Abstract
Background and Objectives:
Metabolic disorders, oral precancerous conditions and oral cancer are accompanied by alterations in the concentration of one or more trace elements like copper, iron, zinc, magnesium etc., in some body fluids, especially blood serum or plasma, which can help not only in the early diagnosis and treatment but also in prognosis. The objective of the study is to evaluate the levels of circulating trace elements (copper, iron, magnesium, zinc and calcium) in serum of patients with Oral Submucous Fibrosis (OSF) and Oral Squamous Cell Carcinoma (OSCC), to analyze the alteration and identify the best predictors amongst these parameters for disease occurrence and progression and their association with areca nut and betel quid chewing habits.
Materials and Method:
Serum levels of trace elements (copper, iron, magnesium, zinc and calcium) were estimated using electronic absorption colorimetric method. These levels were compared with controls and statistically evaluated using ANOVA and POST-HOC TUKEY tests.
Results:
The data analysis revealed that serum copper levels increased gradually from precancer to cancer, as the duration of betel quid chewing habit increased. However, serum iron, magnesium, zinc levels were decreased significantly in both the groups. Serum calcium levels were increased in the cancer group owing to bone resorption in the later stages of the disease, whereas it was close to normal in OSF patients. Among all the trace elements, the best predictor for occurrence of both the lesions was copper.
Conclusion:
The present study shows that the above trace elements may be associated with the pathogenesis and progression of OSF and OSCC. Betel quid and areca nut chewing habits are frequently associated with both disease states and may play a role in altering the serum levels of these trace elements. Concerted efforts would, therefore, help in early detection, management and monitoring the efficacy of treatment.
Keywords: Arecanut, betel quid chewing habit, oral squamous cell carcinoma, oral submucous fibrosis, serum trace elements
INTRODUCTION
Oral cancer is one of the 10 most common forms of cancer among men in developed countries and is ranked as the sixth most common cancer around the world.[1,2] Oral cancer accounts for approximately 4% of all cancers and 2% of all cancer deaths worldwide. The World Health Organization (WHO) reported oral squamous cell carcinoma (OSCC) as having one of the highest mortality ratios amongst all malignancies.[3] In India OSCC accounts for 50-70% of all cancers.[4] Around 90-95% of oral cancers occur predominantly in alcohol and tobacco users, between the 6th and 7th decades of life.[5] Despite the large amount of research data in cellular and molecular biology and advances in oncology and surgery, the mortality and morbidity rates in OSCC patients remain unchanged.[6]
Oral submucous fibrosis (OSF) is an insidious, chronic and debilitating high risk precancerous condition affecting any part of the oral cavity and sometimes the pharynx, commonly found in patients of south Asian origin.[7] It is characterized by changes in the connective tissue fibers of the lamina propria and deeper parts leading to stiffness of the mucosa and restricted mouth opening. Various studies have shown that areca nut chewing, nutritional deficiencies, immunity of the person and genetic predisposition play a part in the initiation and progression of the disease process. OSF is strongly associated with a risk of OSCC with a malignant transformation rate of 7.6% over a period of 17 years.[8] The prevalence of oral precancerous lesions is much higher than that of oral cancer and these lesions provide useful clinical markers for oral cancer.[9]
Trace elements are required in small concentrations as essential components of biological enzyme systems or structural portions of biologically active constituents.[10] Many metabolic disorders, oral precancerous conditions and oral cancer are accompanied by alterations in the concentration of one or more trace elements like copper, iron, zinc, magnesium, etc., in some body fluids, especially blood serum or plasma.[11]
Serum trace element levels also give an idea about necessary elements to be given as supplements to counteract the oxidative stress induced by free radicals which can cause serious damage to cells in oral precancerous and cancerous conditions.[12] Biochemical alterations in the serum of patients with precancerous condition and oral cancer can help not only in the early diagnosis and treatment but also in prognosis, as the disease progresses.[9]
The objective of the present study was to measure and compare with a control group the concentrations of selected trace elements namely copper (Cu), iron (Fe), magnesium (Mg), zinc (Zn) and calcium (Ca) in the serum of untreated patients with OSCC and OSF.
