Abstract
Recent advances in the study of alcoholism have thrown light on the involvement of various neurotransmitters in the phenomenon of alcohol addiction. Various neurotransmitters have been implicated in alcohol addiction due to their imbalance in the brain, which could be either due to their excess activity or inhibition. This review paper aims to consolidate and to summarize some of the recent papers which have been published in this regard. The review paper will give an overview of the neurobiology of alcohol addiction, followed by detailed reviews of some of the recent papers published in the context of the genetics of alcohol addiction. Furthermore, the author hopes that the present text will be found useful to novices and experts alike in the field of neurotransmitters in alcoholism.
Keywords: γ-amino butyric acid, alcoholism, dopamine, glutamate, serotonin
Introduction
Alcohol is a multi-dimensional entity. It has been around for thousands of years and has been known for its many stimulating and mind altering effects. Alcohol is in essence, a drug, pure and simple. It is a drug which is so commonly available in so many different forms and guises that it is often hard to even look at it in that way.
Alcohol is truly in a class of its own. It doesn’t carry the same kind of stigma or social abhorrence which other drugs of abuse such as cocaine, methamphetamines, lysergic acid diethylamide (LSD) etc., carry. Alcohol is widely accepted in the society and consumed by everyone, young and the old alike, women and men included. In some societies, alcohol consumption is even accepted as part of normal social etiquettes. Alcohol is thus, all pervasive and is in this way is the most dangerous drug known to mankind.
Alcohol is the first thing people go for when they are at a social gathering and are looking to have a pleasant time. It is the first choice in the long list of things which can make a person feel intoxicated and give that feeling of high. Being milder in its 1st time effects when compared with other drugs such as nicotine, people falsely believe that there is very little chance of getting addicted to alcohol. However, the brain's reward pathways are rarely under voluntary control. For once the brain senses a certain activity giving it pleasure; it will rewire the brain chemistry in a way which makes the person want to have more of that activity. The activity here in this case being consumption of alcohol.
Slowly over a period of time, the person craves more of the drug, to achieve the same kind of high as earlier. He thus starts consuming more and more alcohol until a point comes when normal brain chemistry simply cannot function without alcohol. The brain's neurobiology has been permanently changed. As an example of the kind of brain chemistry changes which take place, the following image shows the brain scan of a methamphetamine addict and a non-addict [Figure 1].
Figure 1.
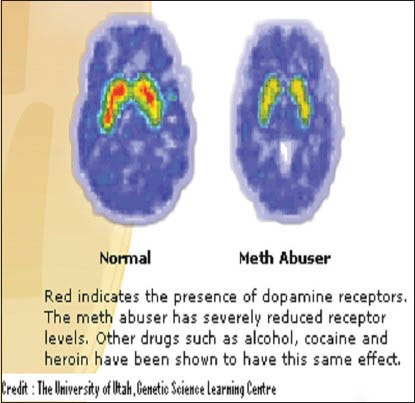
Diagram depicting the difference in the quantities of dopamine receptors in the brain of a methamphetamine addict (right) and non-addict (left)
Thus, alcohol is a powerful drug. It affects several neurological pathways and causes significant changes in the brain. Some of the neurological pathways known to be affected by alcohol consumption include the dopaminergic, serotoninergic, γ-amino butyric acid (GABA) and glutamate pathways.
Alcohol, as a drug, like all the other drugs affects the central nervous system (CNS). The type of alcohol commonly consumed is ethanol with different alcoholic beverages containing different percentages of it. Ethanol acts to depress brain function, very much in the style of an anesthetic. Ethanol at low blood concentrations releases behaviours that are otherwise inhibited and usually produces feelings of relaxation and good mood which may facilitate socializing. Thus at low doses, ethanol is possibly useful. Caution however, needs to be exercised as even low quantities of alcohol affect the ability of hippocampus to process information, which in turn impairs memory formation. Higher doses of alcohol affect the brain further by inducing intoxication wherein the person may experience temporary loss of coordination and judgment.[3]
It must be kept in mind, however that the simple consumption of alcoholic beverages does not make a person alcohol-dependent (AD). The difference between an alcohol addict and an alcohol non-addict goes beyond the quantity and intensity of alcohol consumed. Long-term alcohol abuse produces physiological changes in the brain such as tolerance and physical dependence. These changes in the brain chemistry maintain the alcoholic's compulsive inability to cease alcohol consumption drinking and results in alcohol withdrawal syndrome (AWS) upon discontinuation of alcohol. An alcoholic is therefore aware of the harm caused by alcohol on his or her health but, is unable to control such compulsive drinking impulses. Diagnosis of alcoholism is performed via questionnaire based screening methodologies wherein destructive drinking patterns are ascertained. Furthermore, due to both physical and societal factors, the alcoholic woman is more susceptible, compared to the alcoholic man, at suffering the consequences of uncontrolled alcohol consumption.[1,2]
Discussion
Neurobiology of alcoholism
Alcohol addiction takes place primarily through two means. The first is a positive reinforcement method and the second is a negative reinforcement method. Positive reinforcement represents an environmental situation in which a rewarding stimulus or experience (e.g., alcohol-induced euphoria) increases the chances that the individual displays a certain response (e.g., alcohol-seeking behavior). Negative reinforcement refers to an increase in behavioural patterns, such as alcohol ingestion, if the behavior facilitates the individual to circumvent or avoid an aversive stimulus. An alcoholic trying to abstain from drinking may experience a range of aversive stimuli in the form of alcohol withdrawal symptoms: irritability, anxiety and dysphoria. It is precisely such symptoms which make abstinence difficult and a relapse possible.[4]
Hence, what begins as a mild way to seek pleasure, soon turns into a full-fledged addiction as the alcohol begins to cause widespread neuroadaptations in the brain, causing the person to convert from an alcohol non-addict to an alcohol addict. Such changes in the reinforcing value of alcohol during the transition from alcohol use to dependence reflect adaptive neural changes resulting from chronic exposure to high alcohol quantities. Thus, while on one hand, the early stages of nondependent alcohol use is largely motivated by alcohol's positive reinforcing effects, the drinking behavior in the dependent state is likely driven by both the positive and negative reinforcing effects of the drug. Neuroadaptations leading to dependence are driven by a constellation of processes which heighten motivation for alcohol consumption. Such neuroadaptations cause alcohol withdrawal symptoms upon cessation of drinking.[4]
It has been posited by[5] that the negative-affective state induced by alcohol withdrawal and especially the increase in anxiety[6] is a major driving force in the propensity for relapse to alcohol-seeking behavior. The mechanisms involved behind alcohol sensitization, tolerance, withdrawal and dependence are discussed in the following sections.
