available (but limited) information on the specific connectivity of glycinergic and GABAergic neurons, has attempted to assign potential roles to glycinergic and GABAergic neuron populations in the pre-BötC and BötC. According to our model (see Fig. 1), glycinergic inhibitory neurons in these regions are involved in both rhythm generation and respiratory pattern formation. This is consistent with the finding that local pharmacologic blockade of GlyRs in the pre-BötC of intact in vivo preparations disturbs the inspiration terminating mechanisms (Richter et al., 1979) and can lead to complete respiratory apnea with weak oscillatory or tonic discharges of phrenic nerves (Pierrefiche et al., 1998). Our computational network simulations, with the specific network connections proposed, provide a “proof-of-principle” that selective blockade of all glycinergic inhibition can produce inspiratory apneusis.
Can the experimental evidence and our models of disturbances with attenuated inhibitory interactions in the respiratory network provide an explanation of clinical disorders, including pathological respiratory network responses to prolonged hypoxia? It is important to note that some of the changes in neuronal activities and the output respiratory pattern observed after suppression of glycinergic inhibition also occur in patients with GlyR deficiency during hyperekplexia (Chung et al., 2010; Harvey et al., 2008; Markstahler et al., 2002), Rett Syndrome (Stettner et al., 2007), and developmental disturbances such as OPCA (Richter et al., 2003). The clinical symptoms are prolonged breath holdings (apneusis, but in the hospital often described as apnea). The present study describes simulation of the potential consequences when glycinergic inhibition fails gradually (Fig. 2). The disturbances start with doublet or multiple short-duration bursting of ramp-I neurons and output activity (Fig. 2B and C) that then lead to terminal apneusis (breath-holding, Fig. 2D and E). Our model also predicts that apnea may not occur even after complete blockade of GlyRs, but it may result from accompanying hypoxia.
The experimental finding that inspiratory apnea may result from GlyR blockade lead us to a comparison of hypoxic or ischemic responses that involve endogenous blockade of glycinergic inhibition, which causes expiratory activity-based apnea (Richter et al., 1991; Thoby-Brisson and Ramirez, 2000). We therefore examined single-cell intracellular recording data on progressive blockade of GlyRs delineating alterations of spiking patterns and membrane potential trajectories with data from disturbances arising from hypoxia. A comparison of the hypoxic responses in the in vivo cat with strychnine-mediated blockade of GlyRs in the rat brainstem in situ shows interesting similarity. For example, there is indirect evidence that early in hypoxia the post-I discharge shifts into inspiration (see Fig. 4A-C) similar to that following pharmacological suppression of glycinergic inhibition (Büsselberg et al., 2001b). Such early blockade of glycinergic inhibition may be the primary cause for the successive disturbances in hypoxia. These include recurring bursting of the ramp-I output neurons, which might be responsible for intractable hiccups indicating onset of brainstem damage during hypoxia (Mandala et al., 2010).
FIGURE 4.
Hypoxic responses indicating early blockade of glycinergic inhibition ultimately causing expiratory apnea (anesthetized cat, Richter, unpublished). (A) With prolonged hypoxia, early-I inhibition of the aug-E neuron is progressively shortened to early-inspiratory periods (see gray regions). This indicates a shift of postinspiratory discharge into inspiration which continues throughout the apneustic inspiratory after-discharges. (B) An aug-E neuron revealing oscillatory fluctuations of EPSPs and IPSPs during the apneustic period of phrenic nerve discharge. (C) With enduring hypoxia, aug-E neuronal discharges shift into the inspiratory phase, when the duration of early-I inhibition is very short. Later during hypoxia, aug-E neurons discharge almost tonically, producing expiratory apnea. Normal activity patterns are fully recovered shortly after restoration of normoxia.
The striking difference to the partial blockade of GlyRs, however, is the persistence of tonic expiratory discharges. The final disturbance of network activity during progressive hypoxia is shifting of late expiratory neuronal discharges into the period of long apneustic inspiratory bursts and their vanishing after-discharges (Fig. 4A and B). After a short interval of hypoxia, however, blockade of GlyR seems to reach levels beyond 70% and the phrenic nerve output declines to very weak tonic discharges, while expiratory neurons fire tonically (Fig. 4C). This may result from breakdown of mutual inhibition after additional failure of GABAergic inhibition (Khazipov et al., 1995). In severe hypoxia, there is only a transient increase and then collapse of GABA release (Richter et al., 1999). Therefore, from a therapeutic stand-point, it seems reasonable to start protective treatment already before complete breakdown of inhibitory processes by pharmacotherapeutical reinforcement of glycinergic inhibition as successfully performed in a patient with surgical lesions in the pontine-brainstem junction (Wilken et al., 1997), and also in a patient with OPCA (Richter et al., 2003) and in brainstem stroke patients (El-Khatib et al., 2003).
