Abstract
A multidrug resistant Chyresobacterium indologenes was isolated from blood in a case of septicemia. The organism was resistant to carbapenems and was positive for blaNDM-1; transferable through plasmid.
Keywords: blaNDM-1, Chyresobacterium indologenes, IncN type
INTRODUCTION
Nonfermenting, Gram-negative bacilli poses a serious therapeutic challenge for treatment of both community-acquired and nosocomial infections in immune-suppressed individuals and selection of the appropriate antibiotic to initiate a therapy is essential to optimizing the clinical outcome in these patients. Chyresobacterium indologenes has been recognized since the last decade as an opportunistic pathogen, particularly associated with individuals who are debilitated or immune-suppressed and is involved in causing blood stream infections, ventilator associated pneumonia, intraabdominal and surgical site infections.[1,2,3]
CASE REPORT
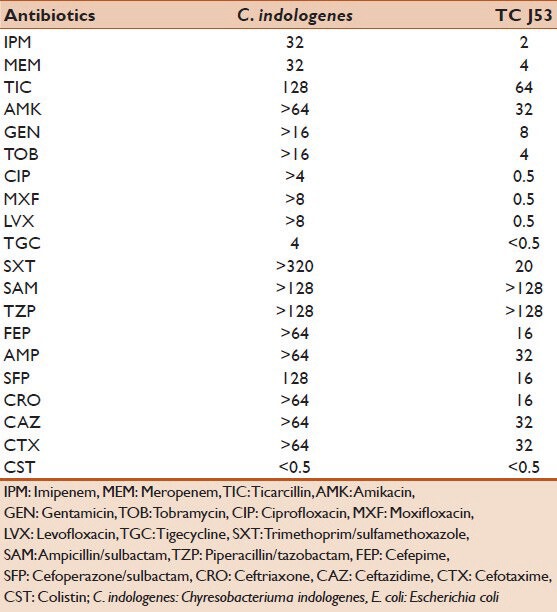
A 77-year-old male, a known case of type-II diabetes mellitus, sustained a penetrating injury after an accidental, fall from height. He was admitted to a peripheral hospital where he developed features of septicemia with high-grade fever, hypotension and coagulopathy. The patient was transferred to our tertiary care hospital. On admission, patient was found to be febrile (temperature 101°F), pulse 101/min, respiratory rate 18/min and blood pressure of 138/86 mm on Hg. Laboratory investigation showed total leucocyte count-15,800/cu mm3, platelet count-100,000/cu mm3 with neutrophils 85% and 9% of band forms. Toxic granules were present. C-reactive protein-30 mg/L, pro-calcitonin level of 32 pg/ml. Random blood sugar was 160 mg/dl. Urine examination showed trace albumin, absence of sugar with occasional pus cells. The patient was diagnosed to have paraplegia due to fracture vertebra (L1-L2). Indwelling urinary catheter was placed in order to facilitate drainage of urine. On clinical suspicion of sepsis, two blood cultures taken at 1-h interval and carried out using BACTEC 9120 system (BD, Country Clare, Ireland) which grew C. indologenes in both samples. Species identification was done manually and by using VITEK-GNI cards. Blood agar showed yellow pigmented colonies. Acid is produced from D-fructose, D-glucose, glycerol, maltose, trehalose, glycogen and mannose, but not from lactose, L-arabinose and sucrose, positive for catalase, oxidase, phosphatase, esculin hydrolysis, and produces indole. It does not, however produce β-galactosidase. Antibiotic sensitivity test was performed by using standard Kirby-Bauer disc diffusion technique as per the guidelines of the Clinical Laboratory Standards Institute (CLSI) with commercially available discs (Hi Media, Mumbai, India) on Mueller-Hinton agar plates.[4] Minimum inhibitory concentrations (MIC) of antibiotics were determined by VITEK-2 (Biomérieux, Marcy l’Etoile, France) and interpreted as per CLSI breakpoints for nonfermenters[4] [Table 1]. As per EUCAST isolate showed 100% susceptibility to colistin by E-test.[5] MICs of meropenem and imipenem by E-test showed, a level of >32 μg/ml for both. Screening for carbapenemase production, by the modified Hodge's test showed negative carbapenemase production but double-disc synergy tests, combined-disc synergy test and metallo-beta lactamase (MBL) production by MBL (IP/IPI) E-test method (bioMérieux, Marcy l’Etoile, France) for MBL production were positive. Polymerase chain reaction (PCR) amplification for detection of extended-spectrum beta-lactamase genes (blaCTXM, blaOXA, blaSHV and blaTEM), Ambler class (B and D) carbapenemases blaIMP, blaVIM, blaSPM, blaGIM, blaSIM, blaNDM-1, blaOXA-23 and blaOXA-24 was carried out on the strain in a GeneAmp 9700 PCR System, (Applied Biosystems, Singapore) by using primers described earlier[6] which revealed the presence of blaNDM-1 gene. The amplicon was purified and sequenced with the ABI 3730XL capillary sequencer (Applied Biosystems, Foster City, CA, USA). Sequencing results confirmed the presence of blaNDM-1 gene. The isolate, was further subjected to 16S rRNA gene sequence analysis and the isolate showed a sequence identity value of 100% to C. indologenes. Plasmid analysis using the Kieser technique[7] revealed that C. indologenes harbored 50 kb plasmids. Transconjugation experiment using Escherichia coli J53 as recipient strain showed a MBL phenotype. Plasmid DNA from the donor and recipient strains was separated by electrophoresis on horizontal 0.8% agarose gels at 50 V for 3 h. The size of the plasmid compared by coelectrophoresis with plasmids of known sizes from E. coli strains V517 and 39R861. DNA bands visualized after staining with 0.05% ethidium bromide. The presence of blaNDM-1 gene in a transconjugant, confirmed by PCR and sequencing analysis. PCR-based replicon typing[8] demonstrated that plasmid belonged to the IncN type.
