Summary
Memory impairment is the most commonly reported cognitive symptom associated with major depressive disorder. Decreased hippocampal volume and neurogenesis in depression link hippocampal dysfunction with deficits in memory. Stress decreases hippocampal dendritic spine density and long-term potentiation (LTP) at glutamate synapses, a cellular correlate of learning and memory. However, elevated plasma levels of 17β estradiol (E2) during proestrus increase hippocampal structure and function, directly opposing the negative consequences of stress. In women, significant fluctuations in ovarian hormones likely increase vulnerability of hippocampal circuits to stress, potentially contributing to the greater incidence of depression compared to men. Using the learned helplessness model of depression and ovariectomized female rats, we investigated whether acquisition of helplessness and hippocampal synaptic dysfunction is differentially impacted by the presence or absence of plasma E2. We find that inescapable shock induces a greater incidence of helplessness in vehicle- versus E2-treated OVX rats. In the vehicle-treated group, LTP was absent at CA3-CA1 synapses in slices only from helpless rats, and CA1 spine density was decreased compared to resilient rats. In contrast, significant LTP was observed in slices from E2-treated helpless rats; importantly, spine density was not different between E2-treated helpless and resilient rats, dissociating spine density from the LTP magnitude. We also find that E2 replacement can reverse previously established helpless behavior. Thus, our results show that E2 replacement in OVX rats increases resilience and improves hippocampal plasticity, suggesting that E2 therapy may increase resilience to stress and preserve hippocampal function in women experiencing large fluctuations in plasma estrogen levels.
Keywords: Stress, Learned helplessness, Estrogen, LTP, Hippocampus, Dendritic spines
1. Introduction
Major depressive disorder (MDD) is a complex psychiatric disorder where biological factors and adverse life events contribute to development of the illness. Women are twice as likely as men to develop depression (Kessler, 2003), and cyclical changes in ovarian estrogen (17β estradiol, E2) in women are believed to be a contributing factor (Shors and Leuner, 2003; Cohen et al., 2005). In fact, women have an increased risk of experiencing a depressive episode when E2 levels are low or fluctuating such as during puberty, post-partum, peri-menopause, and post-menopause (Rubinow et al., 1986; Rubinow and Schmidt, 1987; Schmidt and Rubinow, 2009; Perez-Lopez et al., 2013; Weber et al., 2013). Additionally, the risk of depression is increased in women undergoing prophylactic oophorectomy for cancer prevention and benign disease (Rocca et al., 2008; Parker, 2010; Chen et al., 2013). Precisely how alterations in circulating E2 participate in or contribute to depression are not known.
Stress-induced alterations in synaptic efficacy in hippo-campus, amygdala, and prefrontal cortex likely contribute to depression symptoms (Duman et al., 2000; Kim et al., 2005; Baller et al., 2013). Memory impairment is the most noted stress-induced cognitive abnormality, which is likely due in part to deficits in hippocampal synaptic function caused by increased glucocorticoids (Rabin et al., 1990; Pavlides et al., 1993; McEwen and Magarinos, 1997). In fact, MDD is often accompanied by decreased hippocampal volume and neuro-genesis (Vythilingam et al., 2002; Malberg and Duman, 2003; Campbell et al., 2004; Gass and Henn, 2009). In rodents, stress and elevated glucocorticoids decrease long-term potentiation (LTP) and increase long-term depression (LTD) at hippocampal synapses, decrease dendritic spine density, and induce hippocampal learning deficits, alterations which increase risk of depression-like behavior (Foy et al., 1987; McEwen and Magarinos, 1997; Joels et al., 2004; Holderbach et al., 2007). In contrast, proestrous levels of E2 increase CA1 dendritic spine density, LTP magnitude, and hippocampal-dependent learning and memory (Smith and McMahon, 2005, 2006; Frye et al., 2007; Vedder et al., 2013), effects that could oppose the detrimental consequences of stress and glucocorticoids on hippocampal structure and function. Therefore, low plasma E2 in women could leave hippocampal circuits vulnerable to the harmful effects of chronically elevated glucocorticoids that occur during stress and in depression.
