Abstract
Diabetes is associated with complications like neuropathy, nephropathy, cardiomyopathy, and retinopathy due to increased oxidative stress and serum lipids. In the present study, rosuvastatin, a HMG-CoA inhibitor, was investigated for its protective effect in neuropathy, nephropathy, and cardiomyopathy based on the lipid-lowering property along with its pleiotropic effects such as improved blood flow to the organ and antioxidant defense. Type 2 diabetes was induced in Wistar rats by single i.p. administration of streptozotocin (50 mg/kg). These diabetic rats were treated with daily doses of rosuvastatin (10 mg/kg) alone, metformin (120 mg/kg) and glimepiride (1 mg/kg) and rosuvastatin in combination with metformin (120 mg/kg) and glimepiride (1 mg/kg) for a period of 6 weeks. The biochemical parameters involved in neuropathy, renopathy, and cardiopathy were estimated. Treatment resulted in significant (P < 0.05) decrease in thiobarbituric acid reactive substances (TBARS) and increase in levels of glutathione peroxidise and catalase in brain and kidney homogenates. Significant (P < 0.05) increase in high-density lipoproteins and decrease in creatinine kinase, triglycerides, total serum cholesterol represents the cardioprotective action, whereas significant (P < 0.05) increase in the latency in the hotplate model shows the neuroprotective activity, and significant (P < 0.05) decrease in blood urea nitrogen, creatinine levels and increase in serum total protein levels suggested the renoprotective actions. The unique properties of rosuvastatin such as antioxidant defense and lipid-lowering nature might have resulted in cardio, neuro, and renoprotective activity in type 2 diabetic rats treated with metformin and glimepiride.
Keywords: Cardiomyopathy, diabetes, nephropathy, neuropathy, serum proteins
INTRODUCTION
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels.[1] Long-term complications of diabetes include retinopathy with potential loss of vision; nephropathy leading to renal failure; peripheral neuropathy with risk of foot ulcers, amputations, and Charcot joints; and autonomic neuropathy causing gastrointestinal, genitourinary, and cardiovascular symptoms and sexual dysfunction. Patients with diabetes have an increased incidence of atherosclerotic cardiovascular, peripheral arterial and cerebrovascular disease. Hypertension and abnormalities of lipoprotein metabolism are often found in people with diabetes.[2] The main reason for complications of diabetes is with the flow of blood through the blood vessels that supply energy for every organ. High blood sugar levels will damage the blood vessels and nerves. This causes problems in association with body organs like kidneys, nerves, feet, eyes, heart, bones, skin problems, digestive problems, sexual dysfunction, and problems with teeth and gums.[3]
Metformin is a biguanide that lowers blood glucose primarily by decreasing hepatic glucose output and reducing insulin resistance. When used as monotherapy, metformin does not cause hypoglycemia and is thus termed as an “anti-hyperglycemic”.[4] Metformin acts by increasing the sensitivity of liver, muscle, fat, and other tissues to the uptake of glucose and effects of insulin. These actions lower the level of sugar in the blood.[5]
Glimepiride is more selective for the β-cell K+-ATP channel than for the cardiovascular tissue K+-ATP channel. All the sulfonylurea's exhibit both insulin-secreting and extrapancreatic activities. Glimepiride relies on extrapancreatic effects for a greater proportion of its hypoglycemic effect, and it is possibly because of this that it is considered less likely to produce unwanted hypoglycemia.[6] A specific sulfonylurea receptor (SUR) closely linked to the ATP-sensitive potassium ion channel exists on the β-cell. Sulfonylureas are believed to inhibit this potassium ion channel, thus blocking the efflux of potassium and lowering the membrane potential to cause depolarization. The voltage-dependent calcium channels then open, increasing intracellular calcium concentration. The increased intracellular concentration of calcium ultimately stimulates insulin secretion.[7]
