Abstract
Context:
The correlation of serum and salivary lipid profile has been poorly characterized. The most commonly used laboratory diagnostic procedures for lipid profile involve analysis of cellular and chemical constituents of blood/plasma. As a diagnostic aid, saliva offers many advantages over serum.
Aims:
To evaluate and compare the serum and salivary lipid profile levels in healthy individuals and to validate the role of saliva as a non-invasive diagnostic tool for assessing lipid profile.
Settings and Design:
The present study was a prospective study.
Materials and Methods:
A total of 100 healthy study subjects who had no complaint or any major illness in recent past were selected. The parameters assessed included serum and salivary: total cholesterol (TC), high-density lipoprotein-cholesterol (HDLC), low-density lipoprotein-cholesterol (LDLC), very low-density lipoprotein-cholesterol (VLDLC) and triglycerides (TGL).
Statistical Analysis Used:
Evaluation of results and statistical analysis was carried out using descriptive, correlation and regression analysis.
Results:
There was a moderate level of correlation between serum and salivary TC, TGL, HDLC and VLDLC and there was a low and quite small correlation between serum and salivary LDLC. For all the five parameters assessed as a part of lipid profile, the correlation coefficients were highly significant statistically and also, with an increase in the serum mean values, corresponding increase in the saliva mean values for all the five parameters was noted.
Conclusions:
From the present study we conclude that saliva can be used as a non-invasive diagnostic tool for assessing lipid profile.
Keywords: Lipids, saliva, serum
INTRODUCTION
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. The biological functions of the lipids are as diverse as their chemistry.[1] The lipid profile is a group of tests that typically includes total cholesterol (TC), triglycerides (TGL), high-density lipoprotein-cholesterol (HDLC), low-density lipoprotein-cholesterol (LDLC) and very low-density lipoprotein-cholesterol (VLDLC).
The correlation of serum and salivary lipid profile has been poorly characterized. The most commonly used laboratory diagnostic procedures for lipid profile involve analysis of cellular and chemical constituents of blood/plasma. Other biological fluids have also been used for similar biochemical tests, among which saliva offers some distinct advantages.[2] Because lipids are secreted in saliva, it can be used for evaluation of lipid profile.[3,4]
As a clinical tool, saliva has many advantages over serum. It includes ease of collection by individuals with modest training, ease of storing and shipping. For patients, the non-invasive collection techniques dramatically reduce anxiety, discomfort and simplify procurement of repeated samples for monitoring overtime. Saliva is easier to handle for diagnostic procedures because it does not clot, thus lessening the manipulations required. No special equipment is needed for collection of saliva. Further, analysis of saliva may provide a cost-effective approach for the screening of large populations.[2,5,6,7]
Thus, the present study aimed to evaluate and compare the serum and salivary lipid profile levels in healthy individuals and to validate the role of saliva as a non-invasive diagnostic tool for assessing lipid profile.
MATERIALS AND METHODS
The institutional ethical committee approved this study. A total of 100 healthy individuals who had no complaint or any major illness in recent past were included in the study; medically compromised patients and patients with other systemic illness were excluded. Since investigation of saliva for diagnostic purposes has been used in various diseases such as bacterial, viral and fungal infections, cancer, hereditary diseases, cardiovascular diseases and also in autoimmune diseases, saliva was used in the present study to assess the various lipid parameters.[2,5,7]
After obtaining prior informed written consent, the detailed history of the patients was taken and oral examination was done and subsequently recorded. Saliva (2 ml) and blood (2 ml) samples were collected from each participant after overnight fasting (12-14 h). Blood samples were drawn from antecubital vein with minimal trauma under aseptic conditions. Saliva samples were collected under resting conditions following flushing of mouth with 100 ml of distilled water.
The patients were given detailed information about the collection protocol: the importance of the exact timing of the samples, to brush the teeth properly without toothpaste by using bass technique and to use dental floss before the collection, to avoid fluid (apart from water) ingestion and chewing gum for at least 30 min before collection and to rinse the mouth with water (preferably distilled).
In this study, unstimulated saliva was used as stimulation affects quantity, concentration and pH of saliva. The best two ways to collect whole saliva are the draining method, in which the saliva is allowed to drip off the lower lip and the spitting method, in which the subject expectorates into a test tube.[2] In this study spitting method was used.
Lipid analysis was done on a fully automated analyzer based on spectrophotometric principle using kits obtained from ERBA diagnostics (Transasia Bio-Medicals Ltd, Germany). The serum and salivary lipid profile was analyzed on the same day of the withdrawal of blood and saliva.
