Abstract
Non-Hodgkin's lymphoma (NHL) is primarily a disorder of lymph nodes, when limited primarily to extranodal sites it is referred to as primary extranodal lymphoma and accounts for 24-48% of all cases of NHL. The development of peripheral T-cell lymphoma is poorly understood and is thought to be associated with effector T cells involving chromosomal translocations. It has been suggested by few authors that the continued increased risk for NHL among highly active antiretroviral therapy (HAART)-treated human immunodeficiency virus (HIV)-infected persons may be a reflection of incomplete immune reconstitution and possibly a chronic state of B-cell hyperactivation. Here, we present one such rare case of extranodal NHL in a known HIV patient.
Keywords: Extranodal NHL, HIV, non-Hodgkin's lymphoma
INTRODUCTION
Non-Hodgkin's lymphomas (NHLs) are a heterogeneous group of lympho-proliferative disorders originating in B, T, or natural killer (NK) lymphocytes.[1] NHL has slowly grown from a rare cancer to the fifth most common cancer (incidence of 19.1 per 100,000 in United States of America (USA)) in the world over a period of 30 years. In India its incidence is on the upsurge with the current figure standing at 5.1 per 100,000 in urban registries.[2,3] NHL is the 11th most common cancer in developed countries compared with developing countries, usually fatal, with a 5-year survival rate of less than 35%.[4]
NHL has been reported to occur as a primary lesion or can be associated with human immunodeficiency virus (HIV) infections since the beginning of the acquired immune deficiency syndrome (AIDS) epidemic in 1981. The Global Burden of Disease Study 2010 estimates 1.5 million AIDS-related deaths in 2010. HIV seropositivity increases the risk of developing NHL by 60-165-fold. AIDS-related lymphomas (ARLs) tend to present with high-grade B-cell histology, advanced-stage disease and an aggressive clinical course. With the introduction of highly active antiretroviral therapy (HAART) in 1996, the survival of patients with ARL has improved substantially.[5,6]
NHL is an important neoplasm, as it is an AIDS-defining condition. Here, we present a rare case report of extranodal NHL in a known HIV patient.
CASE REPORT
A male patient aged 68 years with a known HIV seropositivity since 5 years and who is on antiretroviral therapy reported to the Department of Oral and Maxillofacial Pathology, with the chief complaint of swelling in the right lower back region of the jaw since 2 months. Patient also complained of loose molar teeth (46, 47 and 48) in the same region. A detailed medical and dental history was taken. The patient complained of fever, night sweats, fatigue and loss of body weight. The patient was tested for cluster of differentiation (CD) 4/CD8 ratio, which was decreased (0.24). The absolute CD8 count was 3123 and CD4 T-lymphocyte count was 16.9%. CD4/CD8 ratio is a reflection of immune system health. A normal ratio is between one and four. The normal CD4 count is 500-1600 and CD8 count is 375-1100. The normal CD4/CD8 ratio is below 15%.
On extraoral clinical examination, the swelling was located on the right mandibular region leading to facial asymmetry [Figure 1]. Intraorally there was an ulcero-proliferative growth in relation to 46, 47 and 48 region on free and attached gingiva measuring about 6 × 4 cm in size, reddish in color, soft, sessile and oval in shape. It was non-tender and fixed to underlying structures [Figure 2]. Bleeding on gentle probing was present. A single right submandibular lymph node measuring about 1 × 1 cm, roughly oval shape was tender on palpation. Orthopantamogram showed a diffuse radiolucent area in the lower right molar area in relation to 46, 47 and 48.
Figure 1.
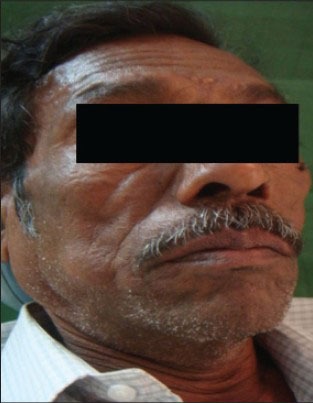
Patient showing growth on right lower back region with extraoral swelling on right cheek region
Figure 2.
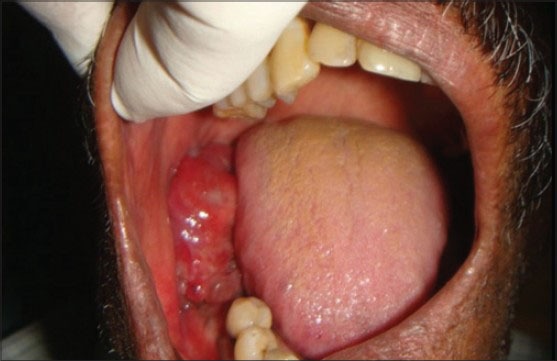
Ulcerative growth seen on the right buccal mucosa measuring about 5 × 6 cm in size extending anteroposteriorly from retromolar area to distal aspect of 46 region.