MATERIALS AND METHODS
A total of 90 individuals from our dental college and other Dental Sciences and Research Centre, Bangalore and other dental colleges were included in the present study who were divided into study and control group. The study group consisted of 30 patients diagnosed with OSCC (Group I) and 30 patients diagnosed with OSF (Group II). Both groups had habit of areca nut and betel quid chewing. Control group consisted of 30 individuals with no lesion and no habit (Group III). After obtaining a detailed case history and duly signed informed consent form from all the participants, 5 ml of venous blood was obtained and centrifuged at 2,500 rpm and serum was collected in sterile vaccutainers. The chemical reagents for specific trace elements and serum samples were pipetted into clean test tubes labeled as blank (B), standard (S), test (T) and mixed well and incubated at room temperature (25°C) for 5 min to ensure the formation of colored complexes specific for each trace element. The electronic absorption colorimeter was adjusted to specific wavelengths between 510 and 580nm at room temperature (25°C) as specified in the manual obtained along with the reagent kits (Crest Biosystems Ltd). The absorbance of the standard (Abs.S) and test sample (Abs.T) against the blank (Abs.B) was measured within 30 min. The absorbance values thus obtained were substituted in the formula mentioned in the kit manual to obtain the serum levels and then were compared with the normal serum levels mentioned in the manual.
RESULTS
Observations
Serum levels of the various trace elements were statistically analyzed in all the three groups included in the study using analysis of variance (ANOVA) test and intergroup relationship was assessed using post-hoc Tukey test.
The mean serum copper, iron, zinc and magnesium levels in group I and II were equal, but comparison between Groups I and III and Groups II and III showed a p < 0.001 suggesting strong statistical significance [Figure 1a–d and Table 1].
Figure 1.
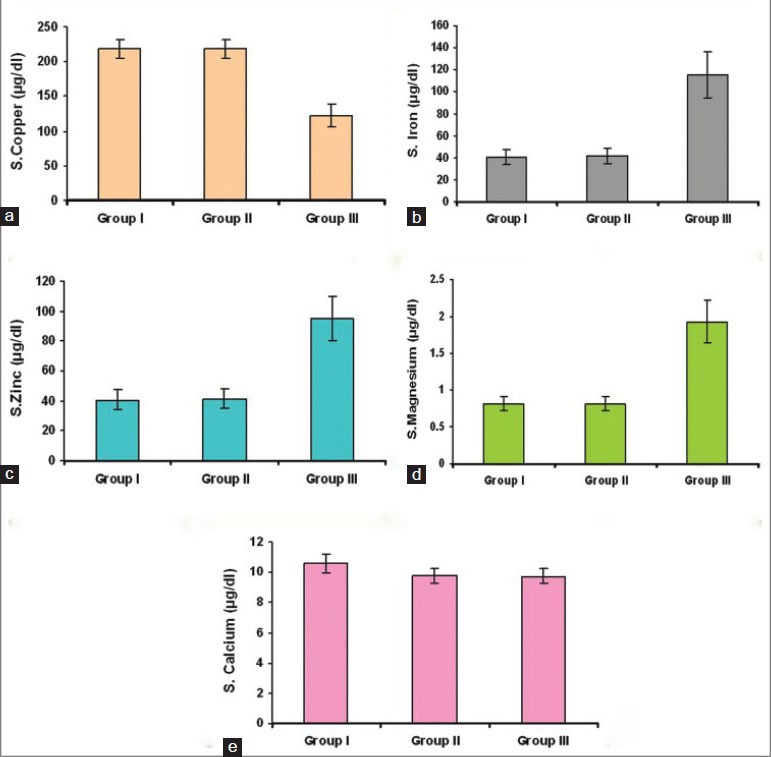
(a) Bar diagram showing serum levels of copper in the three groups, (b) Bar diagram showing serum levels of iron in the three groups, (c) Bar diagram showing serum levels of zinc in the three groups, (d) Bar diagram showing serum levels of magnesium in the three groups, (e) Bar diagram showing serum levels of calcium in the three groups
Table 1.
Mean and standard deviation of serum levels of all the five trace elements and intergroup comparison with statistical values in the three groups
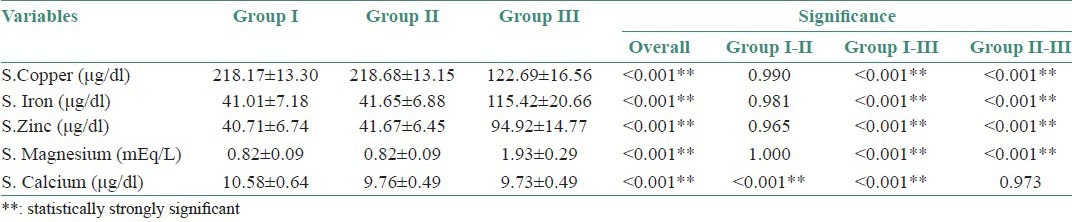
The mean and standard deviation of the serum levels of calcium in Group II and III were almost equal. Comparison between Groups I and II and Group I and III showed a P < 0.001 suggesting high statistical significance [Figure 1e, and Table 1].