The serum and salivary TC was estimated by taking 10 μl of distilled water, 10 μl of sample and 10 μl of cholesterol standard in separate test tubes. In all, 1000 μl of cholesterol reagent was added to all test tubes. The mixtures were incubated at 37°C for 10 min and the absorbance of standard and sample was measured against the blank at 505 nm in the analyzer.
The serum and salivary TGL was estimated by taking 10 μl of distilled water, 10 μl of sample and 10 μl of TGL standard in separate test tubes. 1000 μl of TGL reagent was added to all test tubes. The mixtures were incubated at 37°C for 10 min and the absorbance of standard and sample was measured against the blank at 505 nm in the analyzer.
Serum and salivary HDLC was estimated by mixing 250 μl of serum and saliva sample with 500 μl of HDL-precipitating reagent in separate test tubes, followed by 10 min incubation at room temperature. Mixtures were centrifuged at 4000 rpm for 10 min to obtain a clear supernatant. In all, 50 μl of distilled water, 50 μl of supernatant and 50 μl of HDLC standard were taken in separate test tubes. In all, 1000 μl of cholesterol reagent was added to all test tubes. The mixtures were incubated at 37°C for 10 min and the absorbance of standard and sample was measured against the blank at 505 nm in the analyzer.
Serum and salivary LDLC and VLDLC levels were calculated as shown below:[8]
LDLC = total cholesterol−(VLDLC)−(HDLC)
VLDLC = triglycerides/5
Statistical analysis
Evaluation of results and statistical analysis was carried out using descriptive, correlation and regression analysis. In all the above-mentioned tests, P < 0.05 was taken to be statistically significant.
RESULTS
The minimum values, the maximum values, the average values and the lower and upper limits for 95% confidence interval (CI) observed for each of the five parameters assessed as a component of lipid profile are depicted in Table 1.
Table 1.
Lipid profile values in serum and saliva in study subjects (mg/dl)
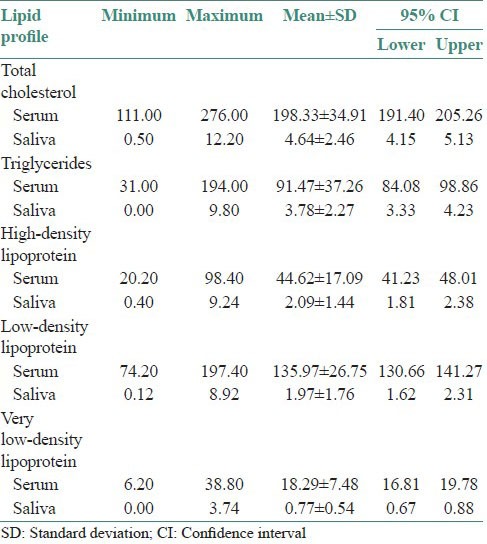
In the present study, as can be appreciated in Table 2, we observed a moderate level of correlation between serum and salivary TC (r = 0.5384), TGL (r = 0.4822), HDLC (r = 0.5689) and VLDLC (r = 0.4388), whereas as far as LDLC is concerned, we observed a low and quite small correlation (r = 0.3182) between serum and salivary values. But for all the five parameters assessed as a part of lipid profile, the correlation coefficients were highly significant statistically (P < 0.01).
Table 2.
Correlation between serum and salivary lipid profile in study subjects using Pearson's correlation
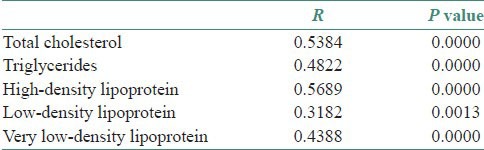
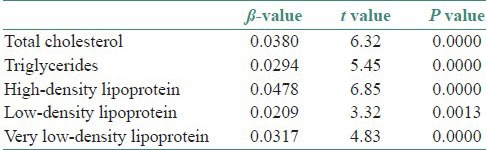
Table 3 shows β-value and corresponding t and P values for serum and salivary lipid profile in study subjects. The principle used for interpretation in this table is that for every one unit change in independent variable there will be change in the dependent variable to an extent of ί-value. The serum values of parameters assessed served as independent variable and the salivary values served as dependent variable in our study. The table depicts the quantum of change in the salivary values of TC, TGL, HDLC, LDLC and VLDLC, when there was one unit change in the serum values.
Table 3.