Rosuvastatin is a synthetic lipid-lowering agent. It is a competitive inhibitor of 3-hydroxy-3- methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-determining enzyme in cholesterol biosynthesis via the mevalonate pathway. This enzyme catalyzes the conversion of HMG-CoA to mevalonate. It acts primarily in the liver. It decreases hepatic cholesterol, increases hepatic uptake of cholesterol, and reduces plasma cholesterol levels.[8] Statins were also shown to exhibit non-lipid-modifiable effects called pleiotropic ones, which could be responsible for additional benefits. The most important pleiotropic anti-atherogenic effects of statins are improvement of endothelial dysfunction, antioxidative properties, anti-inflammatory, anti-proliferative, anti-thrombotic effects and neoangiogenesis.[8,9] Statins have also been suggested to reduce inflammatory cytokines production like tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β).[10] In addition, statins can disrupt the oxidative stress/inflammation cycle by decreasing the release of inflammatory mediators and lipid peroxidation. Chronic administration of statins can also inhibit peroxisome proliferator-activated receptor (PPAR) α and γ, which are known inflammatory mediators.[11] These pleiotropic actions may help in reducing vascular inflammation and in anti-rejection regimens following graft arterial disease. Statins, by increasing the production of nitric oxide, in the endothelium, have local vasodilatory property in addition to anti-thrombogenic, anti-proliferative, and leukocyte adhesion inhibiting effects.[12] Other mechanisms by which statins favorably influence atherosclerosis include enhancement of endothelium-dependent relaxation, inhibition of platelet function, and inhibition of endothelin-1, a potent vasoconstrictor and mitogen.[13]
MATERIALS AND METHODS
Animal procurement and maintenance
Wistar Albino rats, aged 4 months (body weight: 160-180 g), were used for the present study, procured from Sanzyme Ltd., Hyderabad, India. The animals were housed in acrylic poly cages (38 cm × 23 cm × 10 cm) with not more than 6 animals per cage, at ambient temperature of 18 ± 2°C with 12-h-light/12-h-dark cycle. Rats have free access to standard chow diet and water ad libitum. The maintenance and the handling of animals were performed according to the rules and regulations of Institutional Animal Ethical Committee, (Regd. No. 36/SPIPS/IAEC/12) Kakatiya University.
Chemicals and reagents
Rosuvastatin and metformin HCl were kindly gifted by Dr. Reddy's Laboratories, Hyderabad, India. Glimepiride was procured as a gift sample by MSN Laboratories, Hyderabad, India. Streptozotocin and Thiobarbituric acid were purchased from Himedia Laboratories, Mumbai, India, and all other chemicals of highest purity grade available were purchased from S.D. Fine Chemicals, Mumbai, India. For estimation of various biomarkers, Glucose kit, Creatinine kit, Total protein kit, Urea kit, CK-MB kit, Triglycerides kit, Total cholesterol kit, HDL cholesterol kit were purchased from Coral Diagnostics, Mumbai, India
Experimental protocol and methods
Albino Wistar rats of either sex weighing between 180 ± 10 gms were used for the experiment and were allowed to acclimatize for a week. Six rats were formed into one group; 5 such different groups of rats were formed and labeled (Group – I: Normal control, Group – II: Diabetic control, Group – III: Rosuvastatin-treated, Group – IV: metformin- and glimepiride-treated, and Group – V: metformin-, glimepiride-, and rosuvastatin- treated). Diabetes in rats was induced by single dose of streptozotocin (50 mg/kg) administered intraperitoneally (i.p.).[14] Blood glucose levels were estimated after 3 days, and the animals with glucose levels more than 200 mg/dl were used for the study. The diabetic rats were treated with metformin - 120 mg/kg/d; p.o., glimepiride - 1 mg/kg/d, p.o. and rosuvastatin – 10 mg/kg/d, p.o. based on their treatment group for about 6 weeks.
After 6 weeks of treatment, blood samples were collected by retro-orbital sinus puncture, under mild ether anesthesia, from all the groups of animals. Plasma was obtained by immediate centrifugation of blood samples using BIOFUGE cooling centrifuge at 4000 rpm for 10 minutes at 4°C temperature. All samples were stored at –20°C until analysis. Blood samples were used for the estimation of different biochemical parameters i.e. Glucose, Creatine kinase- MB, Serum lipids (triglycerides, LDL and HDL), Creatinine, BUN, and Total protein using semi-autoanalyzer (Biochemical systems international) and the corresponding diagnostic kits. Later, the rats were sacrificed by cervical dislocation, and their brains and kidneys were harvested and then rinsed with ice-cold saline. Then, the organs were homogenized with ice-cold phosphate buffer (pH 7.4). The homogenates (10% w/v) were then centrifuged at 10,000 g for 15 min, and the supernatant so obtained was used for the estimation of antioxidant parameters such as malondialdehyde (MDA) as the thiobarbituric acid reactive substances (TBARS), glutathione peroxidase (GPx) and catalase (CAT) with the help of UV-Visible spectrophotomer (ELICO limited).
