Abstract
BACKGROUND:
BCR-ABL fusion oncogene is a hallmark of Chronic Myeloid Leukemia (CML). It results due to translocation between chromosome 22 and chromosome 9 [t (9; 22)(q34; q11)]. It gives rise to translation of a 210 KDa chimeric protein (p210), leading to enhanced tyrosine kinase activity and activation of leukemogenic pathways, ultimately causing onset of CML. In case of CML, the classic fusions are b2a2 or b3a2, fusing exon 13 (b2) or exon 14 (b3) of BCR, respectively, to exon 2 (a2) of ABL. The type of BCR-ABL transcripts are thought to be have different prognosis and hence useful in clinical decision-making. The frequencies of different fusion oncogenes associated with leukemia can vary in different ethnic groups and geographical regions due to interplay of genetic variation in different ethnic populations, diverse environmental factors and living style. Moreover, earlier relevant studies from our region were carried out in small subset of patients. Therefore, objective of this study was to find out frequencies of different BCR-ABL splice variants in larger subset of CML patients.
METHODS:
A nested reverse transcriptase polymerase chain reaction (RT-PCR) was established to detect BCR-ABL splice variants in 130 CML patients. Sensitivity of RT-PCR and ability to detect BCR-ABL fusion gene in least possible time was studied.
RESULTS:
BCR-ABL detection using our optimized RT-PCR protocol could be completed in 8 hours, starting from RNA extraction to Gel electrophoresis. Sensitivity of RT-PCR assay was of the order of 10−6. Out of 130 Pakistani patients, 83 (63.84%) expressed b3a2 while 47 (36.15%) expressed b2a2 transcript.
CONCLUSION:
Our RT-PCR was proved to be very quick to detect BCR-ABL fusion oncogene in CML patients within one working day. Because of its sensitivity, it can be used to monitor complete molecular response in CML. BCR-ABL RT-PCR and BCR-ABL splice variants frequency in our study differs from other ethnic groups. It shows that ethnic and geographical differences exist in BCR-ABL splice variant frequency, which may have a profound effect on disease biology as well as implications in prognosis and clinical management of BCR-ABL positive leukemias.
Keywords: b2a2, b3a2, breakpoint cluster region-abelson, breakpoint cluster region-abelson alternative splicing
Introduction
Cytogenetically chronic myeloid leukemia (CML) is characterized by the presence of Philadelphia (Ph) chromosome, the diagnostic hallmark of CML, which is present in the majority of CML patients. It originates from the reciprocal translocation of chromosome 9 and 22 t (9;22)(q34;q11).[1] This reciprocal translocation gives rise to breakpoint cluster region-abelson (BCR-ABL) fusion oncogene, which translates a chimeric protein, p210BCR-ABL that is characterized by constitutive activation of its tyrosine kinase activity. In CML, the classic fusion is b2a2 or b3a2 fusing exon 13 (b2) or exon 14 (b3) of BCR to exon 2 (a2) of ABL.[2,3] Both b3a2 and b2a2 transcripts can be formed as a result of alternative splicing. These transcripts lead to the production of an 8.5 kb transcript coding for a 210-kDa (p210) chimeric protein.[4,5] This constitutively active cytoplasmic tyrosine kinase does not block differentiation, but enhances proliferation and viability of myeloid lineage cells and leads to the development of CML.[6]
Genetic abnormalities lead to the formation of fusion oncogenes and this phenomenon is driven by the environmental factors and living style which differ in different geographical regions, the frequencies of different fusion oncogenes associated with leukemia can vary in different ethnic groups,[7] which can have a significant effect on the management and prognosis of this type of leukemia.[8,9] This study was conducted to calculate the frequency of BCR-ABL gene splice variants associated with CML. Reverse transcriptase polymerase chain reaction (RT-PCR) was chosen as the method of choice to detect BCR-ABL gene as this technique is one of the most sensitive methods for this purpose.[10]
Materials and Methods
Sample collection
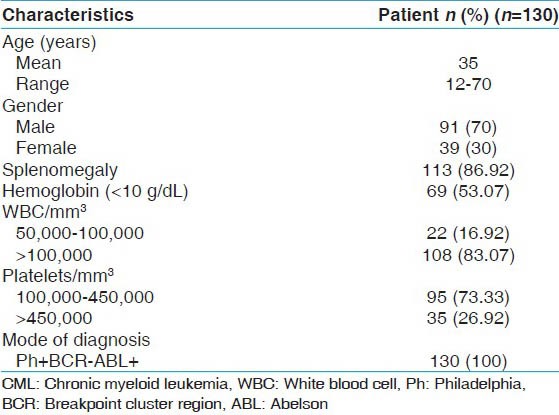
Blood samples of 130 CML patients were collected from different hospitals of Lahore. Clinical features of the patients are given in Table 1. All the samples were stored at 20°C. The procedure for isolation of ribonucleic acid (RNA) was adapted from Chomczynski and Sacchi with slight modification.[11,12]
Table 1.