ß-value and corresponding t and P values for serum and salivary lipid profile in study subjects
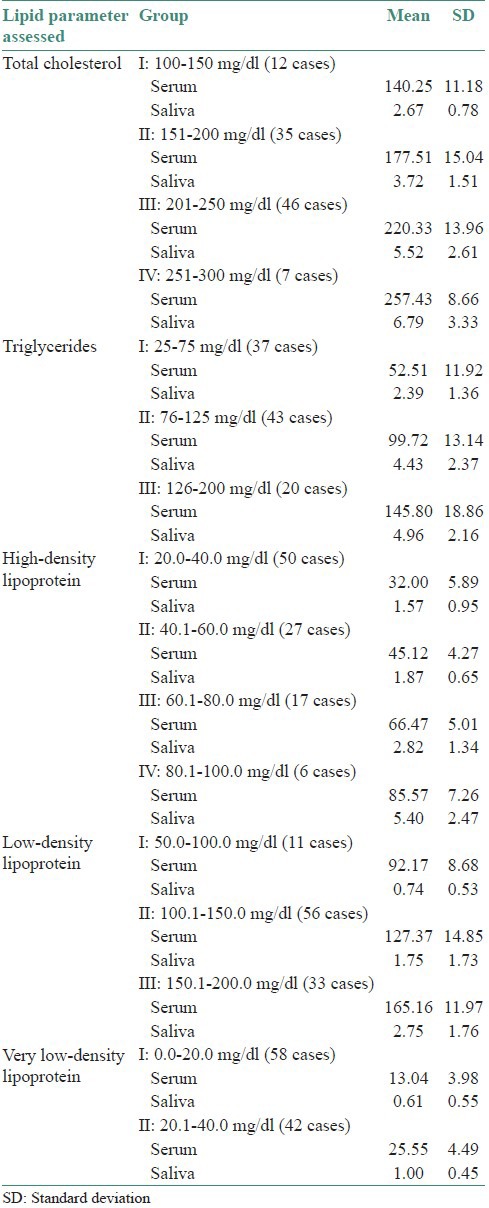
Based on the values obtained, the data were divided into different groups in an ascending order, as can be appreciated in Table 4. The table depicts that as we move from the first group to the last group, in each of the five parameters assessed, there is an increase in the serum mean values. Correspondingly there is an increase in the saliva mean values for all the five parameters.
Table 4.
Division of lipid parameters assessed in serum and saliva into different groups
DISCUSSION
Lipids are a heterogenous group of biomolecules that are generally insoluble in water but readily dissolve in non-polar solvents, such as ether and chloroform. They are major cell membrane components essential for various biological functions including cell growth and division of normal and malignant tissues. Cholesterol is essential for maintenance of the structural and functional integrity of all biological membranes. It is also involved in the activity of membrane-bound enzymes and is important for stabilization of DNA helix.[8,9,10]
In the present study, significant positive moderate degree of correlation was found between serum and salivary TC, TGL, HDLC and VLDLC levels and a significant but low and quite small correlation was found between salivary and serum LDLC levels.
Karjalainen et al., (1997)[3] conducted the study based on relationship of salivary cholesterol of healthy adults in relation to serum cholesterol concentration and oral health. Weak positive correlations between saliva and serum cholesterol concentrations and saliva and serum non-HDLC concentrations were found. It was concluded that in healthy adults saliva cholesterol concentrations reflect serum concentrations to some extent and can be used to select individuals with high serum cholesterol levels. In our study, instead of weak positive correlations, moderate positive correlation was seen between saliva and serum cholesterol concentrations. Thus, it was inferred that salivary lipid profile values do reflect serum lipid values.
Alagendran et al., (2009)[11] in their study to assess the usefulness of saliva as a biomarker of ovulation detection showed that saliva can be used to test cholesterol and phospholipids instead of blood. The present study also suggests that saliva may be used for TC, TGL, HDLC, VLDLC and to some extent for LDLC monitoring, as these follow the course of plasma lipid profile and show a positive correlation.
Al Rawi (2010, 2011),[12,13] did two different studies and compared plasma and salivary lipid profile in individuals with ischemic heart stroke and the diabetes mellitus and suggested that lipid fractions particularly TGL can be assessed in saliva and may be used alone or in combination with other lipid parameters for monitoring disease activity and severity in such studies. The results of our study suggest that saliva can be used to assess, not only TGL but also TC, HDLC, VLDLC and to some extent LDLC.
In the present study, there was an increase in the saliva mean values corresponding to the increase in serum mean values for all the parameters. This means that some amount of plasma lipid components are filtered into the saliva in proportion to the plasma lipid levels. There are several possible mechanisms by which serum lipids can reach saliva.