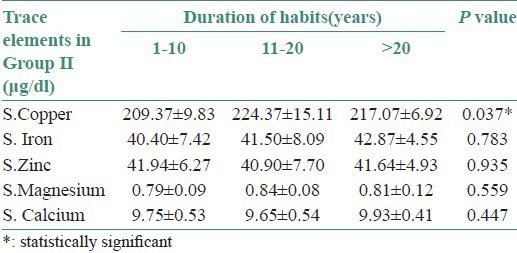
The serum levels of iron, magnesium, zinc and calcium were decreased (no statistical significance) and copper levels were increased in both Groups I and II with a duration of habit between 11 and 20 years (moderate statistical significance) [Figure 2a and b and Tables 2 and 3].
Figure 2.
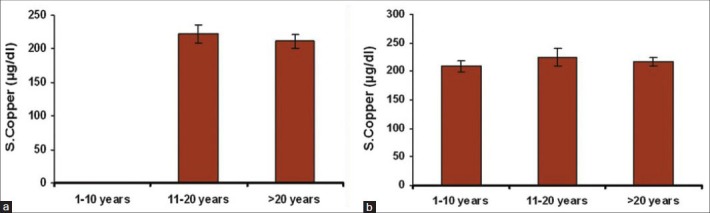
(a) Bar diagram showing correlation of mean serum copper levels with the duration of habits in Group I, (b) Bar diagram showing correlation of mean serum copper levels with the duration of habits in Group II
Table 2.
Correlation of mean serum levels of all the trace elements with duration of habits in Group I
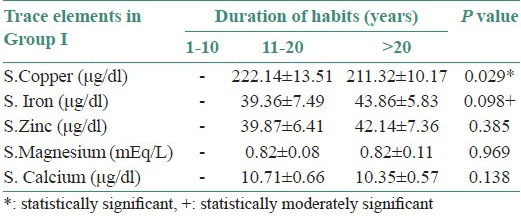
Table 3.
Correlation of mean serum levels of all the trace elements with duration of habits in Group II
In Group III, the serum levels of all the trace elements were almost in the normal range.
DISCUSSION
The rate at which oral precancerous and cancerous lesions are spreading like an epidemic is alarming. The prevalence of oral precancerous lesions is much higher than that of oral cancer and these lesions provide useful clinical markers for oral cancer.[13]
Currently, areca nut chewing is considered to be the most important etiologic factor for OSF.[14] The etiology of OSCC includes various carcinogens in tobacco and related products such as polynuclear aromatic hydrocarbons and nitrosamines.[5]
Over the years, awareness that trace elements play a very important role, either beneficial or harmful, in human health has increased. Any constituent whose concentration is equal to or less than 0.01% (100 parts per million) of the total matrix has been defined as “trace constituent”. In many studies, it was found that three-dimensional active conformations of many proteins like thymidylate synthetase, dihydrofolate reductase, p53, p16, K-ras, etc., are maintained by trace elements. Many metabolic disorders in man are accompanied by alterations in the concentration of one or more trace elements in some body fluids, especially blood serum or plasma.[11,15]
In our study, we observed that the mean age of disease presentation was clustered around 56 years in Group I patients with a percentage of 53.3 and around 44 years in Group II patients with a percentage of 33.3. The predominant habit prevalent in the two study groups was use of betel quid, areca nut and slaked lime (21.7%). Similar observations have been reported in the studies conducted by Pindborg et al., (1984); Seedat and Van Wyk (1988); Sinor et al., (1990); Maher et al., (1994); Murti et al., (1995); Merchant et al., (1997); Shah and Sharma (1998); Farrand et al., (2001); IARC (2004); and Jacob et al., (2004); that areca nut is the main etiological factor for OSF.[16] Betel quid chewing was seen more in males with a percentage of 60 in Group I and a percentage of 70 in Group II patients when compared to females. A majority of studies on OSF with betel quid habit published prior to 1990 revealed a female predominance, while more recent studies indicate a male predilection (Afrozet al., 2006; KiranKumar et al., 2007).[17]
The serum copper levels were increased in Groups I and II as compared to Group III, which was statistically significant. Similarly, Haines et al., (1982) presented data from a study of 28 cases in the Northwick Park Heart Study and 84 controls to assess the relationship between prediagnostic serum copper levels and subsequent cancer risk where serum copper levels among the 28 cases was higher than the control group.[18] Jaydeep et al., (1997);Haider et al., (2000); and Khanna (2006) have also reported raised copper levels in the sera of patients with oral premalignant and malignant lesions.[19] Margalith et al., (1983) suggested that role of copper ions in biological damage is due to superoxide radicals or other reducing agents such as ascorbate, which reduce the copper complex. These complexes react with hydrogen peroxide to form hydroxyl radicals that cause damage to protein, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) that are not repairable by cellular mechanisms, thus initiating the malignant process. Ma et al., in 1995 suggested that the initiating role of copper in OSF is stimulation of fibrogenesis by upregulation of lysyl oxidase, thereby causing inhibition of degradation of collagen. Jaydeep et al., in 1997 in their study reported that the rise in serum copper may be due to increased turnover of ceruloplasmin (a copper carrying globulin with essential oxidase activity) in the serum of carcinoma patients.[17,20,21]