The ability to reproduce ‘‘depression-like’’ symptomatology in rodents has helped pinpoint specific genes, receptors, and biomarkers involved in depression (Nestler et al., 2002; Vollmayr et al., 2007). The learned helplessness paradigm has the advantage over other depression models in that, in addition to animals displaying a sense of “giving up” similar to humans with depression, they fail to learn behavioral contingencies, thus modeling some cognitive deficits associated with depression (Miller and Seligman, 1975; Seligman and Beagley, 1975; Jackson and Minor, 1988). Thus, following exposure to inescapable shock, rats that are unable to learn to escape shock are considered “helpless” and have learning deficits, while those that learn to escape, despite exposure to the same stressor, are considered “resilient”. The learned helplessness model therefore is useful in assessing cognitive dysfunction in depression. Indeed, adult male rats exposed to inescapable foot shock using a protocol similar to that which induces learned helplessness, experience deficits in hippo-campal LTP recorded in vivo (Ryan et al., 2010), although this study did not separately evaluate rats that acquired helplessness from those that did not. Because not all rats experiencing inescapable shock become helpless, thoroughly evaluating possible deficits in both helpless and resilient phenotypes is necessary to separate the deleterious consequences of acute stress from depression-like symptoms.
E2 has anxiolytic and antidepressant effects in open field, inhibitory avoidance, forced swim, and sucrose preference assays (Rachman et al., 1998; Estrada-Camarena et al., 2003; Walf and Frye, 2007; Romano-Torres and Fernandez-Guasti, 2010). However, very few studies have investigated the potential benefits of E2 in the learned helplessness model, and unfortunately the reported results are inconsistent (Jenkins et al., 2001; Dalla et al., 2008; Hajszan et al., 2010). In addition, the learned helplessness literature has only focused on animals that have acquired helplessness with a complete lack of information on hippocampal synaptic function in animals behaviorally resilient to the effects of inescapable stress.
Here we used the learned helplessness model of depression to test the hypothesis that hippocampal structure and function is differently affected in adult OVX rats acquiring helplessness versus resilience, and that proestrous plasma E2 levels decrease acquisition of helplessness and improve hippo-campal synaptic function. Our findings suggest the possibility that E2 replacement may be a therapeutic strategy to increase resilience and reduce the cognitive deficits in women who are susceptible to stress and hormonal fluctuations.
2. Materials and methods
2.1. Animals
Sprague-Dawley rats (6—10 weeks old; Charles River) were housed 2 per cage with access to water and standard chow ad libitum for 7—10 days prior to behavioral assessments. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham, in accordance with NIH guidelines. Please see Supplemental Information for additional methodological details.
2.2. Ovariectomy and estrogen treatment
Female rats were OVXed at 6—8 weeks as previously described (Smith and McMahon, 2005). Our previously published work confirms the effects of E2 on hippocampal spine density and synaptic plasticity at 14 days, 9 or 15 months post-OVX (Smith and McMahon, 2005; Smith et al., 2010). In the present study, at 14 days post-OVX, rats received 2 subcutaneous injections (24 h interval) of 17-β estradiol (E2, Sigma—Aldrich) at 10 μg/250 g in 100 μl cottonseed oil. Vehicle-treated rats received cottonseed oil alone. This injection protocol produces proestrous-like levels of plasma E2 (80—120 pg/ml) 24 h after the 2nd injection (Woolley and McEwen, 1993). Estrogenic response was confirmed by measuring uterine weights at the time of sacrifice (Hall et al., 1992; Smith and McMahon, 2005). Uterine weights in all E2-treated rats were significantly increased compared to vehicle-treated animals (0.20 ± 0.09 vs. 0.10 ± 0.05 g; p < 0.0001).