Hotplate model was performed, and paw withdrawal latency time for all the groups of animals was recorded after 6 weeks of treatment prior to sacrifice in order to assess the neuropathy.[15]
Statistical analysis
Statistical analysis of all the obtained results was performed by one-way ANOVA using graph pad prism software version 5.0 followed by Bonferroni's multiple comparison test. All the results were expressed as mean ± SEM, and a probability of P < 0.05 was considered as significant.
RESULTS
Effect on blood glucose levels
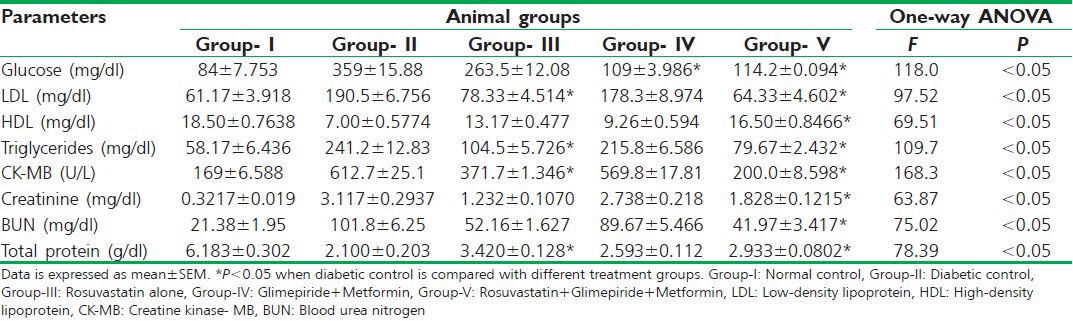
In the streptozotocin-induced type 2 diabetic rats, blood glucose levels were significantly raised when compared with normal control group and were considered as diabetic. Treatment (for about 6 weeks) with rosuvastatin alone rendered no significant decrease in the blood glucose levels. Treatment with oral hypoglycemic agents (metformin plus glimepiride) and the combination of a rosuvastatin with oral hypoglycemic agents (triple therapy) both rendered a significant (P < 0.05) decrease in the blood glucose levels to a near normal level when compared with diabetic control [Table 1].
Table 1.
Effect on various biochemical parameters in different animal groups
Effect on serum lipids
In the diabetic rats, there was a significant and pathological abnormality in the levels of serum lipids (LDL, HDL, and triglycerides) when compared with normal control. Treatment with rosuvastatin alone significantly (P < 0.05) decreased the levels of LDL and the triglycerides, whereas rendered no significant improvement in the levels of HDL when compared with diabetic control. Treatment with oral hypoglycemic agents yielded no significant reversal in the pathologically abnormal levels of serum lipids. Triple therapy significantly (P < 0.05) improved the levels of HDL; furthermore, yielded a significant reduction in the levels of LDL and the triglycerides when compared with diabetic control [Table 1].
Effect on CK-MB levels
Creatine kinase- MB (CK-MB) levels were significantly raised in the diabetic rats when compared with normal control group. Triple therapy and rosuvastatin monotherapy decreased the CK-MB levels significantly (P < 0.05) to a near normal level when compared with diabetic control. Treatment with oral hypoglycemic agents rendered no significant decrease in this cardiac risk marker [Table 1].
Effect on renal parameters
Renal risk markers (creatinine, BUN, and total protein) were found to be pathologically anomalous in the diabetic rats when compared with normal control. Triple therapy significantly (P < 0.05) brought down the levels of creatinine to a level, closer to the physiological range. Rosuvastatin monotherapy and anti-diabetic drug therapy both yielded no significant reversal in the serum creatinine levels. On the other hand, none of the therapies rendered the levels of urea nitrogen to a near normal range. A significant (P < 0.05) improvement in the total protein levels was found after the stipulated statin monotherapy and triple combination therapy when compared with diabetic control, whereas oral hypoglycemic therapy failed to do so [Table 1].
