Abstract
Myeloid sarcoma (MS) is a rare extramedullary tumor composed of primitive granulocytic cells. These lesions are commonly associated with other hematologic disorders such as myeloid leukemia and other myeloproliferative neoplasms. Although extremely rare in the oral cavity, this lesion was reported in gingiva, palate, buccal mucosa and extraction sockets. MS is an aggressive lesion associated with poor prognosis. Early identification and prompt treatment holds the key for increasing the disease-free period in these patients. In this context, we report a rare and aggressive case of MS, which ran a fatal course in a 45-year-old female patient.
Keywords: CD117, gingiva, granulocytic sarcoma, myeloid sarcoma, myeloperoxidase
INTRODUCTION
Myeloid Sarcoma (MS) was first reported by Burns as “chloroma” in 1811 due to its characteristic green color resulting from the presence of myeloperoxidase (MPO) in the tumor cells.[1] However, up to 30% of these tumors are white, gray or brown in color. Hence, the more appropriate term granulocytic sarcoma was proposed by Rappaport in 1967.[2] MS is a rare solid tumor composed of immature cells of granulocyte series.[3] It is frequently seen in association with other myeloproliferative disorders such as acute myeloid leukemia (AML) and the blast crisis stage of chronic myeloid leukemia.[2,3,4] MS is frequently mistaken for non-Hodgkin's lymphoma, small round cell tumors, such as neuroblastoma, rhabdomyosarcoma, Ewing's sarcoma and medulloblastoma and undifferentiated carcinoma, which may cause misdiagnosis in about 50% of cases when immunohistochemistry (IHC) is not used.
There is no age predilection for MS, as these lesions are reported from infants to older individuals. Analysis of previously reported cases shows a female predilection as majority of cases are seen in them. In oral cavity, this lesion has been reported in gingiva, palate, buccal mucosa, extraction sockets, tonsils, tongue, lips and periapical regions.[5,6,7]
MS is an aggressive lesion characterized by rapid growth and extensive bone destruction.[8] Diagnosis of MS is difficult through routine hematoxylin and eosin (H and E)-stained sections and is usually supplemented with IHC, flow cytometry and electron microscopy. IHC markers used for the diagnosis of MS include MPO, lysozyme and other markers targeted against granulocytes.[7,8,9] Radiotherapy and chemotherapy form the mainstay of treatment for MS. Despite aggressive treatment, the prognosis for this lesion is poor with majority of cases having a fatal outcome.[7,8,9]
CASE REPORT
A 45-year-old female patient came to the Department of Oral Medicine and Radiology with a chief complaint of gingival enlargement in the anterior region of upper jaw. History revealed that the growth had started 1 month back and rapidly enlarged to the present size. Her past medical history revealed that the patient was under treatment for hypothyroidism with levothyroxine sodium (100 mcg/day) for the past 5 years. No other medical disorders were observed apart from hypothyroidism.
On extraoral examination, the patient's face was asymmetrical due to an ovoid swelling present on the left side of her face. The swelling was approximately measuring 5 × 5 cm in size, extending from the philtrum of the upper lip to the zygomatic arch anteroposteriorly. Superiorly the swelling was seen extending almost to the floor of the orbit. Inferiorly the swelling extended upto the line joining the corner of the mouth to the tragus of the ear [Figure 1].
Figure 1.
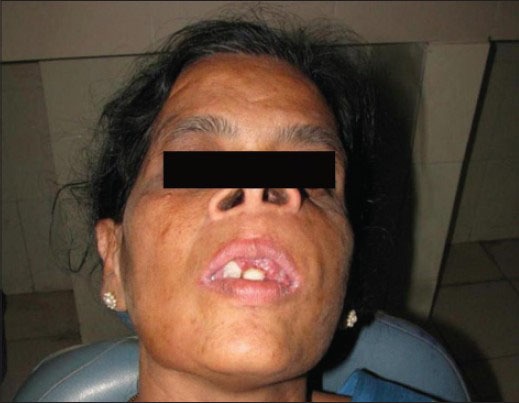
Lesion over the left maxilla producing facial asymmetry
On palpation, the swelling was firm in consistency without any tenderness. Patient's left submandibular lymph nodes were also palpable, nontender and hard in consistency measuring 2 × 2 cm in size.