The reward pathways
Underlying the brain changes and neuroadaptations are the reward and stress circuits of the brain. A neural circuit comprises of a series of neurons which send electro chemical signals to one another. An activated neuron sends chemical signaling molecules called neurotransmitters through the neural circuit which bind to specific molecules called the receptors. Depending upon the circuit involved, the binding of these neurotransmitters may cause excitatory or inhibitory signals to be passed further along the circuit.
Alcohol interacts with several neurotransmitter systems in the brain's reward and stress circuits. These interactions result in alcohol's acute reinforcing effects. Following chronic exposure, these interactions in turn cause changes in neuronal function that underlie the development of alcoholism. The following text introduces some of the neural circuits relevant to AD, categorized by neurotransmitter systems. These neural circuits include the dopaminergic, serotoninergic, glutamatergic and GABAergic neural circuits.
Dopamine pathway
Dopamine is a neurotransmitter primarily involved in a circuit called the mesolimbic system, which projects from the brain's ventral tegmental area to the nucleus accumbens. This circuit affects incentive motivation, i.e., how an organism reacts to incentive changes in the environment.
Studies have shown that dopamine has a role in the incentive motivation associated with acute alcohol intoxication. This is so because alcohol consumption can be blocked by injecting low doses of a compound that interferes with dopamine's normal activity (i.e., a dopamine antagonist) directly into the nucleus accumbens.[7,8] Furthermore, the consumption of alcohol and simply the anticipation of availability of alcohol results in production of dopamine in the nucleus accumbens, determined by the increased levels of dopamine in the fluid outside neurons.[9] However, lesions of the mesolimbic dopamine system do not completely abolish alcohol-reinforced behavior, indicating that dopamine is an important, but not essential, component of alcohol-reinforcement.[10] Finally, alcohol withdrawal produces decreases in dopamine function in dependent individuals and this decreased dopamine function may contribute to withdrawal symptoms and alcohol relapse.[11]
Serotonin pathway
The neurotransmitter serotonin (also known as 5-hydroxytryptamine or 5-HT) has been a target of interest for potential pharmacotherapy for alcoholism for a long time because of the well-established link between serotonin depletion, impulsivity and alcohol-drinking behavior in rats and humans.[12] According to[13] pharmacological compounds that target the serotonin system by inhibiting neuronal reuptake of serotonin, thereby prolonging its actions, or by blocking specific serotonin receptor subtypes have been shown to suppress alcohol-reinforced behavior in rats. During alcohol withdrawal, serotonin release in the nucleus accumbens of rats is suppressed and this reduction is partially reversed by self-administration of alcohol during withdrawal.[14]
GABA pathway
GABA is the major inhibitory neurotransmitter in the brain. It acts through two receptor subtypes called GABAA and GABAB. Alcohol acts to increase GABA activity in the brain and it does so through two general mechanisms. It can for example, act on the GABA-releasing (i.e., presynaptic) neuron, causing an increase in GABA release; or it can act on the signal-receiving (i.e., postsynaptic) neuron facilitating the activity of the GABAA receptor. The consumption of alcohol is suppressed by compounds that interfere with the actions of the GABAA receptor (i.e., GABAA receptor antagonists) as well as compounds that stimulate the GABAB receptor (i.e., GABAB agonists) in the nucleus accumbens, ventral pallidum, bed nucleus of the stria terminalis and amygdala.[15]
Among these regions, the central nucleus of the amygdala is an important brain region involved in the regulation of emotional states. This region is particularly sensitive to suppression of alcohol drinking by compounds acting on the GABA systems (i.e., GABAergic compounds).[16] It has been found that acute and chronic alcohol exposure indeed results in increases in GABA transmission in this region.[17,18] In addition, compounds that target a specific component of the GABAA receptor complex (i.e., the α1-subunit) help reduce consumption of alcohol when injected directly into the ventral pallidum, a brain region which receives signals from neurons located in the extended amygdala.[19,20]
The GABA systems in the brain are altered in situations of chronic alcohol exposure. As an example, in some regions of the brain, the expression of genes that encode components of the GABAA receptor is affected due to alcohol. This has been proven by the changes observed in the subunit composition of the receptor in those regions, the most consistent of which are decreases in α1- and increases in α4-subunits.[21] The function of GABAA receptors also is regulated by molecules known as neuroactive steroids[22] that are produced both in the brain and in other organs (i.e., in the periphery). There is a marked increase in the levels of many neuroactive steroids following exposure to alcohol.[23] Furthermore,[24] stated that the increase in the activity of neuroactive steroids in the brain is not dependent on their production by peripheral organs. These findings therefore indicate that neuroactive steroids are potential key modulators of the altered GABA function which occurs during development of AD by acting directly at GABAA receptors.[24]
Glutamate pathway
Glutamate is the major excitatory neurotransmitter in the brain and it exerts its effects through several receptor subtypes, including one called the N-methyl-D-aspartate (NMDA) receptor. Glutamate systems have been known for a long time to be involved in the acute reinforcing actions of alcohol and the effect of alcohol on an organism can be mimicked with the help of NMDA receptor antagonists.[3] Unlike the case with GABA, alcohol inhibits glutamate activity in the brain. This can be stated from the fact that acute alcohol exposure causes a drop in the extra cellular glutamate levels in a region of the brain called striatum which contains the nucleus accumbens and other structures.[25] Glutamate mediated signal transmission is suppressed in the central nucleus of the amygdala following acute administration and it is an effect which is enhanced following chronic alcohol exposure.[26] The glutamate transmission is most likely affected due to alterations in the functions of both NMDA receptors[27] and another receptor subtype known as metabotropic glutamate subtype 5 receptors.[28] The fact that NMDA receptors are involved in alcoholism is something to take note of as they also play a role in neuroplasticity, a process characterized by neural reorganization that likely contributes to hyper excitability and craving during alcohol withdrawal.[29] Compounds targeting the glutamate systems have also begun to be used for treating AD. As an example, the agent acamprosate modulates glutamate transmission by acting on NMDA and/or metabotropic glutamate receptors.[30] Therefore, by reducing excessive glutamate activity, acamprosate blocks excessive alcohol consumption.