The serum levels of iron were decreased in Groups I and II as compared to Group III, which was statistically significant. Anuradha et al., (1993) observed that patients with OSF (all of whom were heavy tobacco and arecanut users) showed decreased serum iron levels, whereas, the total tissue collagen content increased significantly in patients with advanced disease. They attributed the decrease in iron concentration to utilization of the same for collagen synthesis.[16] Stevens et al., (1988-1994) reported cancer incidence in the First National Health and Nutrition Examination Survey of 41,276 men and women aged between 20 and 74 years and its follow-up study. They found a positive association of cancer risk with lowered serum iron levels.[22] Rogers et al., (1993); Negri et al., (2000); Petridou et al., (2003); and Bhattathiri (2006) conducted case-control studies that have linked low iron intake with high oral cancer risk.[23] In most OSF cases, clinical anemia may be a contributing factor (Ramanathan et al., 1981). Serum iron levels are considered as biochemical indicators for nutritional assessment. Utilization of iron in collagen synthesis (Huang et al., 1997) by the hydroxylation of proline and lysine leads to decreased serum iron levels in OSF patients. Occurrence of iron deficiency is known to present in oral cancer, which may be due to malnutrition caused by the tumor burden.[17]
Serum magnesium levels were decreased in Groups I and II as compared to Group III which was statistically significant. Reinhart et al., (1985); Rubeiz et al., (1993); Chernow et al., (1995); and Guerin et al., (1996) observed 20-61% incidence of hypomagnesemia in cancer patients in postoperative intensive care unit (ICU).[24] Mg deficiency may trigger carcinogenesis by altering fidelity of DNA replication and increasing membrane permeability (Blondell 1980). Anghileri et al., (1981), using cells from induced cancers, found that there is much less Mg++ binding to membrane phospholipids of cancer cells, than to normal cell membranes, which might lead to precancerous changes. Magnesium deficiency impairs phagocytic activity as well as lymphocytic function. Membrane lipid abnormalities in Mg deficient and in neoplastic cells involve peroxidation of unsaturated fatty acids (Rayssiguier et al., 1986).[25]
In the present study, serum levels of zinc were decreased in Groups I and II as compared to Group III which was statistically significant. Umesh Kapil et al., (2003) observed that 53% of oral cancer patients in Jharkand had serum zinc deficiency and the deficiency was higher in females as compared to males.[26] Zinc deficiency causes upregulation of cyclooxygenase-2 (COX-2) and over expression of COX-2 enhances cell proliferation, inhibits apoptosis and modulates angiogenesis, thereby contributing to carcinogenesis (Barch 1986).[27] Zinc is an integral part of biomembranes, may control membrane integrity and may be involved in the stability of membranes and in lipid peroxidation-related injury. The role of zinc in RNA and DNA polymerase, its inhibitory effects on phosphodiesterase and its activating effect on membrane-bound adenylcyclase, suggests a role of zinc in carcinogenesis. Zinc has also been shown to stabilize ribosomes and the DNA double helix. Zinc deficiency also contributes to cancer initiation through activation of NF-kB and the consequent induction of tumorigenic signaling (Louise et al., 2006).[28]