On intraoral examination, a massive, lobulated, firm, nontender and erythematous growth involving the gingiva was seen extending from the left maxillary lateral incisor to the left maxillary third molar [Figure 2]. The growth was extensive and lobulated, covering the teeth on the left side of the maxilla. Patient's peripheral blood picture was completely normal except for anemia (hemoglobin 7.8 g/dl).
Figure 2.
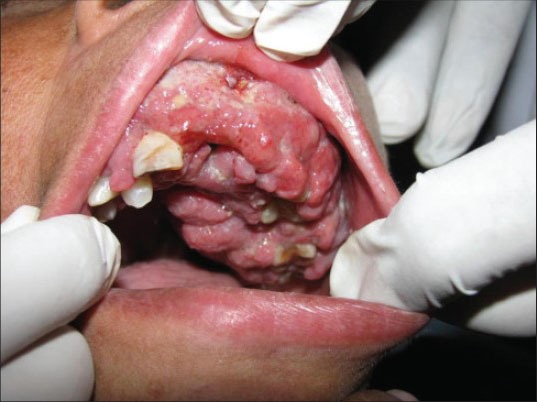
Extensive gingival proliferation of left maxilla covering the teeth in the involved area
Orthopantomograph (OPG) revealed the presence of root stumps in relation to 21, 22 and 25 teeth (Federation Dentaire Internationale World Dental Federation notation) in the lesiona area. However, gross resorption of bone was not evident at the area of lesion [Figure 3]. PNS view (paranasal sinuses radiograph) revealed opacification of the left maxillary sinus, indicating the spread of the lesion to the maxillary sinus. A provisional diagnosis of metastatic tumor of unknown origin was made based on the clinical presentation of rapid growth with enlarged and fixed submandibular lymph nodes.
Figure 3.
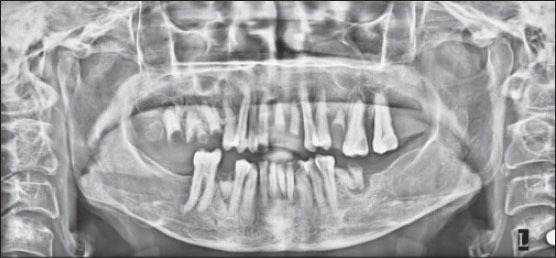
OPG showing root stumps without much destruction of bone
An incisional biopsy was done and the tissue was sent for routine histopathological evaluation. H and E-stained sections showed hyperplastic surface epithelium with extensive aggregates of small round cells with eosinophilic cytoplasm in the underlying connective tissue [Figures 4 and 5]. Tumor cells showed atypical features in the form of cellular and nuclear pleomorphism, hyperchromatism and numerous abnormal mitotic figures. An initial diagnosis of a small round cell tumor was made based on the routine H and E-stained sections.
Figure 4.
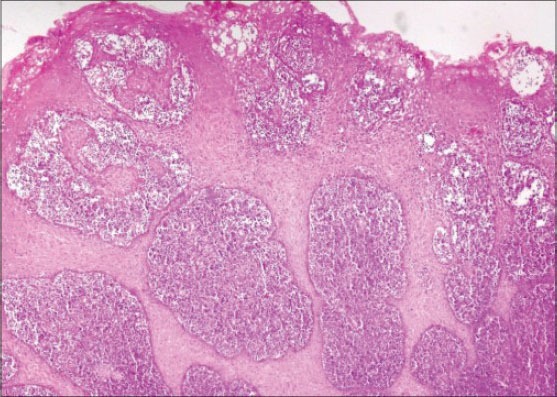
Hyperplastic epithelium with acanthosis (H&E stain, ×40)
Figure 5.