This process appears to depend on the involvement of genes such as Per2, which is typically involved in maintaining the normal daily rhythm (i.e. the circadian clock) of an organism.[31] Acamprosate's capability to reduce alcohol consumption has been seen across different species and the drug has been approved for treatment of alcoholism in humans. This is primarily due to its perceived ability to bring about a reduction in alcohol cravings in abstinent alcoholics.[30]
Genetics of the reward pathways
Alcohol addiction and dependence of late has been shown to be affected by the influence of genes. The presence of such genes does not confirm whether a person will turn into an alcohol addict, but there is a high correlation amongst carriers of such genes and alcohol addiction.
Candidate genes suggested in the development of alcohol addiction are involved in the dopaminergic, serotoninergic, GABA and glutamate pathways.
Dopamine pathway
In the dopaminergic pathway, one such gene is a dopamine receptor D2 (DRD2) which codes for a receptor of dopamine.
Dopamine is an important neurotransmitter involved in reward mechanism in the brain and thereby influences the development and relapse of AD. The dopamine and serotonin pathways are shown as under [Figure 2].
Figure 2.
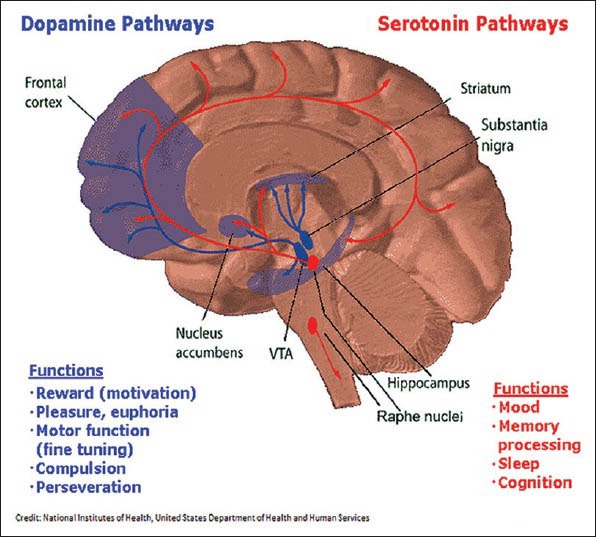
Diagram depicting the dopamine (blue) and serotonin pathways (red) in the brain along with the respective functions of each
It is classified as a catecholamine (a class of molecules that serve as neurotransmitters and hormones). It is a monoamine (a compound containing nitrogen formed from ammonia by replacement of one or more of the hydrogen atoms by hydrocarbon radicals). Dopamine is a precursor (forerunner) of adrenaline and a closely related molecule, noradrenalin.
The DRD2 gene on chromosome 11 (q22-q23) has been found to be associated with increased alcohol consumption through mechanisms involving incentive salience attributions and craving in alcoholic patients.[32] The DRD2 is a G protein-coupled receptor located on postsynaptic dopaminergic neurons that is centrally involved in reward-mediating mesocorticolimbic pathways.[33] The DRD2 gene encodes 2 molecularly distinct isoforms with distinct functions.[34] Signaling through dopamine D2 receptors governs physiologic functions related to locomotion, hormone production and drug abuse.
