Abstract
Myofibroblasts (MFs) are modified fibroblasts that express features of smooth muscle differentiation and were first observed in granulation tissue during wound healing. These cells play a key role in physiologic and pathologic processes like wound healing and tumorigenesis. The presence of MFs has been reported in normal oral tissues and pathologic conditions like reactive lesions, benign tumors, locally aggressive tumors and malignancies affecting the oral cavity. This article briefly reviews the important hallmarks related to the discovery, characterization and tissue distribution of MFs in oral health and disease.
Keywords: Myofibroblast, oral health and disease, oral lesions, tumorigenesis, wound healing
INTRODUCTION
Myofibroblasts (MFs) are specialized fibroblasts with smooth muscle-like features characterized by the presence of contractile apparatus.[1] These are extremely heterogeneous and multifunctional cell population exhibiting a different phenotype and first discovered by electron microscopy in experimental granulation tissue by Gabbiani et al.[2] MFs play a key role in extracellular matrix (ECM) synthesis, re-organization and tissue contraction during both physiologic and pathologic processes like wound healing and tumorigenesis.[3] Cytokines like transforming growth factor-beta 1 (TGF-β1) play an important role in transdifferentiation of fibroblasts into MF and the platelet-derived growth factor (PDGF) is mainly responsible for their maturation.[4] Their presence has been reported in normal oral tissues and pathologic conditions like reactive lesions, benign tumors, locally aggressive tumors and malignancies affecting the oral cavity. This review mainly focuses on the important hallmarks related to the discovery, characterization and tissue distribution of MFs in oral health and disease.
Discovery
For years it was believed that collagen is the main element responsible for wound contraction. This concept changed in 1950 and it was found that fibroblasts, under certain conditions, were capable of contraction in vitro suggesting that cells were central to wound contraction. Electron microscopic observation of granulation tissue fibroblasts revealed numerous bundles and aggregates of microfilaments similar to smooth muscle cells and the term MF was proposed.[5]
Structural characterization and immunophenotype
MFs disclose several typical histologic traits characterized by large, spindle-shaped stellate cells with long cytoplasmic extensions, amphophilic cytoplasm and indented nucleus with conspicuous nucleoli. But transmission electron microscopy remains the method of choice for identification of MFs.[5] Ultrastructurally, these cells have irregular, stellate cellular outlines with numerous long cytoplasmic connections connected by intermediate or adherens junctions and are connected to the ECM by cell-to-stroma attachment sites through fibronexus.[6] They contain bundles of cytoplasmic filaments arranged parallel to the long axis of the cell, a well-developed rough endoplasmic reticulum, Golgi and indented nucleus with prominent nucleoli.[7]
MFs present in various tissues like granulation tissue and pathologic tissue disclosed five cytoskeletal phenotypes: phenotype V, represented by cells expressing only vimentin; phenotype VA represented by cells expressing vimentin and alpha smooth muscle actin (α-SMA); phenotype VAD represented by cells expressing vimentin, α-SMA and desmin; phenotype VD represented by cells expressing vimentin and desmin; phenotype VAM represented by cells expressing vimentin, α-SMA and myosin.[5] Most of the MF express α-SMA, an actin isoform found in vascular smooth muscle cells and regulated by TGF-β and is considered as the main immunohistochemical marker of myofibroblastic differentiation [Figure 1].[8]
Figure 1.