Within the salivary glands, transfer mechanisms include intracellular and extracellular routes. The most common intracellular route is passive diffusion, although active transport has also been reported. Ultrafiltration, which occurs through the tight junctions between the cells, is the most common extracellular route. In contrast, a serum molecule reaching saliva by diffusion must cross five barriers: The capillary wall, interstitial space, basal cell membrane of the acinus cell or duct cell, cytoplasm of the acinus or duct cell and the luminal cell membrane.
Lipids may also be found in whole saliva as a result of gingival crevicular fluid outflow. Lipids may also originate from several membranes such as secretory vesicles, microsomes, lipid rafts and other plasma and intracellular membrane fragments of lysed cells and bacteria, although the lower percentage of phospholipids indicates that the salivary lipids are not primarily of membrane origin. A large portion of salivary lipids is associated with proteins, especially to high molecular weight glycoproteins (i.e., mucins) and to proline rich proteins (PRPs).[2,14,15] Keeping these factors in mind, the study samples were given detailed information about the collection protocol.
The results of the present study support that saliva can be used as a non-invasive diagnostic tool in assessing lipid profile levels, to support this concept are the findings of earlier studies that have been done in other diseases such as detecting various infections; assessing tumor markers; monitoring pharmaceutical and abuse drugs; analyzing hormone levels and also in hereditary and autoimmune diseases.[2,5,7]
CONCLUSION
The results of the present study show that there was a moderate level of correlation existing between serum and salivary TC, TGL, HDLC and VLDLC; a low and quite small correlation exists between serum and salivary LDLC. For all the five parameters assessed as a part of lipid profile, the correlation coefficients were highly significant statistically. With an increase in the serum mean values, correspondingly there was an increase in the saliva mean values for all the five parameters.
From the present study we conclude that saliva can be used as a non-invasive diagnostic tool for assessing lipid profile. However, before a salivary diagnostic test can replace a more conventional one, its diagnostic value has to be determined in terms of sensitivity, specificity, correlation with established disease diagnostic criteria and reproducibility.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nelson DL, Cox MM. Lipids. In: Nelson DL, Cox MM, editors. Lehninger principles of biochemistry. 3rd ed. New York: Worth Publishers; 2000. pp. 363–88. [Google Scholar]
- 2.Kaufman E, Lamster IB. The diagnostic applications of saliva: A review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 3.Karjalainen S, Sewon L, Soderling E, Larsson B, Johansson I, Simell O, et al. Salivary cholesterol of healthy adults in relation to serum cholesterol concentration and oral health. J Dent Res. 1997;76:1637–43. doi: 10.1177/00220345970760100401. [DOI] [PubMed] [Google Scholar]
- 4.Slomiany BL, Zdebska E, Murty VL, Slomiany A, Petropoulou K, Mandel ID. Lipid composition of human labial salivary gland secretions. Arch Oral Biol. 1983;28:711–4. doi: 10.1016/0003-9969(83)90105-x. [DOI] [PubMed] [Google Scholar]
- 5.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159–78. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc. 2006;137:313–21. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 7.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel PS, Shah MH, Jha FP, Raval GN, Rawal RM, Patel MM, et al. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J Cancer. 2004;41:25–31. [PubMed] [Google Scholar]
- 9.Lohe VK, Degwekar SS, Bhowate RR, Kadu RP, Dangore SB. Evaluation of correlation of serum lipid profile in patients with oral cancer and precancer and its association with tobacco abuse. J Oral Pathol Med. 2010;39:141–8. doi: 10.1111/j.1600-0714.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Ramesh V, Premalatha B, Prashad KV, Ramadoss K. Alterations in serum lipid profile patterns in oral cancer. J Nat Sci Biol Med. 2013;4:374–8. doi: 10.4103/0976-9668.116994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alagendran S, Archunan G, Prabhu SV, Orozco BE, Guzman RG. Biochemical evaluation in human saliva with special reference to ovulation detection. Indian J Dent Res. 2010;21:165–8. doi: 10.4103/0970-9290.66625. [DOI] [PubMed] [Google Scholar]
- 12.Al-Rawi NH. Salivary lipid peroxidation and lipid profile levels in patients with recent ischemic stroke. J Int Dent Med Res. 2010;3:57–64. [Google Scholar]
- 13.Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–8. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- 14.Drobitch RK, Svensson CK. Therapeutic drug monitoring in saliva. An update. Clin Pharmacokinet. 1992;23:365–79. doi: 10.2165/00003088-199223050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]