This DRD2 gene shows polymorphisms of 3 kinds namely: −141c ins/del; Taq1B; Taq1A. The −141c ins/del allele and Taq1A allele have been implicated with higher risks of AD. With regards to the Taq1A allele, AD patients with the DRD2 A (1) allele, are characterized by greater severity of their disorder across a range of problem drinking indices, when compared with patients without this allele.[35] The Taq1A polymorphism has also been implicated in conduct disorder, behavioral phenotype of impulsivity and problematic alcohol/drug use amongst adolescents.[36] Furthermore, this particular allelic variant has been implicated with increased mortality over a 10 year period in AD individuals.[37] The A1 allele of the DRD2 was significantly associated with paternal history of alcoholism (χ2 (1) = 4.66; P = 0.031) and male, first-degree, collateral history of alcoholism (χ2 (1) = 4.40; P = 0.036). Age at the onset of alcohol-related problems as main discriminator between type I and type II AD does not seem to be associated by the Taq1A DRD2 polymorphism. However, the A1 allele of the DRD2 may be a marker of male familial alcoholism, which has been associated with type II AD.[38]
Despite its positive correlation, some studies have produced contradictory results. A study conducted by[39] to assess the association of Taq1A polymorphism and AD in south Indian population yielded negative results.[40,41] also did not find any association with Taq1A polymorphism and AD amongst Mexican-Americans. Amongst other studies which have found a negative correlation between Taq1A polymorphism and alcoholism are ones carried out by.[42,43,44] Study conducted by[43] found conflicting results regarding Taq1A allele frequency amongst assessed and non-assessed controls and assessed and non-assessed alcoholics in a population study comprising of Han Chinese, Caucasians and Europeans. The Taq1A allele frequency of non-assessed controls was more than that of non-assessed alcoholics. However, the allele frequency of assessed alcoholics was found to be 3 times that of assessed controls. The study by[42] found conflicting results for male and female subjects, with female subjects showing AD only on the basis of alcohol disorder.[44] In their study of alcohol-dependence in Polish population reported negative association between Taq1A allele and AD.
The second allele, −141c ins/del has produced much more contradictory results. For example, a study conducted by[45] on Spanish Caucasian AD patients did not find any association with the gene and treatment outcome of AD patients. Even[46] did not find any association with the − 141c ins/del allele and AD Caucasian men. According to them, evidence cannot be provided that in AD Caucasian men a genetic predisposition for alcoholism along with functional variants of the DRD2 and DRD3 genes are associated with differences in dopamine receptor sensitivity. However, a study by[40,41] in a Mexican-American population had found a significant correlation between −141c ins/del polymorphism and AD patients. The genotype frequency for the DRD2 −141C ins/del allele was significantly different between alcoholic and control subjects (P = 0.007). Furthermore, a study conducted by[47] came up with interesting results. According to them, although there were no significant differences in allele frequency between the entire group or subgroups of alcoholics and healthy controls, the − 141c del variant of DRD2 might be a protective factor against development of withdrawal symptoms. However, it might also be a risk factor in a highly burdened subgroup of alcoholics with a paternal and grand paternal history of alcoholism and it might contribute to the substantially higher likelihood of suicide in alcoholics.
Single-nucleotide polymorphism Taq1B is closer to the regulatory and structural coding regions (5’ region) of the DRD2 and thus supposed to play an important role in gene function.[32] It has been rarely investigated for its association with AD. Two studies by[40,41] carried out in Mexican-American population reported conflicting results with regard to the association of this polymorphism with AD. In the study conducted by[32] no allelic or genotypic association of Taq1B polymorphism with AD in North Indians was found, concurring with the findings of[40] which also reported negative association of Taq1B with AD in Mexican-Americans. However, in a subsequent study, the same group reported an association of Taq1B polymorphism with early age of onset for alcohol drinking in Mexican-Americans.[41]
Serotonin pathway
Apart from the dopamine pathways, the addiction to alcohol has also been suggested through the serotonin pathways. Serotonin is another neurotransmitter that is affected by many of the drugs of abuse, including cocaine, amphetamines, LSD and alcohol. Serotonin is produced by neurons in the raphe nuclei. Raphe nuclei neurons extend processes to and dump serotonin onto almost the entire brain, as well as the spinal cord. Serotonin plays a role in many brain processes, including regulation of body temperature, sleep, mood, appetite and pain. Problems with the serotonin pathway can cause obsessive-compulsive disorder, anxiety disorders and depression. Serotonin also modulates the behavioral response to unfairness.[48] Most of the drugs used to treat depression today work by increasing serotonin levels in the brain.[49] The image below, shows, the regions of the brain where serotonin reaches [Figure 3].
Figure 3.
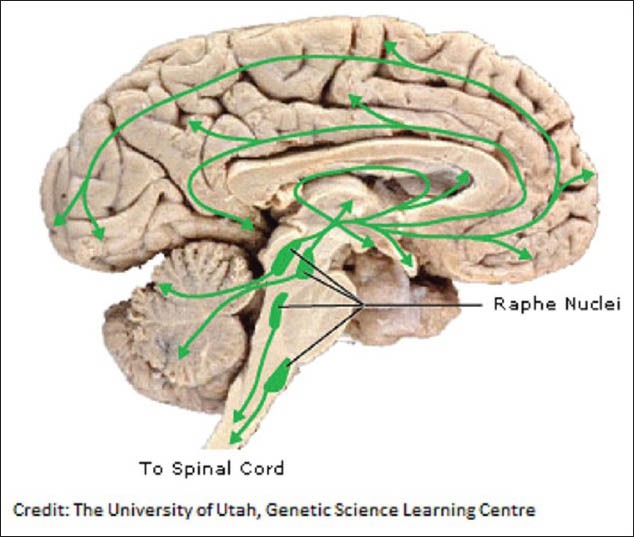
Diagram depicting the various regions of the brain under the influence of serotonin
Chemically, serotonin is a monoamine neurotransmitter, known as 5-HT. It is a derivative of tryptophan and is extensively found in the gastrointestinal tract, platelets and the CNS. Some of the functions of serotonin in the CNS include the regulation of mood, appetite, sleep, as well as muscle contraction. Serotonin also has some cognitive functions, including in memory and learning. Most of the brain serotonin is not degraded after use but is collected by serotonergic neurons by serotonin transporters on their cell surfaces. Studies have revealed nearly 10% of the total variance in anxiety-related personality depends on variations in the description of where, when and how many serotonin transporters the neurons should deploy[50] and the effect of this variation was found to interact with the environment in depression.[51,52] Serotonin is released into the space between neurons and diffuses over a relatively wide gap (>20 μm) to activate 5-HT receptors located on the dendrites, cell bodies and presynaptic terminals of adjacent neurons. Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT, serotonin transporter (SERT), on the presynaptic neuron.