2.3. Induction of learned helplessness
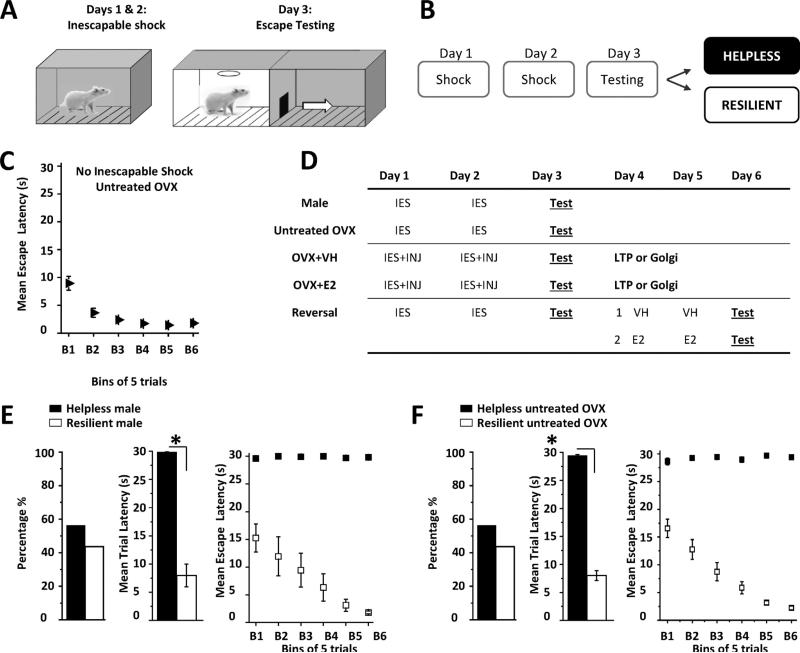
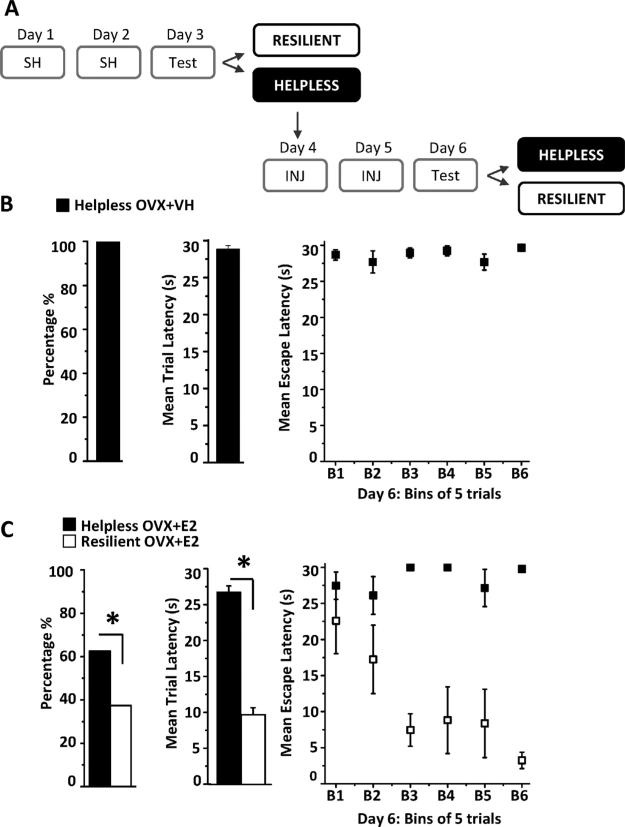
All behavior experiments were conducted between 9 am and 12 noon. On Days 1 and 2 (Fig. 1A and B), rats were exposed to 60 inescapable foot shocks at 0.65 mA (25—35 s duration, 15— 35 s intervals) in a dark solitary cage (Fig. 1A, 12”W × 10”D × 12”H, Coulbourn Instruments H10-11R-TC). On Day 3, rats were tested for helpless behavior using 30 escape trials in a novel shuttle cage (20”W 10”D 12”H; H10-11R-SC) to reduce the possible effects of context dependent fear memory associated with the solitary cage used for inescapable shock. For each escape trial, shock onset was accompanied by a light cue that signaled door opening to permit escape into the adjacent darkened compartment. If the rat crossed through the door, shock was terminated; otherwise the trial ended after 30 s had elapsed. Motion detection lasers were used to record latency for each trial during which an escape was made. If the rat did not escape during the first 20 s of shock, this was counted as a “failed” trial. Behavioral criterion for helplessness was met if the rat failed more than 5 of the last 10 trials during escape testing, while those that did not meet this criterion were considered “resilient” (Fig. 1B) (Vollmayr and Henn, 2001). This protocol was validated using a cohort of male rats and untreated OVX female rats (Fig. 1E and F). A control group of untreated OVX rats were exposed to the solitary cage for 30 min on Days 1 and 2 in the absence of inescapable shock and underwent escape testing on Day 3 (Fig. 1C; no inescapable shock). All rats in this group successfully learned to escape and none reached criteria for helplessness.
Figure 1.