Clinical features and laboratory findings of CML patients (n=130)
RNA extraction and complementary deoxyribonucleic acid (cDNA) synthesis
Quantity of RNA was estimated spectrophotometrically, Glasel,[13] whereas the quality of RNA was checked by native agarose gel electrophoresis and formaldehyde denaturing gel electrophoresis, Sambrook and Russel.[14] RNA was reverse-transcribed to cDNA for using as template in PCR reaction. RT-reaction protocol and other reaction conditions were adapted from.[15] Briefly, 10 μL of RNA was added to 10 μL of RT-reaction mixture containing 5X RT buffer (20 mM Tris HCl, 50 mM KCl, pH: 8.3, 10 mM DTT), 10 mM deoxyribonucleotide triphosphate (dNTPs), 10 mM random hexamer primers, RiboLock™ RNase inhibitor (20 units), M-MuLV RT (40 units) (Fermentas, USA) and DPCE- treated water. Reaction was carried out by incubating at room temperature for 10 min, 37°C for 60 min, 99°C for 3 min and held at 4°C in the last step in the PCR machine.
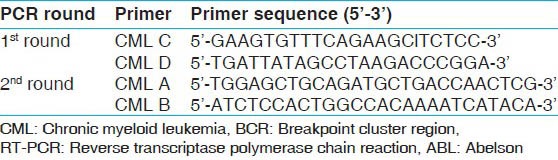
RT-PCR amplifications
PCR primers and nested PCR protocols for the detection of BCR-ABL fusion gene in CML patients were adopted from.[16] The sequences of primers are given in Table 2. The cDNA product was PCR amplified in two rounds with 1 U/μl of Taq DNA polymerase, 125 mM primers, 10X PCR buffer with KCl, 25 mM MgCl2 and 10 mM dNTP mix. Sterile water was used as a negative control. Thermal cycling conditions for nested PCR were: Preliminary denaturation at 95°C for 2 min followed by 35 cycles of denaturation of double stranded DNA at 95°C for 5 s, annealing at 55°C for 10 s and extension at 72°C for 15 s, followed by a final extension at 72°C for 3 min. Round 2 was carried out with the same conditions. The PCR products were run on a 2% agarose gel with ethidium bromide to analyze the size of the amplicons.
Table 2.
Primers used for detection of fusion gene BCR-ABL in CML by RT-PCR
Results
Primer combinations used in amplification allowed amplifying both types of transcripts in a single reaction. Quality of RNA was analyzed on formaldehyde gel electrophoresis [Figure 1] and efficiency of cDNA synthesis on agarose gel electrophoresis. Two types of PCR products were detected as 305 bp and 234 bp for b3a2 and b2a2 respectively [Figure 2]. The frequencies of both fusion transcripts were found to be 63.33% and 36.66% for b3a2 and b2a2, respectively. The sensitivity assay was also performed by generating 10-fold serial dilutions to evaluate the sensitivity of RT-PCR for detection of these fusion transcripts. Using these dilutions, detection limit of the nested PCR was found up to 10 − 6 of cDNA for each sample [Figure 3].
Figure 1.
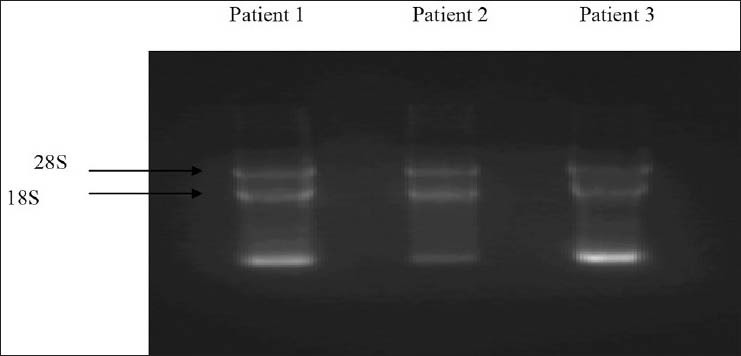
Formaldehyde gel showing bands of ribonucleic acid from three different samples of chronic myeloid leukemia patients
Figure 2.