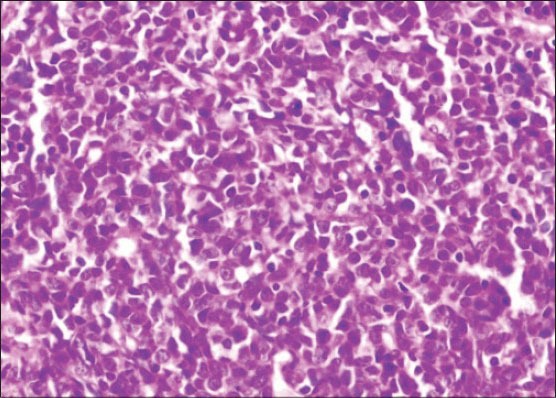
Sheets of tumor cells in the underlying connective tissue (H&E stain, ×400)
On IHC, the lesion stained positive with cluster of differentiation (CD) 45 marker indicating the lesion to be of hematopoietic origin ruling out other small round cell tumors from further consideration. Subsequently, negative staining for CD20 and CD3 markers ruled out B-cell and T-cell lymphomas. Finally, the lesion stained positive with CD68 and CD117 markers establishing the myeloid origin of tumor cells. This favors the diagnosis of AML with minimal differentiation. Because CD68 [Figure 6] and CD117 [Figure 7] are also positive in MS, we used MPO to differentiate the lesion from myeloid leukemia. MPO [Figure 8] was strongly positive in the present case confirming the lesion as MS.
Figure 6.
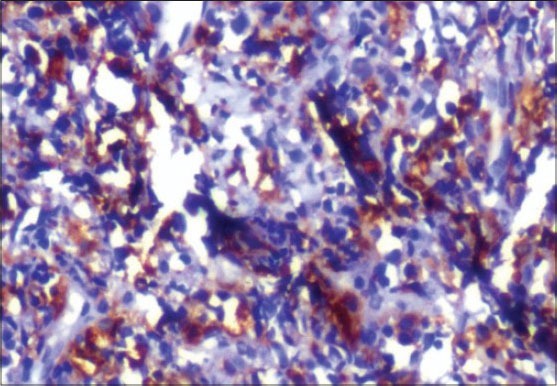
Positive staining with immunohistochemical marker CD68 (IHC stain, ×400)
Figure 7.
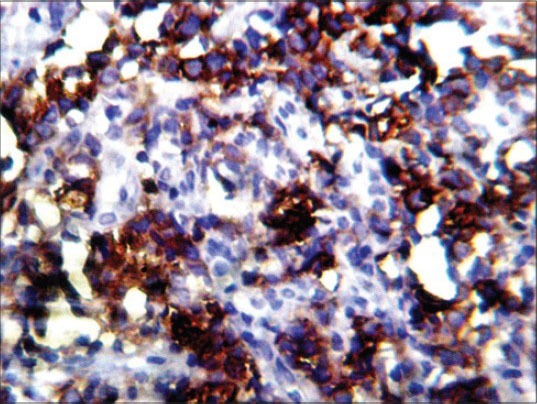
Positive staining with immunohistochemical marker CD117 (IHC stain, ×400)
Figure 8.
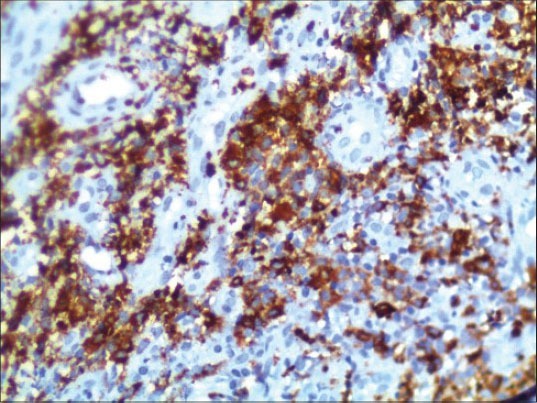
Positive staining with immunohistochemical marker MPO (IHC stain, ×100)
On final diagnosis, the patient was immediately referred to the regional oncology center for further investigations related to the assessment of metastasis and treatment. However, the patient died 2 months after the initial diagnosis of MS on chemotherapy.