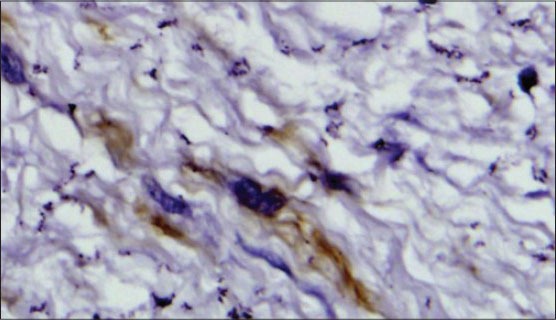
Immunohistochemical staining showing alpha smooth muscle actin (á-SMA) positive myofibroblast (IHC stain, ×400)
Origin and development
The occurrence of MFs in various lesions and conditions, their heterogeneous cytoskeletal composition and various functions, it makes it difficult to assume a common origin for these cells. Over the years it has become evident that MFs arise from variety of sources, according to the involved organ and the physiological or pathologic situation.[9] The various sources include leukocytes,[10] fibroblasts,[11] vascular smooth muscle cells, pericytes,[12] smooth muscle cells by their way along lines of dedifferentiation,[13] fibrocytes,[14] peri-sinusoidal stellate cells[15] and renal tubular epithelial cells.[16] However, the major source of MFs in whatever lesion they appear, is the fibroblast.
The feedback between intracellular and extracellular tension is required for the development of MFs.[1,17] The first step in the development of MF is the formation of the stress fibers and cell matrix adhesions, which is due to mechanical tension and initially these stress fibers are mainly composed of cytoplasmic actins. The term protomyofibroblast was proposed for those fibroblasts with stress fibers that do not express α-SMA. For the transformation of protomyofibroblasts into MFs, mechanical stress along with cytokines like TGF-β is necessary. TGF-β is the major growth factor inducing myofibroblastic differentiation.[4]
MFs in normal tissues and in wound healing
On the basis of ultrastructural and immunohistochemical evidence MFs were discovered in normal human and animal tissues like lymph nodes, blood vessels, uterine submucosa, testicular stroma, intestinal villous core and lung septa.[5] In the oral cavity, these MFs were reported in the human palatal mucosa[18] and granulation tissue during wound healing.[5]
MFs appear to be key cells in the process of wound healing. These cells are more numerous in the exudate layer of granulation tissue. Prostaglandins derived from MF are key factors in promoting healing by restoration of the epithelium. Another important event in wound healing, contraction of the wound is due to the MFs because of the presence of α-SMA filaments in the cytoplasm of these cells.
The ECM is a mixture of collagens, glycoproteins and proteoglycans that form a scaffold for tissue formation. Type I, III, IV and VIII collagens, fibronectin and tenascin are secreted by the MFs and enzymes like matrix metalloproteinases (MMPs) which mediate tissue remodelling following injury are also secreted by these cells.[19] So these cells play an important role in tissue remodelling following injury.
MFs in pathologic tissues
There are many pathologic conditions in which MFs have been described. These include MFs in response to injury and repair phenomenon, proliferative conditions and in stromal response to neoplasia. The various pathological states include.
MFs in inflammatory and reactive lesions of the oral cavity
MFs play an important role in inflammatory response. These cells produce chemokines, inflammatory cytokines and prostaglandins. These cells also secrete adhesion molecules like intercellular cell adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) which helps lymphocytes, mast cells and neutrophils to associate with MF and promote immunologic and inflammatory reactions.[5]
Ultrastructural study of various reactive lesions of the oral cavity like giant cell granuloma,[20,21] giant cell fibroma[22] and phenytoin-induced gingival hyperplasia[23] disclosed fibroblasts that contained intracytoplasmic myofilaments with electron dense bodies similar to smooth muscle cells and these cells were referred to as MFs.
Proliferative myofibroblastic lesions
This group includes proliferations that share predominant myofibroblastic component with variable proliferative potential. These include reactive fasciitis like lesions, benign myofibroblastomas and locally aggressive fibromatosis which may be superficial or deep and sarcomas with myofibroblastic differentiation like inflammatory myofibroblastic tumor and low grade myofibroblastic sarcoma.[24]
Nodular fasciitis is a reactive lesion that has been reported in different parts of the body including the oral cavity; it is characterized by rapid growth with duration of less than 1 month. Histopathologically, this lesion is well-demarcated, nonencapsulated with uniform spindle-shaped cells. Myofibroblastoma is a benign stromal neoplasm with myofibroblastic differentiation. Histologically, this tumor is composed of interlacing bundles of spindle shaped cells with hyalinizing stroma. It is a distinct entity and should be included under the differential diagnosis of oral soft tissue tumors.[25] Inflammatory myofibroblastic lesions encompass a group of myofibroblastic proliferations along with varying amount of inflammatory infiltrate.[26] It rarely occurs in the head and neck region. The concept that inflammatory myofibroblastic tumor is a benign reactive lesion is being questioned because of high recurrences, presence of regional metastasis and cytogenetic abnormalities.[27] These lesions should be differentiated from low-grade sarcomas and require aggressive surgical procedure.