Effect on antioxidant parameters in the brain
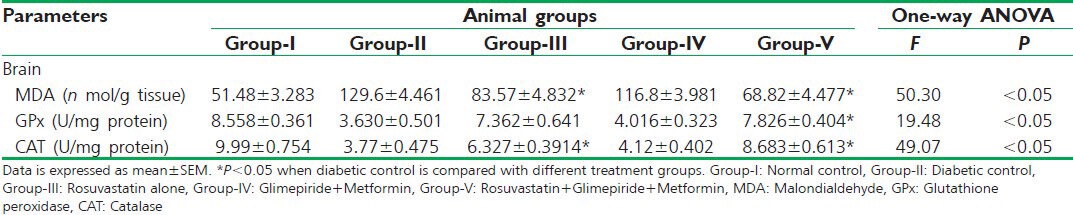
Oxidative stress marker, malondialdehyde (MDA), levels were significantly higher, and the antioxidant defense markers, catalase (CAT) and glutathione peroxidase (GPx), concentrations were significantly lower in the brain homogenates of streptozotocin-induced type 2 diabetic rats when compared with normal control.
Rosuvastatin monotherapy significantly (P < 0.05) curbed the levels of MDA and augmented the concentration of CAT to an almost physiological rage, on the other hand failed to improve GPx concentration when compared with diabetic control. Anti-diabetic drug therapy found to be insignificant (P > 0.05) in reversing the abnormality in the concentrations of antioxidant parameters (MDA, CAT, and GPx) accompanied by type 2 diabetes; in contrary, triple therapy found to be significant (P < 0.05) in doing so when compared with diabetic control [Table 2].
Table 2.
Effect on antioxidant parameters in the brain homogenates of different animal groups
Effect on antioxidant parameters in the kidney
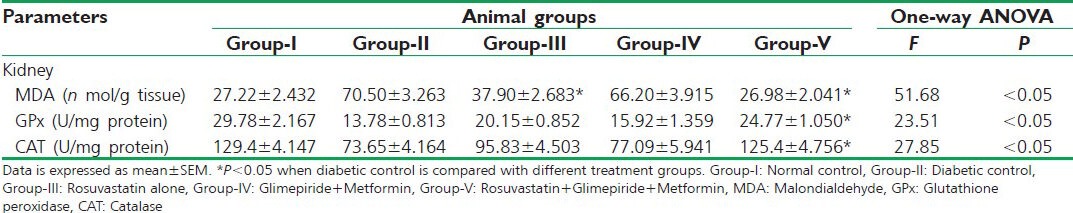
In the kidney homogenates of diabetic rats, there found to be a considerable derailment in the antioxidant parameters (CAT, GPx, and MDA) when compared with normal control. Triple combination therapy and rosuvastatin monotherapy significantly (P < 0.05) reduced the oxidative stress marker (MDA) concentrations, whereas oral hypoglycemic therapy failed to do so. Antioxidant defense marker concentrations (CAT and GPx) were significantly (P < 0.05) improved to a near physiological range by triple therapy when compared with diabetic control. On the other hand, anti-diabetic drug therapy and alone rosuvastatin therapy rendered an insignificant increase in the antioxidant defenses in the kidney homogenates when compared with diabetic control [Table 3].
Table 3.
Effect on antioxidant parameters in the kidney homogenates of different animal groups
Effect on neural functioning (tail-withdrawal reflex)
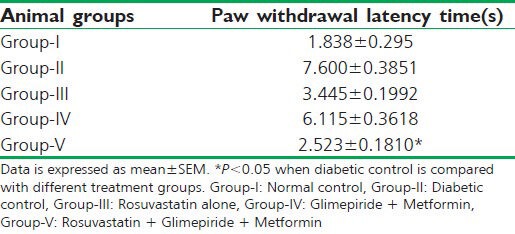
In the streptozotocin-induced type 2 diabetic rats, there was a significant increase in the paw-withdrawal latency time (6 sec) when compared with normal control group. Treatment (for 6 weeks) of the diabetic rats with rosuvastatin alone and with oral hypoglycemic agents (Metformin + glimepiride) both rendered no significant decrease in the paw withdrawal latency time. Treatment with the combination of a statin and the oral hypoglycemic agents (triple therapy) significantly (P < 0.05) lowered the paw withdrawal latency time when compared with diabetic control rats [Table 4].