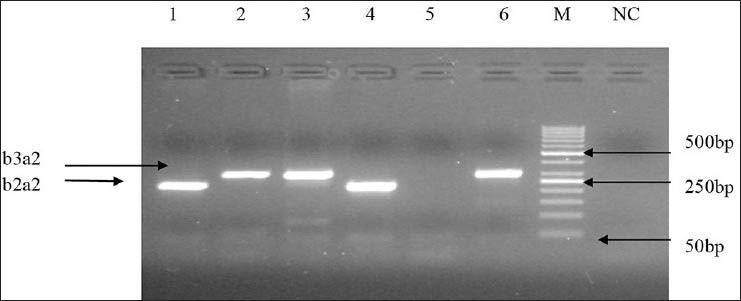
Agarose gel showing two types of characteristics breakpoint cluster region-abelson transcripts b2a2 (lanes 1, 4) and b3a2 (lanes 2, 3, 6), 234 bp and 305 bp respectively (M: 50 bp deoxyribonucleic acid ladder, NC: Negative control)
Figure 3.
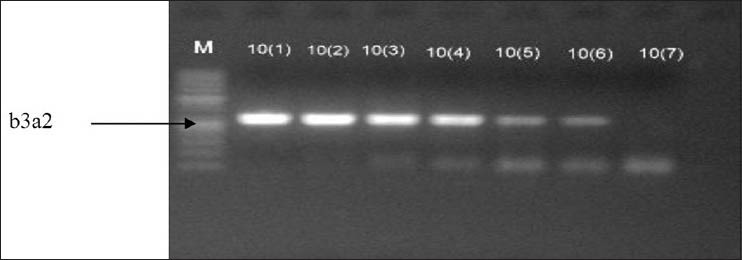
Amplification of breakpoint cluster region-abelson (BCR-ABL) fusion transcript b3a2 (305 bp) from serial dilutions of complementary deoxyribonucleic acid (cDNA) (10−0–10−7) showing sensitivity of nested polymerase chain reaction for the detection of BCR-ABL in chronic myeloid leukemia (M; 50 bp DNA ladder)
Discussion
RT-PCR is one of the most sensitive techniques to detect BCR-ABL transcripts associated with CML.[17] Real time quantitative PCR is increasingly used to assess treatment response in patients with CML. When the level of residual leukemia falls below the level of detection by bone marrow cytogenetic analysis, PCR based assays like the real time quantitative PCR or nested RT-PCR are the methods of choice for further monitoring.[18] In this study, nested PCR was opted as the method of choice due to its sensitivity to detect both b3a2 and b2a2 fusion transcripts in a single reaction. In the sensitivity assay of nested PCR, very high-quality results were obtained and this technique showed sensitivity up to 10−6.
In this study, the frequency of b3a2 and b2a2 was found to be 63.84% and 36.15% respectively. The frequency of b3a2 is approximately 2 times higher than b2a2 with the ratio of 2:1 in our population. The co-expression of transcripts was not found in any of the patients. Spleen was grossly enlarged in most of the patients and mildly enlarged in some but without any correlation to transcript type. Data has been published about the frequency of different types of fusion oncogenes associated with acute lymphocytic leukemia (ALL) in Pakistan.[19] the frequency of BCR-ABL fusion oncogene in Pakistani childhood ALL patients was reported to be 49%, which is higher when compared with other reports from around the world.[19] Frequency of different fusion oncogenes in Pakistani ALL patients is different from other geographic regions, Iqbal.[9] The frequency of fusion oncogenes associated with different leukemia types was compared with western populations and statistically significant differences were observed due to geographical, racial and ethnic differences.[7] In a study carried out by Verschraegen et al.[20] The frequencies of b2a2 and b3a2 transcripts were 30.2% and 67.9%, respectively. Reiter found the incidence of b2a2 and b3a2 transcripts in CML patients to be 31.6% and 68.4% respectively.[21] In Korean CML patients, frequency of b2a2 and b3a2 was collectively found to be 98.18%, corresponding to 67.66% b3a2 and 32.34% b2a2 transcripts,[22] where the number of patients with b3a2 was twice the number of patients with b2a2. Frequencies of fusion transcripts in Iranian CML patients were found to be 21% and 62% for b2a2 and b3a2, respectively. The frequency of b3a2 transcripts was found to be almost 3 times higher than that of b2a2.[23]
Several authors have reported that CML patients with b2a2 BCR-ABL splice variants have a better prognosis and response to imatinib. de Lemos et al.,[24] reported that statistically significant difference was found for the levels of expression of transcripts b2a2 and b3a2 at 6 months of imatinib treatment, which shows that b2a2, may have a better molecular response than b3a2.[25] reported that rare variants like e1a2 have an inferior response to imatinib[26] found that 59% patients with b2a2 type achieved complete cytogenetic response (CGR) when compared with 28% patients with b3a2 (P = 0.04) while in 24 patients with minor or no CGR, 25% had b2a2 compared with 75% b3a2 type (P = 0.04). Moreover, expression of BCR-ABL/ABL% was higher in b3a2 patients compared with b2a2 (P = 0.120). They also found that pre-treatment characteristics such as mean spleen size, mean hemoglobin and mean platelets counts were not significantly different in the b3a2 versus b2a2 transcripts groups,[26] which supports our findings. These observations highlight the need for more extensive studies in different ethnic groups on the role of different BCR-ABL splice variants in biology, Iqbal[27] and treatment response of the BCR-ABL positive leukemia patients, as acquired BCR-ABL point mutations and other factors associated with imatinib resistance do not explain the reason of imatinib resistance in all BCR-ABL positive patients.