Recently mutations in the SERT gene, commonly known as 5’- hydroxtryptamine transporter linked polymorphic region (5’-HTTLPR), has been implicated in cases of alcoholism. This gene is found on chromosome 17 at 17q11.1-q12. The 5’- HTT gene has primarily two mutations. One mutation is known as the “long” allele and the other mutation is known as the “short” allele. The difference between the two alleles is that the “short” version of the allele has a 44 bp deletion in the 5’ regulatory region of the gene. This 44 bp deletion occurs 1 kb upstream from the transcription initiation site of the gene.[53] This is depicted through the following diagram [Figure 4].
Figure 4.
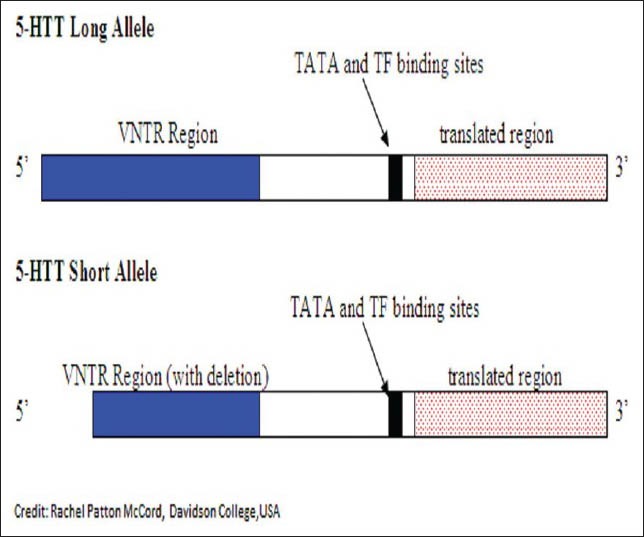
Diagram depicting the difference between the 5’-hydroxtryptamine transporter (5-HTT) long allele and 5-HTT short allele
A study by[54] aimed at looking at the differences in the allele frequency amongst non-alcoholic controls and alcohol-dependent patients in the Yunnan Han population. The study found significant differences in the allele frequency in alcohol-dependent patient and non-alcoholic controls. At (P < 0.05), the proportion of L/L and L/S genotype was significantly smaller in case group than that was in the control group (odds ratio [OR] =0.581, P = 0.026). According to the study, 5’-HTTLPR polymorphism may be associated with AD patients and the genotype L/L or L/S may be a genetic factor that is responsible for decreasing susceptibility of AD in Yunnan Han population.
Another study by[55] aimed to look at the availability of the SERT in patients with AD. In the study, 11 healthy controls and 28 alcoholic patients were recruited. SERT availability was measured in vivo with single photon emission computed tomography and (123) I-labeled 2-((2-((dimethyl-amino) methyl) phenyl) thio)-5-iodophenylamine in the midbrain, thalamus and striatum. In addition to this, each subject was genotyped for the 5’-HTTLPR polymorphism. The study found that when compared with healthy controls, patients with pure AD had a significantly lower availability of SERT in the midbrain. The carriers of one L (long) allele showed a significantly higher availability of SERT in the striatum compared with non-L carriers. The study concludes by stating that pure alcoholics may have lower SERT availability in the midbrain and that the 5’-HTTLPR polymorphism may influence SERT availability in patients with anxiety, depression and AD.
Likewise, in a study on Estonian Children and Adolescents,[56] found a positive correlation between substance abuse amongst the adolescents and the 5’-HTTLPR polymorphism. The study involved 583 children from the Estonian Children Personality Behavior and Health Study who were enrolled at the age of 9 and recalled subsequently at the ages of 15 and 18. According to the study, 5’-HTTLPR had age-dependent effects on alcohol, tobacco and drug use: substance use did not differ by genotype at age 9, but at age 15, the participants with the short (s)/s genotype had higher tobacco use and at age 18, they were more active alcohol, drug and tobacco users.
The findings of the team led by[57] produce similar findings. In their study, 360 treatment-seeking African American male patients with single and co morbid DSM-IV lifetime diagnoses of alcohol, cocaine and heroin dependence and 187 African American male controls were genotyped for the triallelic 5’-HTTLPR functional polymorphism in the 5-HT transporter gene (SLC6A4). The study found that low 5’-HTTLPR activity (P = 0.011, OR = 2.5 [1.3-4.6]) due to the presence of the short allele, were more common in men with alcohol drug dependence compared with controls.
However, the study by[58] produced rather contradictory results. In their study, college students (N = 360; 192 women) self-reported on drinking motives and negative life events for up to 4 years through an Internet survey. Study participants provided saliva for genotyping the triallelic (LA vs. LG or S) variants of 5-HTTLPR. The study found that among men, individuals with two risk alleles (LG or S), compared with individuals with the LA/LA allele displayed lower drinking-to-cope motives. Among women, individuals with one risk allele (either LG or S), compared with individuals with the LA/LA allele, displayed stronger drinking-to-enhance motives. The association between yearly changes in negative life events and drinking-to-cope motives varied across 5-HTTLPR genotype and gender and was strongest in the positive direction for women with the LA/LA variant. The study concludes by stating that their findings are not consistent with prior speculation that stronger positive associations between life stress and alcohol use among individuals with the LG or S allele are the result of increased use of alcohol as a method for coping with stress. The study goes on to add that the more research is needed in understanding the gender differences in relating 5’-HTTLPR polymorphism with substance abuse.