Acknowledgments
This study was supported in the United States by National Institutes of Health, grants R33 HL087377, R01 NS057815, and R01 NS069220 to I. A. R., in part by the Intramural Research Program of the NIH, NINDS (J. C. S.), and in Germany by the Excellence Cluster “Nanoscale microscopy and molecular physiology of the brain” (CNMPB) funded by the DFG and BMBF (D. W. R.).
References
- Büsselberg D, Bischoff AM, Becker K, Becker CM, Richter DW. The respiratory rhythm in mutant oscillator mice. Neurosci. Lett. 2001a;316:99–102. doi: 10.1016/s0304-3940(01)02382-5. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001b;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Richter DW. A combined blockade of glycine and calcium-dependent potassium channels abolishes the respiratory rhythm. Neuroscience. 2003;122:831–841. doi: 10.1016/j.neuroscience.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Chung SK, Vanbellinghen JF, Mullins JG, Robinson A, Hantke J, Hammond CL, Gilbert DF, Freilinger M, Ryan M, Kruer MC, Masri A, Gurses C, Ferrie C, Harvey K, Shiang R, Christodoulou J, Andermann F, Andermann E, Thomas RH, Harvey RJ, Lynch JW, Rees MI. Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J. Neurosci. 2010;30:9612–9620. doi: 10.1523/JNEUROSCI.1763-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Khazipov R, Ben-Ari Y. Direct demonstration of functional disconnection by anoxia of inhibitory interneurons from excitatory inputs in rat hippocampus. J. Neurophysiol. 1995;73:421–426. doi: 10.1152/jn.1995.73.1.421. [DOI] [PubMed] [Google Scholar]
- El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir. Care. 2003;48:956–958. [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: more than startle! Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: comparison of effects of anoxia on excitatory and inhibitory postsynaptic currents. J. Neurophysiol. 1995;74:2138–2149. doi: 10.1152/jn.1995.74.5.2138. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Koshiya N, Chia J, Cao F, Nugent J, Zhang R, Smith JC. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Bötzinger complex respiratory microcircuits. J. Neurosci. 2013;33:2994–3009. doi: 10.1523/JNEUROSCI.4427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala M, Rufa A, Cerase A, Bracco S, Galluzzi P, Venturi C, Nuti D. Lateral medullary ischemia presenting with persistent hiccups and vertigo. Int. J. Neurosci. 2010;120:226–230. doi: 10.3109/00207450903585316. [DOI] [PubMed] [Google Scholar]
- Manzke T, Dutschmann M, Schlaf G, Morschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2589–2602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hulsmann S, Ponimaskin E, Muller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor alpha3 controls breathing in mice. J. Clin. Invest. 2010;120:4118–4128. doi: 10.1172/JCI43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstahler U, Kremer E, Kimmina S, Becker K, Richter DW. Effects of functional knock-out of alpha 1 glycine-receptors on breathing movements in oscillator mice. Respir. Physiol. Neurobiol. 2002;130:33–42. doi: 10.1016/s0034-5687(01)00334-6. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. J. Physiol. 1998;509(Pt. 1):245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J. Exp. Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW, Camerer H, Meesmann M, Rohrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflugers Arch. 1979;380:245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballantyne D, Remmers JE. How is the respiratory rhythm generated? A model. News Physiol. Sci. 1986;1:109–112. [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J. Physiol. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr. Opin. Neurobiol. 1992;2:788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J. Physiol. 1999;514(Pt. 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol. Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JFR, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern: II. Network models of the central respiratory pattern generator. J. Neurophysiol. 1997;7:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog. Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Manzke T, Molkov YI, Bischoff A, Smith JC, Rybak IA, Richter DW. Computational modelling of 5-HT receptor-mediated reorganization of the brainstem respiratory network. Eur. J. Neurosci. 2011;34:1276–1291. doi: 10.1111/j.1460-9568.2011.07825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J. Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J. Neurosci. 2000;20:5858–5866. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J. Pediatr. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]