MFs in odontogenic lesions
The presence of MFs have been reported in the stroma of many odontogenic lesions, including both odontogenic cysts and tumors. Only few studies have been done in this regard. Initial studies using electron microscopy demonstrated that the stromal component of ameloblastoma is composed of MFs associated with collagen and basal lamina like material and proposed that the presence of these cells could contribute to aggressive behavior.[28,29] Later studies conducted using immunohistochemistry to demonstrate α-SMA in odontogenic cysts reported that a variable proportion of the cyst wall fibroblasts were positive for α-SMA and the distribution of these cells was in two positive zones: an inner subepithelial layer and an outer region adjacent to the bone facing surface.[30] The results of this study demonstrated that MFs contribute to cyst wall elasticity constraining cyst expansion.
Studies conducted both on odontogenic cysts and tumors revealed that the cells were particularly more in lesions with locally aggressive behavior like odontogenic keratocyst (OKC) [Figure 2a and b] and solid ameloblastomas [Figure 2c and d]. The mean number of MF were higher in parakeratinized variant of OKC and solid ameloblastoma which were comparable to cases of squamous cell carcinoma and considered that this higher frequency of stromal MFs could contribute to aggressive biologic behavior of these lesions.[8,31]
Figure 2.
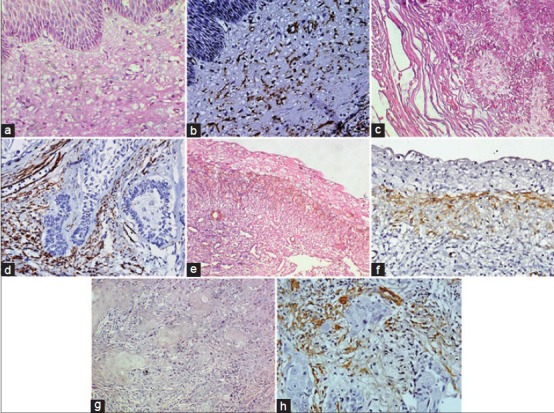
(a) Odontogenic keratocyst (OKC) showing cystic lining epithelium and connective tissue capsule (H&E stain, ×400). (b) Photomicrograph showing á-SMA positive myofibroblasts in the cyst wall of OKC (IHC stain, ×400). (c) Follicular ameloblastoma showing odontogenic epithelial islands in connective tissue stroma (H&E stain, ×400). (d) Photomicrograph showing á-SMA positive myofibroblasts around odontogenic epithelial islands in follicular ameloblastoma (IHC stain, ×400). (e) uminal variant of unicystic ameloblastoma (H&E stain, ×200). (f) Photomicrograph showing á-SMA positive myofibroblasts in unicystic ameloblastoma (IHC stain, ×400). (g) Malignant epithelial islands in well-differentiated oral squamous cell carcinoma (H&E stain, ×400). (h) Photomicrograph showing á-SMA positive myofibroblasts around tumor islands in oral squamous cell carcinoma (IHC stain, ×400)
MFs in lesions like ameloblastomas [Figure 2e and f] were thought to secrete MMP-2 and urokinase plasminogen activator (uPA) and were associated with rupture of osseous cortical.[32] Immunohistochemical and ultrastructural studies showed that the stroma of odontogenic myxoma harbored numerous MFs, which may induce modification in ECM and could contribute to tumor invasion.[33]
MFs in oral precancer and cancer
Oral submucous fibrosis (OSMF) is a potentially malignant disorder characterized by inflammation and progressive submucous fibrosis. The key cellular mediator in various fibrotic disorders was thought to be MFs. Studies done regarding the presence of MFs in OSMF revealed a significant increase in the mean number of MF between early and advanced stages and suggested they can be used as markers for evaluating the severity of OSMF. They also suggested that MFs associated with a fibrotic stroma are thought to promote tumorigenesis compared to fibroblasts derived from normal tissues, thus targeting of these MFs may be beneficial in OSMF.[34]
A continuous molecular cross talk between epithelial and mesenchymal cells is required during embryonic development and probably plays an important role in pathologic process like wound healing and tumor progression.[3] The development of carcinomas is due to the genetic changes within the target epithelium.[35] As there is conversion of normal epithelium to precancerous and to squamous cell carcinoma, the stroma also changes from normal to primed to activated or tumor associated called as the stromal reaction.[36]