Table 1.
Antibiogram of blaNDM-1-positive C. indologenes and its transconjugant E. coli J53 (μg/ml)
DISCUSSION
A 77-year-old male having septicemia is treated with colistin with dosage of 100 mg twice a day for 12 days along with other supporting therapy. Patient recovered well and discharged from the hospital. Appropriate antimicrobial treatment is required for bacteremia and other infections caused by C. indologenes in debilitated or immunosuppressed, individuals because of intrinsic antimicrobial resistance to penicillins, cephalosporins, aztreonam, carbapenems, chloramphenicol, and aminoglycosides chosen empirically to treat such serious Gram-negative infections.[1,2,3,9,10,11] In our case, uncontrolled diabetes mellitus was a risk factor for C. indologenes septicemia. With the worldwide occurrence, increase and rate of dissemination of blaNDM-1, early detection is critical. The benefits of early detection include timely implementation of strict infection control practices as well as clinical guidance regarding the potential risks for therapeutic failure. Here, we report a case of septicemia in 77-year-old male caused by carbapenem resistant C. indologenes having blaNDM-1, which was transferable and treated with colistin for the 1st time in India. The nucleotide sequence of blaNDM-1 gene, from a clinical isolate of C. indologenes reported in this study has been assigned (GenBank nucleotide Accession No: KC004052.1).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bhuyar G, Jain S, Shah H, Mehta VK. Urinary tract infection by Chryseobacterium indologenes. Indian J Med Microbiol. 2012;30:370–2. doi: 10.4103/0255-0857.99511. [DOI] [PubMed] [Google Scholar]
- 2.Lin YT, Jeng YY, Lin ML, Yu KW, Wang FD, Liu CY. Clinical and microbiological characteristics of Chryseobacterium indologenes bacteremia. J Microbiol Immunol Infect. 2010;43:498–505. doi: 10.1016/S1684-1182(10)60077-1. [DOI] [PubMed] [Google Scholar]
- 3.Cascio A, Stassi G, Costa GB, Crisafulli G, Rulli I, Ruggeri C, et al. Chryseobacterium indologenes bacteraemia in a diabetic child. J Med Microbiol. 2005;54:677–80. doi: 10.1099/jmm.0.46036-0. [DOI] [PubMed] [Google Scholar]
- 4.Wayne, PA, USA: CLSI; 2012. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: twenty Second Informational Supplement M100-S22. [Google Scholar]
- 5.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for Interpretation of MICs and zone diameters (Version 2, January 1, 2012) [Last accessed on 2012 Feb 23]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_20_120221.pdf .
- 6.Khajuria A, Praharaj AK, Grover N, Kumar M. First report of an Enterobacter ludwigii isolate coharboring NDM-1 and OXA-48 carbapenemases. Antimicrob Agents Chemother. 2013;57:5189–90. doi: 10.1128/AAC.00789-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12:19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 8.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219–28. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Shah S, Sarwar U, King EA, Lat A. Chryseobacterium indologenes subcutaneous port-related bacteremia in a liver transplant patient. Transpl Infect Dis. 2012;14:398–402. doi: 10.1111/j.1399-3062.2011.00711.x. [DOI] [PubMed] [Google Scholar]
- 10.Bellais S, Poirel L, Leotard S, Naas T, Nordmann P. Genetic diversity of carbapenem-hydrolyzing metallo-beta-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob Agents Chemother. 2000;44:3028–34. doi: 10.1128/aac.44.11.3028-3034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeba B, De Luca F, Dubus A, Delmarcelle M, Simporé J, Nacoulma OG, et al. IND-6, a highly divergent IND-type metallo-beta-lactamase from Chryseobacterium indologenes strain 597 isolated in Burkina Faso. Antimicrob Agents Chemother. 2009;53:4320–6. doi: 10.1128/AAC.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