Table 4.
Effect on nerve fiber conduction (tail-withdrawal reflex) in different animal groups
DISCUSSION
The results of the present study inferred that the treatment of streptozotocin-induced type 2 diabetic Wistar Albino rats with the combination of a statin (rosuvastatin) and oral hypoglycemic agents (metformin + glimepiride) provides cardio protection and alleviates neural and renal complications in addition to better and efficient glycemic control.
This triple therapy (rosuvastatin + glimepiride + metformin) resulted in efficient glycemic control in the diabetic rats. One of the previous studies involving statins reported that atorvastatin improves glycemic control and insulin sensitivity via the activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) via 15-deoxy-delta-12, 14-PGJ2 (15DPGJ2).[16]
This study demonstrated that rosuvastatin when combined with oral hypoglycemic agents reported protective effect in neural complications associated with type 2 diabetes. This was evidenced by better glycemic control along with a significant reduction in the concentration of MDA (biomarker for lipid peroxidation) and a significant rise in the concentrations of antioxidant enzymes (GPx and CAT) in the brain homogenates of diabetic rats treated with triple therapy, which indicates minimal damage to the neural cell membranes.
The mechanisms that are attributable to the antioxidant activity of statin are increase in the bioavailability of nitric oxide (NO), decrease in the lipid peroxidation and the ROS production.[17]
This study also demonstrated that triple therapy improves nerve fiber conduction in diabetic rats, which was evidenced by decrease in the paw withdrawal latency time (s) when the animals were subjected to hotplate test (thermal stimulus model to assess small nerve fiber function). Here, the statins up-regulate the expression and the activity of nitric oxide synthase (NOS).[18]
In this study, rosuvastatin in combination with oral hypoglycemic agents demonstrated a potential cardioprotective action against diabetic dyslipidemia and the consequences associated with diabetic dyslipidemia. Triple therapy rendered significant changes in the raised LDL, triglyceride, and creatine kinase-MB concentrations and reduced HDL concentration in the diabetic rats.
The cardioprotective role of triple therapy might be due to effective control on blood glucose levels and lipid concentrations by the combination of metformin with a sulfonylurea in the diabetic rats.[19] In addition to this, beneficial cardiovascular pleiotropic effects of rosuvastatin, which include improvement of endothelial dysfunction, increased nitric oxide (NO) bioavailability, antioxidant properties, inhibition of inflammatory responses, and stabilization of atherosclerotic plaques, might act in concert with the potent lipid-lowering effect of statins to exert early as well as lasting cardiovascular protective effects.[8] In one of the previous studies, it was reported that cardioprotective activity can be assessed by a significant reduction in the levels of creatine kinase-MB (CK-MB) along with significant changes in the other cardiac risk markers and the antioxidant defense markers like MDA and CAT.[20]
It was also observed by triple therapy to the diabetic animals that this combination mitigates renal anomalies associated with diabetic nephropathy. Significant reduction in the concentration of serum creatinine and an increase in the total protein content (i.e. decrease in proteinuria) along with better glycemic control were the corresponding findings observed with the triple therapy to the diabetic rats. In addition to this, there were also the findings denoting significant positive changes in the concentrations of antioxidant parameters in the kidney homogenates of diabetic rats after the stipulated period of triple therapy.
The renoprotective role of statin combined with oral hypoglycemics was attributed to strict glycemic control, improvement in the insulin sensitivity, lowered serum lipid content, decreased ROS production, and reduced protein overload as well.[21] In the literature, there was an in vivo evidence for the assessment of renoprotective effect wherein the renal risk markers were significantly decreased (creatinine, BUN, and proteinuria) along with a significant reduction in the oxidative stress marker (MDA) and a significant rise in the antioxidant defense markers (GSH, CAT).[22]
At the end, triple therapy (rosuvastatin in combination with glimepiride plus metformin) improved glycemic control and the antioxidant defenses, enhanced nerve fiber functioning, substantially decreased cardiac risk markers (LDL, triglycerides, and CK-MB), and reduced the protein overload, thereby alleviated renal damage, in comparison to glimepiride plus metformin therapy, in streptozotocin-induced type 2 diabetic Wistar Albino rats.