Likewise, in the study carried out by[59] which aimed at understanding the role of 5’-HTTLPR polymorphism with risky alcohol use in adolescence, there was no correlation with drinking to cope motives and the 5’-HTTLPR polymorphism. The study however found a positive correlation with drinking to cope motives and the Taq1A polymorphism of the DRD2 gene.
The results of the aforementioned study was therefore in complete contrast to the results published by[60] which found a positive correlation of the short (S) allele with binge-drinking behavior, drinking more alcohol per occasion, as well as drinking to get drunk more often.
The SERT gene or SERT, also known as SLC6A4 has another polymorphism in intron 2. This polymorphism has therefore appropriately been named as serotonin intron 2 (STin2). It is a variable number of tandem repeats (VNTR) with three distinct alleles. These alleles are of 9 base pair repeats, 10 base pair repeats as well as 12 base pair repeats. The 9 base pair repeat is extremely rare and in statistical studies, often clubbed with the 10 base pair repeat.
Recently, a study by[45] found an association between STin2 polymorphism and treatment outcome in AD patients. According to study, the SLC6A4 STin2 12/12 carriers, showed poor 6-month time point treatment outcome (32.8% in the good outcome group vs. 64.0% in the poor outcome group). On the other hand, patients having the 10/10 genotype had a better treatment outcome. The study concludes by stating that the functional polymorphism of the SLC6A4 gene may have an influence on treatment outcome in AD patients.
However, a subsequent study by[61] found no role of STin2 VNTR polymorphism in AD. In the study, 165 AD patients, 113 heroin dependent patients and 420 healthy controls from a homogeneous Spanish Caucasian population were genotyped using standard methods. The study found that genotypic frequencies of STin2 VNTR polymorphism did not differ significantly across the three groups. The study concludes by stating that their data does not support a role of serotonergic polymorphisms in AD.
GABA pathway
GABA or GABA is the third neurotransmitter whose functioning is critical in understanding the genetics of alcohol addiction. GABA as a neurotransmitter has been long known to be affected by alcohol consumption. Recently, two sub types of the GABAA receptor have come into the spotlight for showing what can possibly be a genetic predisposition to alcohol addiction. These two subtypes are namely GABA A receptor α1 (GABRA1) and GABA A receptor α6 (GABRA6). The gene encoding GABRA1 is located on chromosome 5 at 5q34-35 while the gene encoding GABRA6 is located on the same chromosome at 5q34. According to a study by,[62] a significant correlation was found with the GABRA1 genotype and Collaborative Study of the Genetics of Alcoholism (COGA) AD, history of blackouts, age at first drunkenness as well as the level of response to alcohol. The study concludes by stating that the efforts to characterize genetic contributions to AD may benefit by examining alcohol-related behaviors in addition to clinical AD.
Furthermore, a study on Korean population by[63] found a positive association between alcoholism and the GABRA1 and GABRA6 receptors. According to the researchers, genetic polymorphisms of the GABAA α1 and GABAA α6 receptor gene may be associated with the development of alcoholism and that the GG genotype of the GABAA α1 receptor gene play a vital role in the development of the early onset and the severe type of alcoholism.
Another study on Taiwanese Han population found similar results. In the study conducted by[64] it was found that GABRA6 and GABRA1 genes account for alcohol susceptibility in Han and exert their genetic influences in a somewhat dominant and synergistic fashion.
However, not all studies have produced favorable results. In a study conducted by,[65] which looked at the data collected from a large number of multiplex, alcoholic families under the COGA, no association was found between the GABRA1 and GABRA6 markers and AD. Similarly, another study conducted by[66] found no association between the genes encoding GABRA1 and GABRA6 with alcoholism.
Glutamate pathway
The fourth pathway which interests us and is of note for alcohol addiction is the pathway of glutamate. There have been some studies conducted into the involvement of this pathway in the process of alcohol addiction. According to one study published by[67] physical dependence, which refers to the pharmacological tolerance induced by chronic alcohol intake, results in AWS and is neurobiologically supported by the imbalance between GABA and glutamate-NMDA neurotransmission.
In addition, one of the latest studies on this pathway found an association between a polymorphism in the promoter of a glutamate receptor subunit gene and alcoholism. The study was conducted by[68] and the study found that short alleles were significantly less frequent among AD subjects. The study concludes by stating that it was the 1st time that such an association was found with the stated polymorphism and AD.
Conclusion
The field of neurotransmitters is a highly active field of research nowadays. Different alleles of the genes in the various pathways are being studied in different population groups across the world. However, what remains to be seen is a definitive consensus on a causative allele of alcoholism. There are conflicting reports in this regard with different population groups having different alleles as risk factors. Moreover, new alleles are also being discovered wherein an association exists between the stated allele and alcoholism. As a reviewer, I would suggest one possible way to overcome much of the conflicting reports would be to perform studies with a much larger sample size. Such efforts are hampered by inadequate funding, so collaborative efforts on a national scale, combining the skills and infrastructures of different hospitals and psychiatric care centers could potentially overcome this problem.
A broad consensus does exist as to the involvement of various neurotransmitter pathways, but defining the precise causative alleles or groups of alleles in the genes of the particular neurotransmitter pathways involved in alcoholism is a challenge to be overcome in the coming years.
Limitation of the Review
The review of the papers presented does carry some inherent biases which I shall henceforth disclose:
Publication bias: Papers were selected by a rigorous search of PubMed. Grey literature and papers unavailable in an electronic format were not searched
Selection bias: The pathways of dopamine, serotonin, GABA and glutamate; the specific alleles of the genes involved in the pathways were selected due to prior work performed in my graduate dissertation at a premier hospital in India
Language bias: Only English sources were searched. Non-English sources were not searched.