Learned helplessness protocol and experimental timeline. (A and B) On Days 1 and 2, inescapable shock (IES, 0.65 mA) was conducted in a dark, solitary cage and on Day 3, rats experienced escape testing to determine helplessness or resilience (see “Materials and Methods”). (C) Graph shows average escape latency for each bin of 5 trials during escape testing in untreated OVX rats that were not exposed to inescapable shock. (D) Experimental timeline for all treatment groups: IES, inescapable shock; Test, escape testing; OVX + VH, vehicle injection (INJ); OVX + E2, estradiol injection; LTP/Golgi, sacrifice for LTP recordings or Golgi staining. Reversal: untreated OVX rats that met criteria for helplessness were divided into two separate groups for VH or E2 injections before 2nd escape testing. (E and F) Left bar charts show percentage of helpless rats in male (■ helpless, n = 16; 55.2%; □ resilient n = 13, 44.8%) and untreated OVX control groups (■ helpless, n = 58 56.3%; □ resilient n = 45, 43.7%). There was a significant difference in mean escape latency during the 30 trials of escape testing between helpless and resilient rats in both groups (middle bar charts, *p < 0.0001). Right graphs show average escape latency in helpless and resilient rats for each bin of 5 consecutive trials during escape testing in male (panel E) and untreated OVX rats (panel F).
To examine the role of E2 in acquisition of learned helplessness, OVX rats were treated with subcutaneous injections of either vehicle or E2 immediately following inescapable shock on Days 1 and 2 (Fig. 1D; OVX + VH, OVX + E2). This injection protocol allowed for escape testing to be performed 24 h following the 2nd E2 injection when hippocampal spine density, synaptic function, and learning and memory are increased (Smith and McMahon, 2005, 2006; Vedder et al., 2013). To determine whether E2 treatment reverses previously established helplessness, a subset of untreated OVX rats that met criterion for helplessness during escape testing on Day 3 (Fig. 1D; reversal) were treated with vehicle or E2 on Days 4 and 5, and on Day 6, were tested for helplessness with a 2nd round of escape testing.
2.4. Electrophysiology and spine density
Twenty-four hours following behavioral assessment (Day 4), rats were selected for either electrophysiology or spine density analysis. For electrophysiology, rats were deeply anesthetized with isoflurane (VetOne) in oxygen prior to decapitation and brain removal. Hippocampal slices were prepared and LTP measured as previously described (Smith and McMahon, 2005, 2006). Extracellular excitatory postsynaptic potentials (fEPSPs) were stimulated (0.1 Hz, 100 μs duration) using a bipolar tungsten electrode placed in CA1 stratum radiatum to stimulate Schaffer collaterals. Baseline responses were recorded for 20 min before high frequency stimulation (HFS; 100 Hz, 50 s duration) was used to induce LTP. HFS was delivered 4 times with 20 s intervals at 1.5 times baseline stimulation and fEPSPs were recorded for at least 40 min post-tetanus. To investigate the effects of E2 replacement slices where prepared from vehicle or E2-treated rats 24 h after escape testing (Day 4, Fig. 1D). Raw baseline fEPSP slopes were compared between experimental groups to ensure that differences observed in the LTP magnitude between experimental groups are not a consequence of differences in strength of the initial baseline transmission.
For spine density analysis, Golgi-Cox staining was performed using FD Rapid GolgiStain kit according to manufacturer's directions (FD Neurotechnologies, Inc., Ellicott City, MD). CA1 pyramidal cells were selected for analysis if the apical dendritic arbor was intact and tertiary dendrites in stratum radiatum were distinguishable and could be tracked back to an identified secondary dendrite. Spine density analysis was performed on tertiary dendrites because previous analysis of the effects of E2 was performed on tertiary branches (Gould et al., 1990; Smith and McMahon, 2005). Experimenter was blinded to the identity of the treatment group during analysis. Brightfield, confocal images were acquired at 63× (1.4 na) using a Nikon Eclipse TE-2000U inverted high resolution digital microscope. A 10 μm segment was outlined using MetaMorph Bioimaging software (Universal Imagining Corporation, Molecular Devices). Deconvolved, Z stacked images (30 focal planes, 10 μm intervals) were used to ensure spines above and below the segment could be included and only protrusions with a clearly discernible spine head and neck were counted (Shors et al., 2001; Smith and McMahon, 2005). Spines on 4 segments from 2 different tertiary branches on 2 different neurons were averaged together to represent mean density for each animal.
2.5. Statistics
Statistical analysis was performed using Origin 8.5 (OriginLab). All data are reported as mean ± standard error with significance set at p < 0.05. Percentage of helpless versus resilient rats within each treatment group was compared using the Chi-square test for categorical variables. Significant difference in mean escape latency between helpless and resilient rats within a given treatment group was evaluated with a two sample, independent Student's t-test. To determine if significant LTP was expressed, a one-sample dependent Student t-test was used. Comparison of the LTP magnitude, spine density, paired-pulse facilitation ratio, and steady-state depolarization between helpless and resilient rats within in a given treatment group was achieved using a two sample, independent Student's t-test.