DISCUSSION
MS is an extramedullary localized tumor mass composed of immature cells of the granulocytic lineage (myelocytes). These extramedullary tumors occurring before or during myeloid leukemia were termed granulocytic sarcomas, leukocytic sarcomas, chloromas or myelosarcomas. MS is believed to originate from bone marrow and travel via the Haversian canals to reach the subperiosteal bone region, from where the tumor cells spread to other parts of the body.[8] Genetic alterations that have a higher risk for the development of MS were identified, these include t (8;21), t (15;17) or inv (16). In addition, patients with high peripheral white blood cell counts also have a high risk of developing this lesion.[8] These lesions are commonly associated with other hematologic disorders such as myeloid leukemia and other myeloproliferative disorders. Although extremely rare in the oral cavity, this lesion is reported in gingiva, palate, buccal mucosa and extraction sockets.
Our review includes 15 cases of MS published in the English literature from the year 2000 till date. The present review includes clinical features, history of myeloproliferative disorders, IHC markers used for the diagnosis, treatment given and prognosis of the disease process taken from the published articles [Table 1].
Table 1.
Data from the review of literature from the year 2000
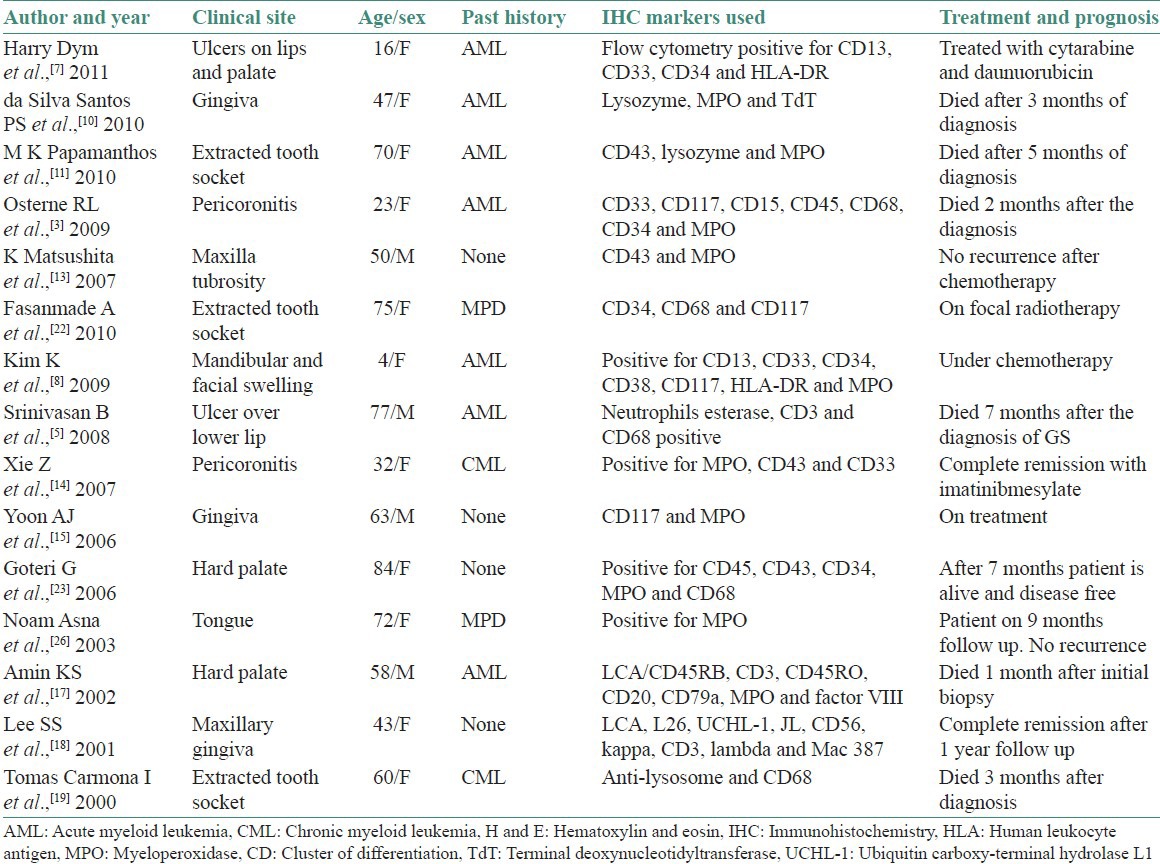
Earlier studies have shown that MS was commonly reported in patients with chronic myeloid leukemia in blast crisis.[9,20,21] However, the current review had shown more number of acute myeloid leukemia transforming into MS.