Serum levels of calcium were increased in Group I as compared to Groups II and III, which was statistically significant. This is in accordance with a study conducted by Iwase et al., (2003) who assessed 225 patients with oral malignancies out of which five (2.2%) had hypercalcemia.[29] The fundamental cause of cancer-induced hypercalcemia is increased bone resorption with calcium mobilization into the extracellular fluid, and secondarily, inadequate renal calcium clearance (Ignoffo and Tseng, 1991;Warrell 1992). Cancer-associated hypercalcemia (CAH) frequently occurs in patients with advanced oral cancer and indicates that the patients have entered the terminal stage of the disease (Warrell 1997).[17]
The copper levels were increased in both Groups I and II with duration of habit between 11 and 20 years. A population-based survey conducted by Yang et al., (2001) in an aboriginal community of southern Taiwan, China, included 312 participants aged 20 years or older. The average duration of chewing was 22 years and correlated with increased levels of serum copper.[17]
CONCLUSION
Serum copper levels were significantly high in OSCC and OSF patients with habit when compared to the control group and gradually increased with the duration of the habit. The serum copper levels in OSF patients were less compared to those with OSCC. Serum iron, magnesium and zinc were significantly decreased in OSCC and OSF patients with habit when compared to the control group and gradually decreased with the duration of the habit. The levels of these trace elements were lower in patients with OSCC than in patients with OSF, but no statistical significance was observed. Serum calcium levels were elevated in OSCC patients with habit when compared to OSF patients with habit and control group and gradually increased with the duration of the habit and in older age individuals.
Hence, the altered serum levels of trace elements in the presence of areca nut and betel quid chewing habit may be associated with the pathogenesis and progression of the premalignant lesions, OSF and OSCC. Concerted efforts of assessing serum levels of trace elements would, therefore, help in early detection, management and monitoring the efficacy of treatment.
ACKNOWLEDGEMENTS
We sincerely thank all the staff and students of Departments of Oral Pathology and Oral Medicine and Radiology, PMNM Dental College, Bagalkot and Departments of Oral Pathology, Oral Medicine and Radiology and Oral and Maxillofacial Surgery, GDC and H, Bangalore, for their efforts, support and contribution towards this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yan W, Wistuba I, Emmert-Buck MR, Erickson HS. Squamous cell carcinoma-similarities and differences among anatomical sites. Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Donnell A, Jin S, Zavras AI. Delay in the diagnosis of oral cancer. J Stomatol Investig. 2008;2:15–26. [Google Scholar]
- 3.Khalili J. Oral cancer: Risk factors, prevention and diagnosis. Exp Oncol. 2008;30:259–64. [PubMed] [Google Scholar]
- 4.Agrawal KH, Rajderkar SS. Clinico-epidemiological profile of oral cancer: A hospital based study. Indian J Community Health. 2012;24:80–5. [Google Scholar]
- 5.Marocchio LS, Lima J, Sperandio FF, Correa L, de Sousa SO. Oral squamous cell carcinoma: An analysis of 1,564 cases showing advances in early detection. J Oral Sci. 2010;52:267–73. doi: 10.2334/josnusd.52.267. [DOI] [PubMed] [Google Scholar]
- 6.Vairaktaris E, Spyridonidou S, Papakosta V, Vylliotis A, Lazaris A, Perrea D, et al. The hamster model of sequential oral oncogenesis- A review. Oral Oncol. 2008;44:315–24. doi: 10.1016/j.oraloncology.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Jayasooriya PR, Jayasinghe KA, Tilakaratne WM. Oral submucous fibrosis. J Investig Clin Dent. 2011;2:171–5. doi: 10.1111/j.2041-1626.2011.00055.x. [DOI] [PubMed] [Google Scholar]