3. Results
3.1. Inescapable shock induces learned helplessness in males and OVX female rats
To confirm that our shock protocol induces helplessness, a cohort of males (Fig. 1E) and untreated OVX female rats (Fig. 1F) were exposed to inescapable shock on Days 1 and 2 and escape testing on Day 3 (Fig. 1A, B, and D). Using this protocol, inescapable shock induced helplessness in 55.2% of males and in 56.3% of OVX females. Chi square analysis indicates no significant difference between the 2 behavioral phenotypes in either the male or OVX female groups (p > 0.05). As expected, the mean escape latency during the 30 trials for helpless rats was significantly increased compared to rats reaching criteria for resilience (males: 29.9 ± 0.1 s vs. 8.0 ± 2.0 s; untreated OVX: 29.0 ± 0.2 s vs. 8.6 ± 6.0 s, p < 0.0001). In fact, this significant difference in escape latencies between helpless and resilient rats was immediately apparent within the first bin of 5 trials during escape testing, suggesting that molecular changes dictating these behavioral phenotypes had occurred as a consequence of the 2 sessions of IES (helpless males 29.6 ± 0.4 s vs. resilient males 15.2 ± 2.5 s p < 0.0001; helpless untreated OVX females 28.1 ± 0.7 versus resilient untreated OVX females 16.6 ± 1.6 s, p < 0.0001). Furthermore, escape latencies of resilient males or untreated OVX females continued to decrease during each successive block of 5 averaged trials (Fig. 1E and F, right graphs), demonstrating learning during escape testing in a novel environment, despite prior exposure to inescapable shock. Thus, helpless rats show an inability to learn behavioral contingencies in a novel environmental context while resilient rats successfully learn to escape. Importantly, rats not exposed to inescapable shock learn to escape and never meet criteria for helplessness (Fig. 1C, 3.3 ± 3.5 s).
3.2. LTP is absent and spine density is decreased only in vehicle-treated OVX rats meeting criteria for helplessness
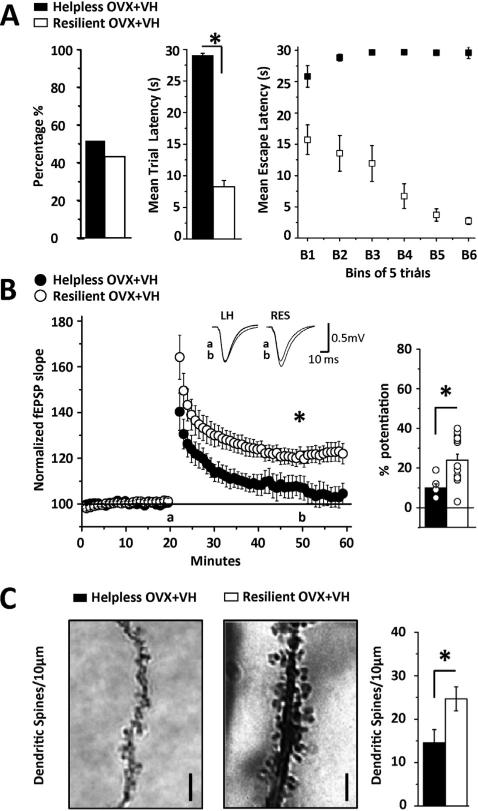
Stress decreases hippocampal synaptic function and learning, and increases the risk of depression-like behavior in rodents (Foy et al., 1987; McEwen and Magarinos, 1997; Joels et al., 2004; Frye et al., 2007; Holderbach et al., 2007; McLaughlin et al., 2008). Because only rats that acquire helplessness have deficits in escape behavior, we predicted that helpless rats would have deficits in LTP and decreased dendritic spine density compared to resilient rats, despite similar exposure to the stress of inescapable shock. To test this hypothesis, a cohort of vehicle-treated OVX rats was exposed to inescapable shock and escape testing as above (see Fig. 1D). On Day 4, acute slices were prepared from a subset of the helpless and resilient rats to measure LTP at CA3-CA1 synapses, and in a separate subset, dendritic spine density was analyzed using Golgi impregnation. In this cohort, 50.8% reached criteria for helplessness and 49.2% were resilient, with no significant difference in numbers of helpless versus resilient rats (Fig. 2A, p > 0.05). The mean escape latency was significantly longer in helpless versus resilient rats (29.0 ± 0.2 s vs. 8.2 ± 0.9; p < 0.001). Furthermore, this significant difference was apparent during the first 5 trials of escape testing (helpless 25.8 ± 1.7 s vs. resilient 15.7 ± 1.8 s; p < 0.0001), suggesting that these behavioral phenotypes were acquired during the 2 sessions of inescapable shock and are not a consequence of the amount of shock experienced by rats reaching criteria for helplessness during escape testing.