MSs are reported in a wide variety of anatomical sites, including breast, ovary, brain, gastrointestinal tract and skull. Involvement of oral cavity by MS is rare when compared with acute leukemia, which is well documented in the literature. At times, an oral lesion may be the first sign of the disease process as seen in the present case. In oral cavity, these lesions are commonly reported in gingiva, jaws, palate, extraction tooth sockets and buccal mucosa.[9,13,20,21,22,23]
The clinical behavior of MS is aggressive. These lesions can enlarge rapidly causing extensive bone destruction (intrabony lesions) and invasion of adjacent tissues causing tooth displacement and root resorption. In addition, pressure on the nerves can cause pain, facial paralysis, deafness and blindness depending on the location of involvement.[8,20]
In the present case, the lesion was seen involving the left maxillary gingiva producing obvious facial asymmetry [Figure 1]. The duration of the lesion was very short (1 month) indicating the aggressive nature of the lesion. The involved gingiva was lobulated with a granular surface. Teeth present in the area of the lesion were totally submerged within the enlarging mass of gingiva [Figure 2].
Radiographically, MS involving the jaws shows radiolucent areas with diffuse margins. Periosteal changes with elevation of the periosteum producing sunburst appearance can also be seen as in the case of Ewing's sarcoma or osteosarcoma.[8] In addition, perforation of cortical plates is reported in case of intrabony lesions. The present case was seen as an extensive enlarging mass of gingiva. An OPG was taken to assess the involvement of bone below the proliferating gingiva. OPG showed mild to modest loss of alveolar bone below the involved gingiva [Figure 3].
In the present case, the patient's peripheral blood smear showed microcytic hypochromic anemia without any evidence of atypical cells. As the patient's clinical, radiographic and peripheral blood smear investigations did not reveal any underlying disease process, the patient was advised for an incisional biopsy for histopathological examination.
The diagnosis of MS is challenging, particularly when there is no history of hematological disorders or peripheral blood or bone marrow involvement. As the clinical features of this lesion are nonspecific, it is hard to suspect this lesion based on clinical and radiographic features alone. However, this lesion should always be suspected in individuals affected by myeloproliferative neoplasms.[8,9,20,21] In the present case, the peripheral smear was normal despite histological and immunohistochemical confirmation as MS.
The diagnosis of MS based on routine H and E stains is confusing, as the histological features are similar to that of Hodgkin's lymphoma, Burkitt's lymphoma, large cell lymphomas and small round blue cell tumors, such as neuroectodermal tumors. Therefore, the diagnosis of this lesion is based on the demonstration of MPO, lysozyme and other markers directed against the cells of myeloid lineage.[8,9,20,21]
MPO can be identified by Sudan black B or by peroxidase or diaminobenzidine reactions. MPO is localized in the primary granules of the myeloid cells and is synthesized early in the differentiation and thus serves as an important marker for the identification of cells with myeloid lineage.[5] Immunoperoxidase, naphthol ASD-chloroacetate esterase and alpha-naphthyl acetate esterase are also useful for detection of MPO in the granules.[21]
IHC can be started with CD45 to confirm the hematological origin of the disease process. Subsequently, one or more markers indicative of myeloid origin can be used to confirm the disease process. These include MPO, lysozyme, CD13, CD 14, CD33, CD34, CD68 and CD117.[8,9,13,16,20,21,22,23]
Alexiev et al., studied the histologic and immunohistochemical profiles of 13 cases of MS on formalin-fixed paraffin-embedded tissue samples.[6] They concluded that immunohistochemical panel including CD43, lysozyme, MPO, CD68 or CD163, CD117, CD3 and CD20 can successfully identify vast majority of MS variants in formalin-fixed paraffin-embedded tissue sections. The use of a large IHC panel has been suggested by most authors when making a differential diagnosis between GS and lymphoid as well as other myeloid tumors.[6,7,8] However, a concise IHC panel including CD20, CD43, CD68 and MPO can successfully identify the majority of extramedullary myeloid tumors.[8,9,20,21] In the present case, H and E-stained sections showed hyperplastic epithelium [Figure 4] with sheets of tumor cells in the underlying connective tissue [Figure 5]. The findings of H and E were not conclusive, as these features might be seen in any of the lymphocytic lesions discussed above. Positive staining with CD45 IHC marker identified this lesion to be of hemopoietic origin. Further positive staining with CD68 [Figure 6] and CD117 [Figure 7] and MPO [Figure 8] confirmed the lesion as MS.