CONCLUSION
Rosuvastatin have a protecting effect in type 2 diabetes-associated neuronal, cardiac, and renal complications in Wister Albino rats when administered in combination with oral hypoglycemic agents. Beneficial pleiotropic effects of rosuvastatin and strict glycemic control with metformin and glimepiride may be resulted in the protective effects against type 2 diabetes and its devastating complications
Further studies are necessary, first, to warrant whether this triple combination therapy provides protection against any other diabetic anomalies in addition to the aforementioned complications, second, to provide insights into explicit molecular mechanisms underlying the outcomes of this study.
ACKNOWLEDGMENT
Authors are thankful to Dr. Reddy Labs and MSN Labs, Hyderabad for providing the API's for this work.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Triplitt CL, Reasner CA, Isley WL. Diabetes Mellitus in Pharmacotherapy: A Pathophysiologic Approach. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. 6th Ed. New York: McGraw Hill Medical Publishing Division; 2005. pp. 1333–67. [Google Scholar]
- 2.American Diabetes Association: Clinical practice recommendations. Diabetes Care. 1998;21:S1–95. [PubMed] [Google Scholar]
- 3.Stern MP, Haffner SM. Prospective assessment of metabolic control in diabetes mellitus: The complications question. JAMA. 1998;260:2896–7. [PubMed] [Google Scholar]
- 4.Melchior WR, Jaber LA. Metformin: An antihyperglycemic agent for treatment of type II diabetes. Ann Pharmacother. 1996;30:158–64. doi: 10.1177/106002809603000210. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-acitvated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dills DG, Schneider J. Clinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. Horm Metab Res. 1996;28:426–9. doi: 10.1055/s-2007-979831. [DOI] [PubMed] [Google Scholar]
- 7.Langtry HD, Balfour JA. Glimepiride: A review of its use in the management of type 2 diabetes mellitus. Drugs. 1998;55:563–84. doi: 10.2165/00003495-199855040-00007. [DOI] [PubMed] [Google Scholar]
- 8.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(Suppl 1):III39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 9.Pella D, Rybar R, Mechirova V. Pleiotropic effects of statins. Acta Cardiol Sin. 2005;21:190–8. [Google Scholar]
- 10.Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–4. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 11.Inoue I, Goto S, Mizotani K, Awata T, Mastunaga T, Kawai S, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: Reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPAR alpha) in primary endothelial cells. Life Sci. 2000;67:863–76. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- 12.Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM, Trocha SD, et al. Simvastatin exerts both anti-inflammatory and cardioprotective effects in Apo E deficient mice. Circulation. 2001;103:2598–603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS. Non-lipid lowering effects of statins on atherosclerosis. Curr Cardiol Rep. 1999;1:225–32. doi: 10.1007/s11886-999-0027-7. [DOI] [PubMed] [Google Scholar]
- 14.Reitman S, Frankel S. Colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–88. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 16.Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, et al. Pravastatin and the development of diabetes mellitus: Evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–62. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 17.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–7. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 18.Santodomingo-Garzon T, Cunha TM, Verri WA, Jr, Valerio DA, Parada CA, Poole S, et al. Atorvastatin inhibits inflammatory hypernociception. Br J Pharmacol. 2006;149:14–22. doi: 10.1038/sj.bjp.0706836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 20.Momin FN, Kalai BR, Shikalgar TS, Naikwade NS. Cardioprotective effect of methanolic extract of Ixora coccinea leaves on doxorubicin-induced cardiac toxicity in rats. Indian J Pharmacol. 2012;44:178–83. doi: 10.4103/0253-7613.93844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buemi M, Allegra A, Corica F, Aloisi C, Giacobbe M, Pettinato G, et al. Effect of fluvastatin on proteinuria in patients with immunologlobulin A nephropathy. Clin Pharmacol. 2000;67:427–31. doi: 10.1067/mcp.2000.105330. [DOI] [PubMed] [Google Scholar]
- 22.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj HG, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009;5:249–55. doi: 10.7150/ijbs.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