[28,29,30] Studies related to the differences in clinical features and response to treatment in CML and Ph positive acute leukemia patients are specially required with the advent and Food and Drug Administration approval of other tyrosine kinase inhibitors such as nilotinib and dasatinib for front-line treatment of BCR-ABL positive CML and ALL.
Conclusion
Frequencies of BCR-ABL splice variants can vary in different geographical regions due to the interplay of natural genetic variations in different ethnic populations, diverse environmental factors and living style. Moreover, the knowledge about the rate of occurrence of these transcripts associated with CML can be of very significance as it can lend a hand to further understand the pathobiology of t (9;22)-positive leukemic cells. Moreover, it will also assist in prognosis and management strategies of these CML transcripts types.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Quintás-Cardama A, Cortes JE. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin Proc. 2006;81:973–88. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the BCR/ABL hybrid gene. Science. 1986;233:212–4. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 3.Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of ABL and BCR genes in chronic myelogenous leukaemia. Nature. 1985;315:550–4. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 4.Arana-Trejo RM, Ruíz Sánchez E, Ignacio-Ibarra G, Báez de la Fuente E, Garces O, Gómez Morales E, et al. BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican patients with chronic myeloid leukaemia (CML) Clin Lab Haematol. 2002;24:145–50. doi: 10.1046/j.1365-2257.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichty BD, Keating A, Callum J, Yee K, Croxford R, Corpus G, et al. Expression of p210 and p190 BCR-ABL due to alternative splicing in chronic myelogenous leukaemia. Br J Haematol. 1998;103:711–5. doi: 10.1046/j.1365-2141.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 6.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal Z, Tanveer T, Iqbal M, Ferhan M, Baig SM, Naqvi MI, et al. Colorado: Proceedings 100th Annual meeting, American Association of Cancer Research; 2009. First comprehensive report of strong interplay of genetic and environmental factors as well as high degree of ethnic and geographical variations in biology of leukemia as manifested by frequencies of common fusion oncogenes of prognostic significance associated with different leukemic subtypes in Pakistani population. [Google Scholar]
- 8.Ariffin H, Chen SP, Kwok CS, Quah TC, Lin HP, Yeoh AE. Ethnic differences in the frequency of subtypes of childhood acute lymphoblastic leukemia: Results of the Malaysia-Singapore Leukemia Study Group. J Pediatr Hematol Oncol. 2007;29:27–31. doi: 10.1097/MPH.0b013e318030ac4c. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal Z. Frequency of chromosomal abnormalities and corrresponding fusion oncogenes in acute lympoblastic leukemia (ALL) patients of Pakistan and its implication in differential diagnosis and prognosis of leukaemia. Haematologica. 2006;91:65. [Google Scholar]
- 10.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532. [PubMed] [Google Scholar]
- 13.Glasel JA. Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques. 1995;18:62–3. [PubMed] [Google Scholar]
- 14.Sambrook JF, Russel DW. New York: Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning. A Laboratory Manual. [Google Scholar]
- 15.van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: Investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–28. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 16.Radich JP, Gehly G, Gooley T, Bryant E, Clift RA, Collins S, et al. Polymerase chain reaction detection of the BCR-ABL fusion transcript after allogeneic marrow transplantation for chronic myeloid leukemia: Results and implications in 346 patients. Blood. 1995;85:2632–8. [PubMed] [Google Scholar]