An incisional biopsy was done along with the extraction of 46, 47 and 48 teeth under local anesthesia (2% Lignocaine with adrenalin, one in 80,000 dilution) and the specimen was submitted for histopathological evaluation [Figures 3 and 4]. Hematoxylin and eosin stained sections (H and E) showed surface-stratified squamous epithelium with ulceration. The connective tissue showed sheets of immature round tumor lymphoid cells of varying sizes and shapes, which were diffusely distributed in the connective tissue [Figure 5]. The round tumor cells showed dysplastic features like nuclear pleomorphism, prominent nucleoli and granular cytoplasm. Two principle cell types observed were small cells with irregular or cleaved nuclear contours and scant cytoplasm referred to as centrocytes and larger cells with open chromatin, several nucleoli and modest amounts of cytoplasm referred to as centroblasts. There was evidence of increased and abnormal mitotic figures at focal areas. With these findings a histopathological diagnosis of NHL was made.
Figure 3.
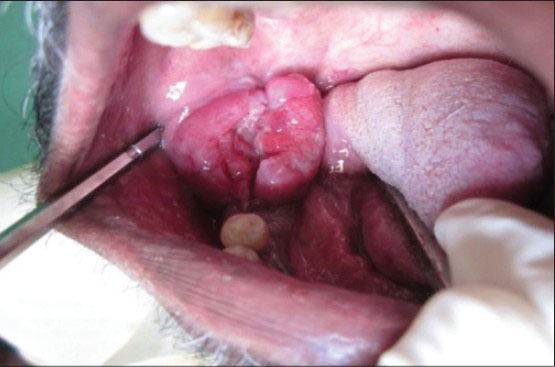
Increased intraoral growth after 2 months
Figure 4.
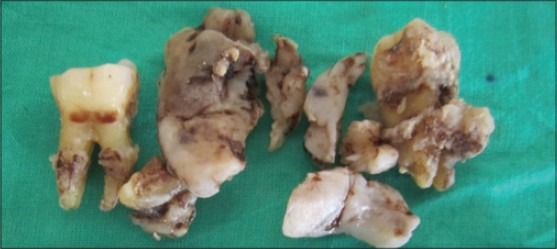
Incisional biopsy specimen along with extracted 46, 47 and 48 teeth
Figure 5.
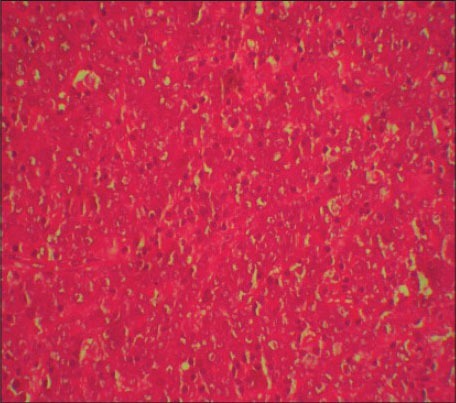
Photomicrograph showing sheets of lymphoid cells (H&E stain, ×400)
To identify the subtype of NHL, immunohistochemistry (IHC) was performed using CD45, CD20 and CD3 markers. The section was positive for CD45 indicating NHL. The positivity was indicated by intense nuclear brown staining on cell membrane surface [Figure 6]. The section was also positive for CD3 indicating T-cell lineage and negative for CD20 confirming that it was not of B-cell lineage [Figures 7 and 8]. The final diagnosis was confirmed as “Extranodal Peripheral T-cell Non-Hodgkin's Lymphoma.” Patient did not turn up for 2 months after the histological diagnosis. After 2 months, when the patient visited the hospital, the growth had increased and also had developed an extraoral swelling in relation to right lower jaw region. The patient was referred for chemotherapy and radiotherapy to Cancer institute.
Figure 6.
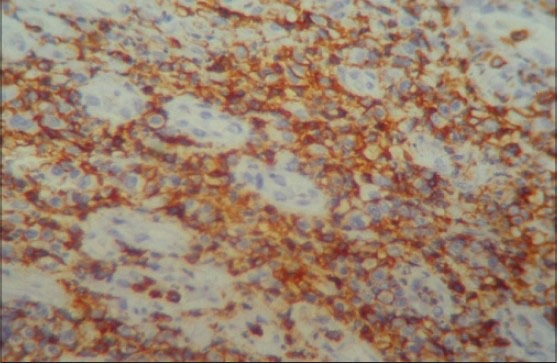
Photomicrograph showing IHC positivity for CD45 with intense nuclear brown staining for cell membrane surface (IHC stain, ×400)
Figure 7.