Figure 2.
LTP is absent and spine density is decreased only in vehicle-treated OVX rats reaching criteria for helplessness. (A) Left bar chart shows percentage of helpless (■, 50.8%, n = 44) and resilient (□, 49.2%, n = 41) rats, with no difference in numbers between phenotypes. Middle bar chart shows mean escape latency during the 30 trials of escape testing. Right graph shows average escape latency in helpless and resilient rats where each bin is an average of 5 consecutive trials across the 30 trials. (B) Summary plot shows a lack of significant LTP in slices from vehicle-treated helpless rats (●, n = 7 slices/5 rats) in contrast to significant LTP in rats meeting criteria for resilience (○, n = 14 slices/9 rats). Inset shows representative fEPSP waveforms at baseline (a) and 40 min post tetanus (b) in helpless and resilient animals. Right bar chart shows average LTP magnitude from each recording included in the data sets. (C) Left, representative images of a tertiary CA1 dendrite from a helpless (■) and resilient (□) rat. Right, bar chart shows CA1 pyramidal cell dendritic spine density is decreased in vehicle-treated helpless versus resilient rats. Spines were counted from 10 mm sections above and below the plane of focus. N = 8 sections/4 rats per group; images show deconvolved, stacked images of all focal planes collapsed into maximum 3D projection. Scale bar represents 2 μm. LTP and spine analysis was performed 24 h following escape testing. Asterisk indicates significance at p < 0.001 for 2A, 2B, and p < 0.05 for 2C. Error bars represent SEM.
As predicted, in slices from vehicle-treated helpless rats, LTP was completely absent (Fig. 2B, 107 ± 1% of baseline fEPSP slope; p > 0.05), while high frequency stimulation (HFS) induced significant potentiation in vehicle-treated resilient rats (Fig. 2B, 120 1% of baseline fEPSP slope, p < 0.001; between phenotypes, p < 0.001). The percent potentiation for each experiment included in the averaged LTP plot is shown in the bar graph in Fig. 2B. Thus, CA3-CA1 synapses in OVX resilient rats are capable of expressing LTP, despite exposure to the same inescapable shock that renders synapses in vehicle-treatedhelplessratsunabletoexpresspotentiation.Ofnote,in a separate cohort of vehicle-treated control OVX rats not exposed to behavior, the LTP magnitude is identical to that measured in resilient rats (see Supplemental Figure 1A, 120 ± 1% of baseline fEPSP slope). Furthermore, CA1 dendritic spine density was significantly decreased in helpless versus resilient rats (Fig. 2C, 14.3 ± 3.6 spines/10 μm vs. 24.6 ± 3.7 spines/10 μm, p < 0.05), and both groups have fewer spines than vehicle-treated control OVX rats not exposed to behavior (Supplemental Figure 1B; 39.5 ± 4.4 spines/10 μm), suggesting that shock decreases spine density and this effect is greater in those rats reaching criteria for helplessness.
3.3. E2 replacement increases resilience and helpless rats have significant LTP with no deficit in spine density
The beneficial effects of E2 replacement in OVX rats on hippocampal LTP, spine density (see Supplemental Figure 1), and learning are in opposition to the effects of stress and glucocorticoids (Foy et al., 1987; Woolley and McEwen, 1993; McEwen and Magarinos, 1997; Joels et al., 2004; Smith and McMahon, 2005; Frye et al., 2007; Holder-bach et al., 2007; Harburger et al., 2009; Vedder et al., 2013). Therefore we predicted that E2 replacement should protect against the detrimental effects of inescapable shock, thereby increasing resilience and facilitating learning during escape testing. To test this, OVX rats were treated with E2 on Days 1 and 2 of inescapable shock, tested for helpless versus resilient behavior on Day 3, and on Day 4, LTP and spine density were measured in separate subsets of helpless and resilient rats (see Fig. 1D).