Acknowledgments
I would like to acknowledge my faculty at Amity Institute of Biotechnology, Dr. Manju Pathak for her unwavering support and encouragement in writing this review paper. She single-handedly inspired me to undertake this task and the work would not have borne fruition without her support and guidance. Thanks are also due to my mother, Dr. Sharmila Banerjee, without whose support and editorial help, I could not have had the will to complete this work. Furthermore, I would like to state that no financial aid in any form was received for undertaking this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Walter H, Gutierrez K, Ramskogler K, Hertling I, Dvorak A, Lesch OM. Gender-specific differences in alcoholism: Implications for treatment. Arch Womens Ment Health. 2003;6:253–8. doi: 10.1007/s00737-003-0014-8. [DOI] [PubMed] [Google Scholar]
- 2.Blum LN, Nielsen NH, Riggs JA. Alcoholism and alcohol abuse among women: Report of the Council on Scientific Affairs. American Medical Association. J Womens Health. 1998;7:861–71. doi: 10.1089/jwh.1998.7.861. [DOI] [PubMed] [Google Scholar]
- 3.Gilpin NW, Koob GF. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res Health. 2008;31:185–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob GF. Alcoholism: Allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 6.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 7.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–91. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–8. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 9.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–8. [PubMed] [Google Scholar]
- 10.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virkkunen M, Linnoila M. Serotonin in early onset, male alcoholics with violent behaviour. Ann Med. 1990;22:327–31. doi: 10.3109/07853899009147915. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–85. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–25. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–9. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- 17.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–8. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–66. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, et al. The GABA (A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–75. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, et al. The reinforcing properties of alcohol are mediated by GABA (A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–37. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- 21.Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–71. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA (A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 23.VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–9. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–30. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carboni S, Isola R, Gessa GL, Rossetti ZL. Ethanol prevents the glutamate release induced by N-methyl-D-aspartate in the rat striatum. Neurosci Lett. 1993;152:133–6. doi: 10.1016/0304-3940(93)90501-b. [DOI] [PubMed] [Google Scholar]
- 26.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: An in vitro and in vivo analysis. J Neurosci. 2004;24:1594–603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 28.Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: Relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–93. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulvirenti L, Diana M. Drug dependence as a disorder of neural plasticity: Focus on dopamine and glutamate. Rev Neurosci. 2001;12:141–58. doi: 10.1515/revneuro.2001.12.2.141. [DOI] [PubMed] [Google Scholar]
- 30.Littleton JM. Acamprosate in alcohol dependence: Implications of a unique mechanism of action. J Addict Med. 2007;1:115–25. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 31.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 32.Prasad P, Ambekar A, Vaswani M. Dopamine D2 receptor polymorphisms and susceptibility to alcohol dependence in Indian males: A preliminary study. BMC Med Genet. 2010;11:24. doi: 10.1186/1471-2350-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 34.Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 35.Connor JP, Young RM, Lawford BR, Ritchie TL, Noble EP. D (2) dopamine receptor (DRD2) polymorphism is associated with severity of alcohol dependence. Eur Psychiatry. 2002;17:17–23. doi: 10.1016/s0924-9338(02)00625-9. [DOI] [PubMed] [Google Scholar]
- 36.Esposito-Smythers C, Spirito A, Rizzo C, McGeary JE, Knopik VS. Associations of the DRD2 TaqIA polymorphism with impulsivity and substance use: Preliminary results from a clinical sample of adolescents. Pharmacol Biochem Behav. 2009;93:306–12. doi: 10.1016/j.pbb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berggren U, Fahlke C, Berglund KJ, Wadell K, Zetterberg H, Blennow K, et al. Dopamine D2 receptor genotype is associated with increased mortality at a 10-year follow-up of alcohol-dependent individuals. Alcohol Alcohol. 2010;45:1–5. doi: 10.1093/alcalc/agp041. [DOI] [PubMed] [Google Scholar]
- 38.Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, et al. The TaqI A DRD2 polymorphism in type II alcohol dependence: A marker of age at onset or of a familial disease? Alcohol. 2009;43:271–5. doi: 10.1016/j.alcohol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Shaikh KJ, Naveen D, Sherrin T, Murthy A, Thennarasu K, Anand A, et al. Polymorphisms at the DRD2 locus in early-onset alcohol dependence in the Indian population. Addict Biol. 2001;6:331–5. doi: 10.1080/13556210020077055. [DOI] [PubMed] [Google Scholar]
- 40.Konishi T, Calvillo M, Leng AS, Lin KM, Wan YJ. Polymorphisms of the dopamine D2 receptor, serotonin transporter, and GABA (A) receptor beta (3) subunit genes and alcoholism in Mexican-Americans. Alcohol. 2004;32:45–52. doi: 10.1016/j.alcohol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Konishi T, Luo HR, Calvillo M, Mayo MS, Lin KM, Wan YJ. ADH1B*1, ADH1C*2, DRD2 (-141C Ins), and 5-HTTLPR are associated with alcoholism in Mexican American men living in Los Angeles. Alcohol Clin Exp Res. 2004;28:1145–52. doi: 10.1097/01.alc.0000134231.48395.42. [DOI] [PubMed] [Google Scholar]