- 17.Guo JQ, Lin H, Kantarjian H, Talpaz M, Champlin R, Andreeff M, et al. Comparison of competitive-nested PCR and real-time PCR in detecting BCR-ABL fusion transcripts in chronic myeloid leukemia patients. Leukemia. 2002;16:2447–53. doi: 10.1038/sj.leu.2402730. [DOI] [PubMed] [Google Scholar]
- 18.Menif S, Zarrouki S, Jeddi R, ben Alaya N, Ali ZB, Ben Abid H, et al. Quantitative detection of BCR-ABL transcripts in chronic myeloid leukemia. Pathol Biol (Paris) 2009;57:388–91. doi: 10.1016/j.patbio.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal Z, Tanveer A. High incidence of BCR-ABL fusion oncogene in Pakistani childhood acute lymphoid leukaemia (ALL) patients reflects ethnic differences in molecular genetics of ALL. Haematologica. 2006;91:65. doi: 10.1097/MPH.0b013e3180f61bcf. [DOI] [PubMed] [Google Scholar]
- 20.Verschraegen CF, Kantarjian HM, Hirsch-Ginsberg C, Lee MS, O’Brien S, Rios MB, et al. The breakpoint cluster region site in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Clinical, laboratory, and prognostic correlations. Cancer. 1995;76:992–7. doi: 10.1002/1097-0142(19950915)76:6<992::aid-cncr2820760612>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Reiter E, Greinix HT, Brugger S, Keil F, Rabitsch W, Mannhalter C, et al. Long term follow up after allogeneic stem cell transplantation for chronic myelogenous leukemia. Bone Marrow Transplant. 1998;22(Suppl 4):S86–8. [PubMed] [Google Scholar]
- 22.Goh HG, Hwang JY, Kim SH, Lee YH, Kim YL, Kim DW. Comprehensive analysis of BCR-ABL transcript types in Korean CML patients using a newly developed multiplex RT-PCR. Transl Res. 2006;148:249–56. doi: 10.1016/j.trsl.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Yaghmaie M, Ghaffari SH, Ghavamzadeh A, Alimoghaddam K, Jahani M, Mousavi SA, et al. Frequency of BCR-ABL fusion transcripts in Iranian patients with chronic myeloid leukemia. Arch Iran Med. 2008;11:247–51. [PubMed] [Google Scholar]
- 24.de Lemos JA, de Oliveira CM, Scerni AC, Bentes AQ, Beltrão AC, Bentes IR, et al. Differential molecular response of the transcripts B2A2 and B3A2 to imatinib mesylate in chronic myeloid leukemia. Genet Mol Res. 2005;4:803–11. [PubMed] [Google Scholar]
- 25.Verma D, Kantarjian HM, Jones D, Luthra R, Borthakur G, Verstovsek S, et al. Chronic myeloid leukemia (CML) with P190 BCR-ABL: Analysis of characteristics, outcomes, and prognostic significance. Blood. 2009;114:2232–5. doi: 10.1182/blood-2009-02-204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Kumar L, Mohanty S, Kochupillai V. Response to Imatinib mesylate in chronic myeloid leukemia patients with variant BCR-ABL fusion transcripts. Ann Hematol. 2010;89:241–7. doi: 10.1007/s00277-009-0822-7. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal Z, Iqbal M, Akhter T. Frequency of BCR-ABL fusion oncogene in Pakistani childhood acute lymphoid leukemia (ALL) patients reflects ethnic differences in molecular genetics of ALL. J Pediatr Hematol Oncol. 2007;29:585. doi: 10.1097/MPH.0b013e3180f61bcf. [DOI] [PubMed] [Google Scholar]
- 28.Gruber FX, Lundán T, Goll R, Silye A, Mikkola I, Rekvig OP, et al. BCR-ABL isoforms associated with intrinsic or acquired resistance to imatinib: More heterogeneous than just ABL kinase domain point mutations? Med Oncol. 2012;29:219–26. doi: 10.1007/s12032-010-9781-z. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal Z, Aleem A, Iqbal M, Naqvi MI, Gill A, Taj AS, et al. Sensitive detection of pre-existing BCR-ABL kinase domain mutations in CD34+ cells of newly diagnosed chronic-phase chronic myeloid leukemia patients is associated with imatinib resistance: implications in the post-imatinib era. PLoS One. 2013;8:e55717. doi: 10.1371/journal.pone.0055717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ, et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94:1362–7. doi: 10.3324/haematol.2009.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]