Many of the epithelial tumors are characterized by the changes in the connective tissue cells and various extracellular components called as the stromal reaction. One of the stromal reactions is the appearance of specialized fibroblasts called MFs. Transdifferentiation of fibroblasts to MFs is a crucial event in tumorigenesis, which is mediated by the growth factors and cytokines secreted by the tumor cells. These MFs in turn secrete numerous growth factors and inflammatory cytokines that stimulate epithelial cell proliferation. These cells were also thought to act together with host immune cells to support blood vessel formation, basement membrane disruption, invasion and metastasis. Therefore, these cellular events play an important role in neoplastic growth and development.[3]
Studies done regarding the presence of MFs in oral epithelial dysplasia and squamous cell carcinoma revealed a higher number of MFs in oral squamous cell carcinoma [Figure 2g and h] compared to normal and dysplastic epithelium, which were devoid of MFs.[36,37,38] These findings suggest that invasion of epithelium beyond the basement membrane is required for myofibroblastic differentiation. Similar findings have been reported in animal studies on oral carcinogenesis.[39] The increased number of MFs in oral squamous cell carcinoma is due to the inductive effect of malignant epithelial cells which secrete numerous growth factors and help in transdifferentiation of MFs. These MFs were in turn thought to produce chemokines, growth factors and matrix degrading enzymes. They were thought to act along with the host immune cells to aid in angiogenesis, basement membrane breakdown and metastasis.[40]
Studies conducted by Kellerman et al.,[41] and Moghadam et al.,[36] revealed no statistically significant difference in the mean number of myofibroblasts between well, moderate and poorly differentiated OSCC and suggested that transdifferentiation of MFs occurs during the invasive stage of carcinomatous epithelium and further loss of tumoral differentiation does not affect the number of cells.[36]
MFs in the stroma of oral squamous cell carcinoma exhibited two morphologic patterns: Spindle and network. In network pattern, the MFs are abundant and occupy entire tissue stroma; whereas in spindle pattern, the cells are spindle-shaped and located at the periphery in one to three concentric layers.[42,43] Morphologic characters of the invasive front may better reflect the tumor prognosis than other parts of the tumor.[44] Few studies done in this regard, reported high levels of collagen fibers and stromal MFs at the invasive front and their number increased with the increasing tumor invasiveness.[45]
The presence of MFs was also thought to be associated with tumor prognosis. Studies done in this regard showed that increased MFs was associated with poor prognosis.[43] MFs in tongue cancer were associated with risk score and prognosis. Patients whose specimens were weakly positive for α-SMA had 5 year mean survival rate of 82%; whereas, patients with samples that were strongly positive with α-SMA had a mean survival rate of 38% indicating that the presence of more number of MFs in the stroma is associated with poor prognosis.[35] Thus, the presence of increased stromal MFs is an effective predictor of oral squamous cell carcinoma patient mortality.[46]
CONCLUSION
MFs are ubiquitous cells and are present in various lesions like reactive, benign and malignant lesions. These cells are particularly important in tumorigenesis and tumor progression. So, it is necessary to clarify by sophisticated techniques how these cells exert their effects on stromal and epithelial tissue compartments, mainly because they can provide new insights into mechanisms associated with tumor growth, invasion and metastasis. Therapeutic targeting of MFs using anti-MF drugs which prevent their transdifferentiation from fibroblasts may be beneficial in oral squamous cell carcinoma patients.