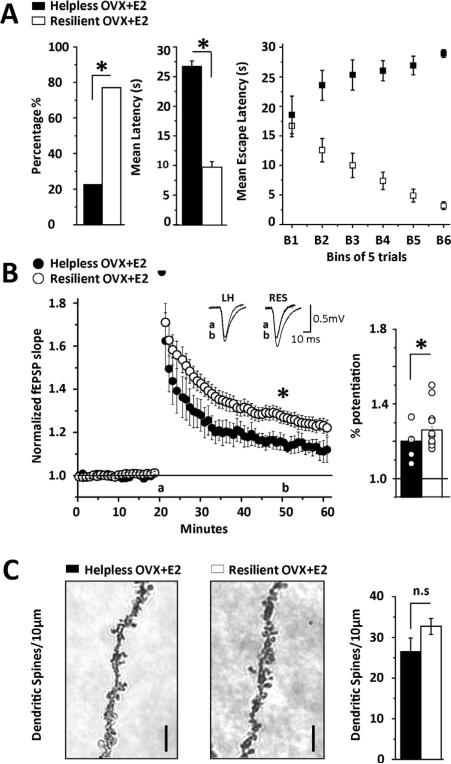
In contrast to the untreated and vehicle-treated OVX cohorts where the numbers of helpless and resilient rats were equal, in the E2-treated group, there were significantly more rats reaching criteria for resilience, such that 77.2% were resilient and only 22.8% demonstrated helplessness (Fig. 3A, p < 0.001; mean escape latencies in helpless vs. resilient: 26.8 ± 0.8 s vs. 9.7 ± 0.9; p < 0.001). In contrast to the vehicle-treated group, the mean escape latency during the first 5 trials is not different between E2-treated helpless and resilient rats (helpless 18.5 ± 3.2 s vs. resilient 16.5 ± 1.8 s; p = 0.66), suggesting that E2 is beneficial even in rats ultimately reaching criteria for helplessness during escape testing.
Figure 3.
E2 replacement increases behavioral resilience. (A) Left bar chart shows percentage of helpless (■, 22.8%, n = 16) and resilient rats (□, 77.2%, n = 54) with significantly more resilient than helpless animals. Middle bar chart shows mean escape latency during the 30 trials of escape testing. Right graph shows average escape latency in helpless and resilient rats where each bin is an average of 5 consecutive trials across the 30 trials. (B) Summary plot shows significant LTP in E2-treated helpless OVX rats (●, n = 5 slices/4 rats), however, the magnitude is significantly less than in E2-treated resilient OVX rats (○, n = 12 slices/10 rats). Inset shows representative fEPSP waveforms at baseline (a) and 40 min post tetanus (b) in helpless and resilient animals. Right bar chart shows average LTP magnitude from each recording included in the data sets. (C) Left, representative images of a tertiary CA1 dendrite from a helpless (■) and resilient (□) rat. Right, bar chart shows no significant difference in dendritic spine density between E2-treated helpless and resilient rats. Spines were counted from 10 μm sections above and below the plane of focus. N = 8 sections/4 rats per group; images show deconvolved, stacked images of all focal planes collapsed into maximum 3D projection. Scale bar represents 2 μm. LTP and spine analysis was performed 24 h following escape testing. Asterisk indicates significance at p < 0.001 except potentiation from baseline in helpless rats where p < 0.05. Error bars represent SEM.
Furthermore, in slices from E2-treated helpless rats, HFS induced significant LTP (Fig. 3B, 114 ± 2% of baseline fEPSP slope; p < 0.001), in contrast to the lack of LTP in vehicle-treated helpless rats (Fig. 2B). However, the LTP magnitude in the E2-treated helpless rats was significantly less than that in E2-treated resilient rats as expected (Fig. 3B, 127 ± 2% of baseline fEPSP slope; p < 0.05; p < 0.001 between pheno-types). Importantly, the LTP magnitude in E2-treated resilient rats is comparable to that measured at CA3-CA1 synapses in slices from a separate cohort of control E2-treated rats not exposed to behavior (Supplement Figure 1A; 135 ± 2% of baseline fEPSP slope). Despite the significant difference in LTP magnitude between E2-treated helpless and resilient rats, there was no difference in dendritic spine density between the 2 phenotypes (Fig. 3C, 26.5 ± 4.1 spines/ 10 μm vs. 31.4 ± 2.1 spines/10 μm; p > 0.05), although both groups have fewer spines than E2-treated control OVX rats not exposed to behavior (Supplemental Figure 1C; 58.5 ± 6.1 spines/10 μm), again demonstrating that shock decreases spine density.