- 8.Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: revisited. J Oral Maxillofac Surg. 2011;10:1–9. doi: 10.1007/s10006-010-0219-8. [DOI] [PubMed] [Google Scholar]
- 9.Khanna S. Immunological and Biochemical markers in oral carcinogenesis: The public health perspective. Int J Environ Res Public Health. 2008;5:418–22. doi: 10.3390/ijerph5050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz MK. Role of trace elements in cancer. Cancer Res. 1975;35:3481–7. [PubMed] [Google Scholar]
- 11.Sadat A. Serum trace elements and immunoglobulin profile in lung cancer patients. J Appl Res. 2008:8. [Google Scholar]
- 12.Swain N, Ray JG. Altered trace element level and antioxidant activity in whole blood of oral leukoplakia and cancer patients in comparison with healthy controls. Int J Oral Maxillofac Pathol. 2011;2:2–6. [Google Scholar]
- 13.George A. Potentially malignant disorders of oral cavity. Oral Maxillofac Pathol J. 2011;2:95–100. [Google Scholar]
- 14.Angadi PV, Rekha KP. Oral submucous fibrosis: A clinicopathologic review of 205 cases in Indians. Oral Maxillofac Surg. 2011;15:15–9. doi: 10.1007/s10006-010-0225-x. [DOI] [PubMed] [Google Scholar]
- 15.Anand VD, White JM, Nino HV. Some aspects of specimen collection and stability in trace element analysis of body fluids. Clin Chem. 1975;21:595–602. [PubMed] [Google Scholar]
- 16.Effiom OA, Adeyemo WL, Omitola OG, Ajayi OF, Emmanuel MM, Gbotolorun OM. Oral squamous cell carcinoma: A clinicopathologic review of 233 cases in Lagos, Nigeria. J Oral Maxillofac Surg. 2008;66:1595–9. doi: 10.1016/j.joms.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 17.IARC working group. Betel-quid and areca-nut chewing. IARC monographs-100E. 2006;85:333–72. [Google Scholar]
- 18.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer Risk in relation to serum copper levels. Cancer Res. 1989;49:4353–6. [PubMed] [Google Scholar]
- 19.Tadakamadla J, Kumar S, Mamatha GP. Evaluation of serum copper and iron levels among oral submucous fibrosis patients. Med Oral Patol Oral Cir Bucal. 2011;16:e870–3. doi: 10.4317/medoral.17083. [DOI] [PubMed] [Google Scholar]
- 20.Gupta MK, Mhaske S, Ragavendra R, Imtiyaz Oral submucous fibrosis - Current Concepts in Etiopathogenesis. People's J Sci Res. 2008;1:39–44. [Google Scholar]
- 21.Dyavanagoudar SN. Oral submucous fibrosis: Review on etiopathogenesis. J Cancer Sci Ther. 2009;1:72–7. [Google Scholar]
- 22.Tiejian W, Sempos CT, Freudenheim JL, Muti P, Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol. 2004;14:195–201. doi: 10.1016/S1047-2797(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 23.Richie JP, Jr, Kleinman W, Marina P, Abraham P, Wynder EL, Muscat JE. Blood iron, glutathione and micronutrient levels and the risk of oral cancer. Nutr Cancer. 2007;60:474–82. doi: 10.1080/01635580801956477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deheinzelin D, Negri EM, Tucci MR, Salem MZ, da Cruz VM, Oliveira RM, et al. Hypomagnesemia in critically ill cancer patients: A prospective study of predictive factors. BrazJ MedBiol Res. 2000;33:1443–8. doi: 10.1590/s0100-879x2000001200007. [DOI] [PubMed] [Google Scholar]
- 25.Heaton FW, Tongyai S, Rayssiguier, Itokawa Y, Durlach J, Libbey J. London, Paris: from 5th Intl Mg Sympos, Kyoto, Japan, 1988 Press; 1989. Membrane function in magnesium deficiency - Magnesium in Health and Disease; pp. 27–33. [Google Scholar]
- 26.Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2:305–12. [PubMed] [Google Scholar]
- 27.Barch DH, Iannaccone PM. Role of zinc deficiency in carcinogenesis. AdvExp Med Biol. 1986;206:517–27. doi: 10.1007/978-1-4613-1835-4_36. [DOI] [PubMed] [Google Scholar]
- 28.Fong LY, Riley M, Farber JL. Zinc deficiency promotes cancer of the upper aerodigestive tract (UADT) by activation of NF-kB and COX-2. Proc Amer Assoc Cancer Res. 2006:47. [Google Scholar]
- 29.Pande S, Anil K, Khatri S, Rao RR. Para neoplastic hypercalcaemia in advanced carcinoma of oral cavity. Indian J Med Paediatr Oncol. 2007;28:44–6. [Google Scholar]