- 42.Lucht M, Barnow S, Schroeder W, Grabe HJ, Rosskopf D, Brummer C, et al. Alcohol consumption is associated with an interaction between DRD2 exon 8 A/A genotype and self-directedness in males. Neuropsychobiology. 2007;56:24–31. doi: 10.1159/000109974. [DOI] [PubMed] [Google Scholar]
- 43.Noble EP. The D2 dopamine receptor gene: A review of association studies in alcoholism and phenotypes. Alcohol. 1998;16:33–45. doi: 10.1016/s0741-8329(97)00175-4. [DOI] [PubMed] [Google Scholar]
- 44.Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jabłoński M, Rommelspacher H, Samochowiec A, et al. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neurosci Lett. 2006;410:1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Florez G, Saiz P, Garcia-Portilla P, Alvarez S, Nogueíras L, Morales B, et al. Association between the Stin2 VNTR polymorphism of the serotonin transporter gene and treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2008;43:516–22. doi: 10.1093/alcalc/agn048. [DOI] [PubMed] [Google Scholar]
- 46.Wiesbeck GA, Dürsteler-MacFarland KM, Wurst FM, Walter M, Petitjean S, Müller S, et al. No association of dopamine receptor sensitivity in vivo with genetic predisposition for alcoholism and DRD2/DRD3 gene polymorphisms in alcohol dependence. Addict Biol. 2006;11:72–5. doi: 10.1111/j.1369-1600.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 47.Johann M, Putzhammer A, Eichhammer P, Wodarz N. Association of the -141C Del variant of the dopamine D2 receptor (DRD2) with positive family history and suicidality in German alcoholics. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:46–9. doi: 10.1002/ajmg.b.30085. [DOI] [PubMed] [Google Scholar]
- 48.Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 51.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 52.Levinson DF. The genetics of depression: A review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang XJ, Zhong SR, Bao JJ, Dou SJ, Wu WY, Jing Q. Association of polymorphism in the serotonin transporter gene promote with the susceptibility to alcohol dependence in Yunnan Han Population. Yi Chuan. 2011;33:48–53. doi: 10.3724/sp.j.1005.2011.00048. [DOI] [PubMed] [Google Scholar]
- 55.Ho PS, Shih MC, Ma KH, Huang WS, Ho KK, Yen CH, et al. Availability of the serotonin transporter in patients with alcohol dependence. World J Biol Psychiatry. 2011;12:134–42. doi: 10.3109/15622975.2010.503813. [DOI] [PubMed] [Google Scholar]
- 56.Merenäkk L, Mäestu J, Nordquist N, Parik J, Oreland L, Loit HM, et al. Effects of the serotonin transporter (5-HTTLPR) and α2A-adrenoceptor (C-1291G) genotypes on substance use in children and adolescents: A longitudinal study. Psychopharmacology (Berl) 2011;215:13–22. doi: 10.1007/s00213-010-2109-z. [DOI] [PubMed] [Google Scholar]
- 57.Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry. 2011;16:1139–46. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armeli S, Conner TS, Covault J, Tennen H, Kranzler HR. A serotonin transporter gene polymorphism (5-HTTLPR), drinking-to-cope motivation, and negative life events among college students. J Stud Alcohol Drugs. 2008;69:814–23. doi: 10.15288/jsad.2008.69.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Zwaluw CS, Kuntsche E, Engels RC. Risky alcohol use in adolescence: The role of genetics (DRD2, SLC6A4) and coping motives. Alcohol Clin Exp Res. 2011;35:756–64. doi: 10.1111/j.1530-0277.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 60.Herman AI, Philbeck JW, Vasilopoulos NL, Depetrillo PB. Serotonin transporter promoter polymorphism and differences in alcohol consumption behaviour in a college student population. Alcohol Alcohol. 2003;38:446–9. doi: 10.1093/alcalc/agg110. [DOI] [PubMed] [Google Scholar]
- 61.Saiz PA, Garcia-Portilla MP, Florez G, Arango C, Corcoran P, Morales B, et al. Differential role of serotonergic polymorphisms in alcohol and heroin dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:695–700. doi: 10.1016/j.pnpbp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Dick DM, Plunkett J, Wetherill LF, Xuei X, Goate A, Hesselbrock V, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006;30:1101–10. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 63.Park CS, Park SY, Lee CS, Sohn JW, Hahn GH, Kim BJ. Association between alcoholism and the genetic polymorphisms of the GABAA receptor genes on chromosome 5q33-34 in Korean population. J Korean Med Sci. 2006;21:533–8. doi: 10.3346/jkms.2006.21.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang YT, Sun HS, Fann CS, Chang CJ, Liao ZH, Huang JL, et al. Association of the gamma-aminobutyric acid A receptor gene cluster with alcohol dependence in Taiwanese Han. Mol Psychiatry. 2002;7:828–9. doi: 10.1038/sj.mp.4001110. [DOI] [PubMed] [Google Scholar]
- 65.Dick DM, Edenberg HJ, Xuei X, Goate A, Hesselbrock V, Schuckit M, et al. No association of the GABAA receptor genes on chromosome 5 with alcoholism in the collaborative study on the genetics of alcoholism sample. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:24–8. doi: 10.1002/ajmg.b.30058. [DOI] [PubMed] [Google Scholar]
- 66.Song J, Koller DL, Foroud T, Carr K, Zhao J, Rice J, et al. Association of GABA (A) receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:39–45. doi: 10.1002/ajmg.b.10022. [DOI] [PubMed] [Google Scholar]
- 67.Rolland B, Karila L, Guardia D, Cottencin O. Pharmaceutical approaches of binge drinking. Curr Pharm Des. 2011;17:1333–42. doi: 10.2174/138161211796150792. [DOI] [PubMed] [Google Scholar]
- 68.Domart MC, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, et al. Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism. Addict Biol. 2012;17:783–5. doi: 10.1111/j.1369-1600.2011.00321.x. [DOI] [PubMed] [Google Scholar]