ACKNOWLEDGMENT
We are thankful to Dr. T. R. Saraswathi for her constant support and encouragement.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 3.Desmouliere A, Guyot C, Gabbani G. The stroma reaction of myofibroblast: A key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:505–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 4.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schurch W, Seemayer TA, Hinz B, Gabbiani G. Myofibroblast. In: Mills SE, editor. Histology for Pathologists. Philadelphia: Lippincott-Williams and Wilkins Publishers; 2007. pp. 123–164. [Google Scholar]
- 6.Hinz B, Gabbani G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodelling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 8.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in the stroma of odontogenic cysts and tumors can contribute to variations in the biologic behavior of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The Myofibroblast: One function multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohnheim J. About inflammation and sepsis. Virchows Arch Path Anat. 1867;40:1–79. [Google Scholar]
- 11.Oda D, Gown AM, Vande Berg JS, Stern R. The fibroblast-like nature of myofibroblasts. Exp Mol Pathol. 1988;49:316–29. doi: 10.1016/0014-4800(88)90004-4. [DOI] [PubMed] [Google Scholar]
- 12.Ronnov-Jessen L, Peterson OW, Koteliansky VE, Bissel MJ. The origin of myofibroblast in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaivegato A, Bochaton-Piallat ML, D’Amore E, Sartore S, Gabbiani G. Expression of myosin heavy chain isoforms in mammary epithelial cells and in myofibroblasts from different settings during neoplasia. Virchows Arch. 1995;426:77–86. doi: 10.1007/BF00194701. [DOI] [PubMed] [Google Scholar]
- 14.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocvte define a new leucocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Blajzewski S, Preaux AM, Mallat A, Brocheriou I, Mavier P, Dhumeaux D, et al. Human myofibroblast like cells obtained by outgrowth are representative of fibrogenic cells in the liver. Hepatology. 1995;22:788–97. [PubMed] [Google Scholar]
- 16.Kalluri R, Neilson EJ. Epithelial- mesenchymal transition and its implication for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Boya J, Carbonell AL, Martinez A. Myofibroblasts in human palatal mucosa. Acta Anat (Basel) 1988;131:161–5. doi: 10.1159/000146506. [DOI] [PubMed] [Google Scholar]
- 19.Powell DW. Myofibroblasts: Paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;11:271–92. [PMC free article] [PubMed] [Google Scholar]
- 20.El-Labban NG, Lee KW. Myofibroblasts in central giant cell granuloma of the jaws: An ultrastructural study. Histopathology. 1983;7:907–18. doi: 10.1111/j.1365-2559.1983.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 21.Dayan D, Buchner A, David R. Myofibroblasts in peripheral giant cell granuloma: Light and electron microscopic study. Int J Oral Maxillofac Surg. 1989;18:258–61. doi: 10.1016/s0901-5027(89)80088-8. [DOI] [PubMed] [Google Scholar]
- 22.Weathers DR, Campbell WD. Ultrastructure of the giant-cell fibroma of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1974;38:550–61. doi: 10.1016/0030-4220(74)90086-3. [DOI] [PubMed] [Google Scholar]
- 23.Dill RE, Iacopino AM. Myofibroblasts in phenytoin-induced hyperplastic connective tissue in the rat and in human gingival overgrowth. J Periodontol. 1997;68:375–80. doi: 10.1902/jop.1997.68.4.375. [DOI] [PubMed] [Google Scholar]
- 24.Dayan D, Narasallah V, Vered M. Clinio-pathologic correlation of the myofibroblastic tumors of the oral cavity: 1 Nodular fasciitis. J Oral Pathol Med. 2005;34:426–35. doi: 10.1111/j.1600-0714.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamada T, Hirano M, Semba I, Kamikawa Y, Sugihara K. Myofibroblastoma of the tongue: A case report with immunohistochemical findings. J Oral Maxillofac Surg Med Pathol. 2012;24:180–3. [Google Scholar]
- 26.Coindre JM. Histologic classification of soft tissue tumors (WHO, 1994) Ann Pathol. 1994;14:426–7. [PubMed] [Google Scholar]