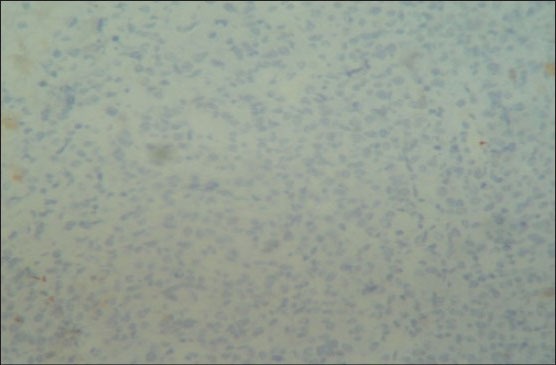
Photomicrograph showing negativity for CD20 staining (IHC stain, ×400)
Figure 8.
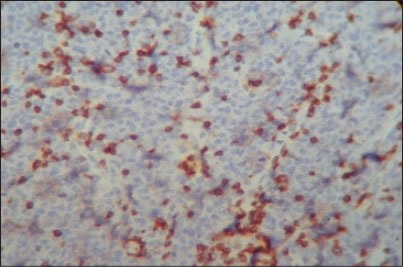
Photomicrograph showing small cell T-lymphocytes positive for CD3 with intense nuclear brown staining for cell membrane surface (IHC stain, ×400)
DISCUSSION
NHL though primarily a disorder of lymph nodes is also known to involve extranodal sites. When limited primarily to extranodal sites, it is referred to as primary extranodal lymphoma and accounts for 24-48% of all cases of NHL. Most common site of extranodal disease in the western countries is reported to be gastrointestinal tract (4-20% of all NHL) as compared to head and neck region, which is the commonest site (50.9%) in India. Different subsites include tonsil, orbit, nasopharynx, sinonasal tract, palate, eyelid, parotid gland and thyroid gland. The male to female ratio ranges between 2.31:1.[7] The case presented here is also of male patient of age 68 years who was HIV positive and the growth was seen in lower right mandible in the retromolar area, which is a very rare site.
Oral lesions are seen in approximately 4% of patients with AIDS-related NHL.[8] The NHL most commonly originates from cells of B -lymphocyte series. The T-cell lymphocyte derivations are less common. A person with AIDS is immunocompromised and can present with any lesions or conditions. A patient can come with oral lesion as initial sign and hence investigations should be performed to confirm diagnosis as NHL. These oral lesions can be used as indicators to determine lymphoma.
Precursor lymphoid neoplasms include both precursor B-lymphoblastic lymphoma/leukemia and T-lymphoblastic lymphoma/leukemia. Both are essentially systemic disorders at presentation that may be manifested as lymphoma or leukemia. Most patients present with lymphadenopathy but nodal versus extranodal should be examined, as the disease can occur in extranodal sites also.[9] About a quarter of cases arising extranodally may be aggressive, but introduction of novel approaches and proper diagnosis will further improve prognosis. Many classifications were given since 30-40 years, which can be difficult to interpret and reproduce.[10] The improved classification systems REAL (Revised European-American Lymphoma) system in 2001 and modified World Health Organization (WHO) classification system in 2008 which are most accepted utilizes immunophenotyping by flow cytometry, cytogenetics and other molecular pathologic tools to designate particular lymphoma subtypes. The B- and T-cell neoplasms are stratified into precursor or lymphoblastic neoplasms and mature (peripheral) neoplasms.[11]
Many forms of AIDS-NHL are characterized by the presence of recurrent genetic alterations, which may be due, in part, to errors in normal processes that occur in activated B cells, which involve modification of somatic DNA, such as immunoglobulin gene (Ig), class-switch recombination (CSR) and somatic hyper mutation (SHM). AIDS is able to produce DNA double-strand breaks, resulting in widespread genome instability, which may contribute to some of the non-Ig-related genetic modifications that are present in AIDS-NHL. The changes in HIV infection that result in chronic B-cell hyperactivation have the potential to contribute to the genesis of AIDS-NHL.[12] The development of peripheral T-cell lymphoma is poorly understood and is thought to be associated with effector T cells involving chromosomal translocations.[13]
Individuals without HIV infection generally have a greater number of CD4 cells than CD8 cells. As individuals get older, the immune system's defense against pathogens is weaker and the CD4/CD8 ratio tends to decrease. Individuals with autoimmune diseases tend to have an increased CD4/CD8 ratio, whereas those with viral infections have a decreased ratio. Factors affecting CD4 count and subsequently the CD4/CD8 ratio are some viral infections, tuberculosis, corticosteroid use, seasonal/diurnal variations and variations in CD4 analyses.[14] It has been suggested by Biggar et al., in 2001 that the continued increased risk for NHL among HAART-treated HIV-infected persons may be a reflection of incomplete immune reconstitution and possibly a chronic state of B-cell hyperactivation.[15]
Antibody therapies for peripheral T-cell lymphoma are emerging as are approaches to improve antibody therapy such as conjugation with radioisotopes and chemotherapy agents.[16] IHC plays a key role in the diagnosis and classification of hemato-lymphoid neoplasms. New cell and lineage markers are constantly being discovered and added to existing list of antibodies. Panel of markers are used for diagnosis of malignant lymphoma versus reactive lymphoid hyperplasia. The peripheral T-cell lymphomas about 90% are positive for CD3 and CD45. The T-cell receptor associated protein βF1 is expressed in approximately 75% of cases.[17] In the present case, IHC using CD3, CD20 and CD45 was done which confirmed the diagnosis of extranodal peripheral T-cell NHL. CD3 confirms the T-cell blast origin and CD45 confirms it as NHL. The CD20 marker is positive for Reed Sternberg cells and B-cell origin, which was negative in our case.