Treatment advocated for MS includes high-dose chemotherapy for the patients who have MS in addition to AML.[12,24] Radiotherapy is advocated for MS not associated with other myeloproliferative disorders.[25,26]
The patient succumbed to the disease shortly after the diagnosis of MS. Earlier studies on MS had shown similar results with respect to the survival of the patients with MS. In most cases, death occurred during the first year after the diagnosis of MS.[12] A retrospective study done by Cankaya et al., in 2001 showed that patients with head and neck MS with associated AML had a 100% mortality rate.[12] A remission period ranging from 2 to 29 months was observed in their review after chemotherapy (one patient had both radiotherapy and chemotherapy) and all the patients succumbed to death after recurrence. Tsimberidou et al., in their study found that the median survival of patients treated with chemotherapy was 15 months and the projected 3-year survival was 30%.[25] Data from the current review of published cases from the year 2000 show that majority of the patients diagnosed with MS are alive and disease free with appropriate treatment [Table 1]; however, the chances of recurrence in these individuals cannot be ruled out in the near future.
CONCLUSION
We report a rare and aggressive case of MS, which ran a fatal course in a 45-year-old female patient. The patient presented with a rapidly enlarging gingiva lesion of 1 month duration. The lesion was diagnosed as MS after extensive immunohistochemical studies that stained positive for MPO, CD45, CD68 and CD117. Our case shows the importance of early diagnosis and prompt treatment of those patients affected by Myeloid sarcoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Burns A. 1st edition. Edinburgh: Thomas Royce and Co; 1811. Observations on the surgical anatomy of the head and neck; pp. 364–66. [Google Scholar]
- 2.Rappaport H. Atlas of Tumour Pathology. Section. III, Fascicle 8. Washington DC: Armed Forces Institute of Pathology; 1967. Tumours of the hematopoietic system; pp. 241–7. [Google Scholar]
- 3.Osterne RL, Matos-Brito RG, Alves AP, Nogueira TN, Rocha-Filho FD, Meneses FA, et al. Oral granulocytic sarcoma: A case report. Med Oral Patol Oral Cir Bucal. 2009;14:E232–5. [PubMed] [Google Scholar]
- 4.Barker GR, Sloan P. Maxillary chloroma: A myeloid leukaemic deposit. Br J Oral Maxillofac Surg. 1988;26:124–8. doi: 10.1016/0266-4356(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan B, Ethunandan M, Anand R, Hussein K, Ilankovan V. Granulocytic sarcoma of the lips: Report of an unusual case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e34–6. doi: 10.1016/j.tripleo.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH, et al. Myeloidsarcomas: A histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;2:42. doi: 10.1186/1746-1596-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dym H, Movahed R. Granulocytic sarcoma of palate. Case report and review of literature. N Y State Dent J. 2011;77:24–7. [PubMed] [Google Scholar]
- 8.Kim K, Velez I, Rubin D. A rare case of granulocytic sarcoma in the mandible of a 4-year-old child: A case report and review of the literature. J Oral Maxillofac Surg. 2009;67:410–6. doi: 10.1016/j.joms.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Tong AC, Lam KY. Granulocytic sarcoma presenting as an ulcerative mucogingival lesion: Report of a case and review of the literature. J Oral Maxillofac Surg. 2000;58:1055–8. doi: 10.1053/joms.2000.8752. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Santos PS, Fontes A, de Andrade F, de Sousa SC. Gingival leukemic infiltration as the first manifestation of acute myeloid leukemia. Otolaryngol Head Neck Surg. 2010;143:465–6. doi: 10.1016/j.otohns.