- 27.Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor--a true neoplasm or reactive lesion? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:460–6. doi: 10.1016/j.tripleo.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Rothouse LS, Majack RA, Fay JT. An ameloblastoma with myofibroblasts and intracellular septate junctions. Cancer. 1980;45:2858–63. doi: 10.1002/1097-0142(19800601)45:11<2858::aid-cncr2820451123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Bartov SA. Ameloblastoma with myofibroblasts: First report. J Oral Pathol. 1986;15:284–6. doi: 10.1111/j.1600-0714.1986.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi T, Morgan PR. Immunohistochemical characterization of odontogenic cysts with mesenchymal and myofilament markers. J Oral Pathol Med. 1995;24:170–6. doi: 10.1111/j.1600-0714.1995.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 31.Mashhadiabbas F, Moghadam SA, Moshref M, Elahi M. Immunohistochemical detection and ultrastructure of myofibroblasts in the stroma of odontogenic cysts and ameloblastoma. Iranian Red Crescent Med J. 2010;12:453–7. [Google Scholar]
- 32.Fregnani ER, Sobral LM, Alves FA, Soares FA, Kowalski LP, Coletta RD. Presence of myofibroblast and expression of matrixmetalloproteinase 2 (MMP-2) in ameloblastomas correlate with the rupture of osseous cortical. Pathol Oncol Res. 2009;15:231–40. doi: 10.1007/s12253-008-9110-4. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Mata M, Mosqueda-Taylor A, Carlos-Bregni R, de Almeida OP, Contreras-Vidaurre E, Vargas PA, et al. Odontogenic myxoma: Clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol. 2008;44:601–7. doi: 10.1016/j.oraloncology.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Angadi PV, Kale AD, Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J Oral Pathol Med. 2011;40:208–13. doi: 10.1111/j.1600-0714.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- 35.Thode C, Jorgensen TG, Dabelsteen E, Mackenzie I, Dabelsteen S. Significance of myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:201–7. doi: 10.1111/j.1600-0714.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 36.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38:639–43. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 37.Zidar N, Gale N, Kambic V, Fischinger J. Proliferation of myofibroblasts in the stroma of epithelial hyperplastic lesions and squamous carcinoma of the larynx. Oncology. 2002;62:381–5. doi: 10.1159/000065071. [DOI] [PubMed] [Google Scholar]
- 38.Seifi S, Shafahi S, Shafigh E, Sahabi SM, Ghasemi H. Evaluation for the presence of α-SMA positive myofibroblasts in oral squamous cell carcinomas, and oral epithelial dysplasia and hyperkeratosis. Asian Pac J Cancer Prev. 2010;11:359–64. [PubMed] [Google Scholar]
- 39.Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts and malignant transformation in a 4NQO rat tongue carcinogenesis model. Oral Oncol. 2006;43:999–1006. doi: 10.1016/j.oraloncology.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66:8026–36. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- 41.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–17. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts accompany modification in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron. 2009;2:49–57. doi: 10.1007/s12307-009-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Myofibroblasts in the stroma of oral squamous cell carcinoma is associated with poor prognosis. Histopathology. 2007;51:849–53. doi: 10.1111/j.1365-2559.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- 44.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 45.Kawashiri S, Tanaka A, Noguchi N, Hase T, Nakaya H, Ohara T, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1346–53. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 46.Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–81. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]