The peripheral T-cell lymphoma associated with human T-cell lymphotrophic virus 1 and peripheral T-cell neoplasms are aggressive and the treatment is single agent and multi agent chemotherapy as well as several bio-immunotherapeutic agents (such as Alemtuzumab and Denileukin diftitox) a recombinant toxin containing interleukin 2 and truncated diphtheria toxin has also been used and are under investigation.[18]
CONCLUSION
The aggressive NHLs are a group of malignancies with symptom being rapid onset and a short natural history. Presenting features vary depending on the site affected. Investigations are aimed at making an accurate diagnosis and the patient's ability to tolerate aggressive therapy. The main stay of treatment is multi agent chemotherapy. The advent of monoclonal antibody therapy has improved outcomes in many B-cell malignancies. The aggressive lymphomas including many T-cell disorders carry a poor prognosis and new treatment strategies are needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, et al. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484–560. doi: 10.6004/jnccn.2011.0046. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Registry Programme: First All India Report 2001-2002. [Last cited on 2012 Oct 20]. Available from: www.canceratlasindia.org/chapter 6_report.asp .
- 3.Lowry L, Smith P, Qian W, Falk S, Benstead K, Illidge T, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother Oncol. 2011;100:86–92. doi: 10.1016/j.radonc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Yeole BB. Trends in the incidence of Non-Hodgkin's lymphoma in India. Asian Pac J Cancer Prev. 2008;9:433–6. [PubMed] [Google Scholar]
- 5.Vishnu P, Aboulafia DM. AIDS-Related Non-Hodgkin's Lymphoma in the Era of Highly Active Antiretroviral Therapy. Adv Hematol 2012. 2012 doi: 10.1155/2012/485943. 485943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 7.Singh D, Kumar L, Goyal H, Raina V, Bijlani L, Wadhwa J, et al. Primary extranodal non-Hodgkin's lymphoma in northern India. Proc Am Soc Clin Oncol. 2003;22:2457. [Google Scholar]
- 8.Neville BW, Damm DD, Allen CM, Bouquot JE. 3rd ed. New York: WB Saunders Elsevier; 2009. Oral and Maxillofacial Pathology; pp. 595–8. [Google Scholar]
- 9.Sander CA, Flaig MJ, Jaffe ES. Cutaneous manifestations of lymphoma: A clinical guide based on the WHO classification. World Health Organization. Clin Lymphoma. 2001;2:86–100. doi: 10.3816/clm.2001.n.014. [DOI] [PubMed] [Google Scholar]
- 10.Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2003;362:139–46. doi: 10.1016/S0140-6736(03)13868-8. [DOI] [PubMed] [Google Scholar]
- 11.Lu P. Staging and classification of lymphoma. Semin Nucl Med. 2005;35:160–4. doi: 10.1053/j.semnuclmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Epeldegui M, Vendrame E, Martínez-Maza O. HIV-associated immune dysfunction and viral infection: Role in the pathogenesis of AIDS-related lymphoma. Immunol Res. 2010;48:72–83. doi: 10.1007/s12026-010-8168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–57. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 14.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type-1-infected cohort. Clin Infect Dis. 2006;42:418–27. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 15.Biggar RJ. AIDS-related cancers in the era of highly active antiretroviral therapy. Oncology (Williston Park) 2001;15:439–48. [PubMed] [Google Scholar]
- 16.Flowers CR, Armitage JO. A decade of progress in lymphoma: Advances and continuing challenges. Clin Lymphoma Myeloma Leuk. 2010;10:414–23. doi: 10.3816/CLML.2010.n.086. [DOI] [PubMed] [Google Scholar]
- 17.Chu PG, Chang KL, Arber DA, Weiss LM. Practical applications of immunohistochemistry in hematolymphoid neoplasms. Ann Diagn Pathol. 1999;3:104–33. doi: 10.1016/s1092-9134(99)80038-0. [DOI] [PubMed] [Google Scholar]
- 18.Shipp, et al. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]