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Papamanthos MK, Kolokotronis AE, Skulakis HE, Fericean AM, Zorba MT, Matiakis AT. Acute myeloid leukaemia diagnosed by intra-oral myeloid sarcoma. A case report. Head Neck Pathol. 2010;4:132–5. doi: 10.1007/s12105-010-0163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cankaya H, Ugras S, Dilek I. Head and neck granulocytic sarcoma with acute myeloid leukemia: Three rare cases. Ear Nose Throat J. 2001;80:224. [PubMed] [Google Scholar]
- 13.Matsushita K, Abe T, Takeda Y, Takashima H, Takada A, Ogawa Y, et al. Granulocytic sarcoma of the gingiva: Two case reports. Quintessence Int. 2007;38:817–20. [PubMed] [Google Scholar]
- 14.Xie Z, Zhang F, Song E, Ge W, Zhu F, Hu J. Intraoral granulocytic sarcoma presenting as multiple maxillary and mandibular masses: A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e44–8. doi: 10.1016/j.tripleo.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Yoon AJ, Pulse C, Cohen LD, Lew TA, Zegarelli DJ. Myeloid sarcoma occurring concurrently with drug-induced gingival enlargement. J Periodontol. 2006;77:119–22. doi: 10.1902/jop.2006.77.1.119. [DOI] [PubMed] [Google Scholar]
- 16.Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in non-leukemic patients. Cancer. 1986;58:2697–709. doi: 10.1002/1097-0142(19861215)58:12<2697::aid-cncr2820581225>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Amin KS, Ehsan A, McGuff HS, Albright SC. Minimally differentiated acute myelogenous leukemia (AML-M0) granulocytic sarcoma presenting in the oral cavity. Oral Oncol. 2002;38:516–9. doi: 10.1016/s1368-8375(01)00085-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Kim HK, Choi SC, Lee JI. Granulocytic sarcoma occurring in the maxillary gingiva demonstrated by magnetic resonance imaging. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:689–93. doi: 10.1067/moe.2001.118287. [DOI] [PubMed] [Google Scholar]
- 19.Tomás Carmona I, Cameselle Teijeiro J, Diz Dios P, Fernández Feijoo J, Limeres Posse J. Intra-alveolar granulocytic sarcoma developing after tooth extraction. Oral Oncol. 2000;36:491–4. doi: 10.1016/s1368-8375(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 20.Koudstaal MJ, van der Wal KG, Lam KH, Meeuwis CA, Speleman L, Levin MD. Granulocytic sarcoma (chloroma) of the oral cavity: Report of a case and literature review. Oral Oncol Extra. 2006;42:70–7. [Google Scholar]
- 21.Eisenberg E, Peters ES, Krutchkoff DJ. Granulocytic sarcoma (chloroma) of the gingiva: Report of a case. Oral Maxillofac Surg. 1991;49:1346–50. doi: 10.1016/0278-2391(91)90317-f. [DOI] [PubMed] [Google Scholar]
- 22.Fasanmade A, Pring M, Pawade J, Guest P, Bell C. Rapidly progressing mass of anterior mandible following a dental extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:330–4. doi: 10.1016/j.tripleo.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Goteri G, Ascani G, Messi M, Filosa A, Segura-Egea JJ, Rubini C, et al. Myeloid sarcoma of the maxillary bone. J Oral Pathol Med. 2006;35:254–6. doi: 10.1111/j.1600-0714.2006.00336.x. [DOI] [PubMed] [Google Scholar]
- 24.Brunning RD, McKenna RW. Washington DC: Armed Forces Institute of Pathology; 1994. Tumors of Bone Marrow, Third Series, Fascicle 9; pp. 19–37. [Google Scholar]
- 25.Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–3. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 26.Asna N, Cohen Y, Ben-Yosef R. Primary radiation therapy for solitary chloroma of oral tongue. Isr Med Assoc J. 2003;5:452. [PubMed] [Google Scholar]