3.4. Differences in LTP magnitude are not due to alterations in paired-pulse facilitation ratio or steady-state depolarization during tetanus
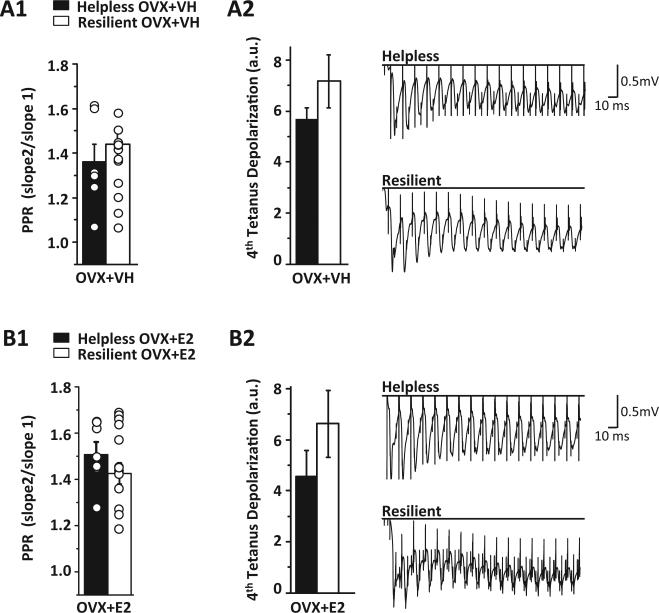
We next investigated whether the deficit in LTP in helpless rats is due to decreased presynaptic release probability or decreased tetanus-induced depolarization needed to release the voltage-dependent Mg2+ block from NMDARs required for LTP induction. However, no significant differences in the paired-pulse ratio, an indirect measure of release probability (Dobrunz and Stevens, 1997), were found in helpless versus resilient rats in either vehicle (Fig. 4A1, 1.36 ± 0.08 vs. 1.43 ± 0.05; p > 0.05) or E2-treated (Fig. 4B1, 1.50 ± 0.05 vs. 1.42 ± 0.04; p > 0.05) OVX rats. Furthermore, there were no differences in steady state depolarization during the 4th round of tetanus between helpless and resilient rats in either the vehicle (Fig. 4A2, 56.8 ± 4.4 vs. 71.6 ± 10.3; p > 0.05) or E2-treated (Fig. 4B2, 45.7 ± 9.9 vs. 66.2 ± 13.1; p > 0.05) groups. Thus, the difference in LTP between helpless and resilient rats is not due to alterations in presynaptic gluta-mate release or the magnitude of depolarization during tetanus.
Figure 4.
No change in the paired-pulse facilitation ratio or steady-state depolarization in helpless versus resilient rats. (A1) No significant difference in the paired pulse facilitation ratio between vehicle-treated resilient (□, n = 14 slices/9 rats) and helpless (■, n = 7 slices/5 rats) OVX rats. (A2) Left bar chart shows no significant difference in average steady state depolarization (arbitrary units) between helpless and resilient rats during the 4th round of tetanus in vehicle-treated rats. Right, representative waveforms from a helpless and a resilient rat. (B1) No significant difference in the paired-pulse facilitation ratio between E2-treated resilient (□, n = 12 slices/10 rats) and helpless (■, n = 5 slices/4 rats) OVX rats. (B2) Left bar chart shows no significant difference in steady-state depolarization during the 4th round of tetanus in E2-treated rats. Right, representative waveforms from a helpless and a resilient rat. Bar charts show averaged data from all recordings used in LTP data sets in Fig. 4. Error bars represent SEM.
3.5. Helpless behavior is reversed by E2 replacement in OVX rats
Finally, we sought to determine whether E2 replacement can reverse previously established helpless behavior. For these experiments, a subset of untreated OVX rats that met criteria for helplessness during escape testing on Day 3 were treated with either vehicle or E2 on Days 4 and 5, then exposed to a 2nd round of escape testing on Day 6 to determine if helplessness can be reversed (Fig. 5A). All helpless vehicle-treated rats (17 of 17 rats) continued to meet criteria for helplessness and mean escape latency during escape testing was 28.9 ± 0.5 s (Fig. 5B), demonstrating that a 2nd exposure to the escape testing environment is not sufficient to facilitate learning and reverse helplessness.
Figure 5.
E2 replacement can reverse previously established helpless behavior. (A) Experimental paradigm. (B and C) Left bar chart shows the percentage of helpless rats that remain helpless following a 2nd round of escape testing on Day 6. Middle bar chart shows the average escape latency from all 30 trials during escape testing on Day 6. Right graph shows escape latency where each bin is an average of 5 consecutive trials across the 30 trials. All vehicle-treated rats (n = 17 of 17) remained helpless while only 62.5% of E2-treated rats were helpless and 37.5% were resilient (6 of 16 rats). In the E2-treated group, mean escape latency significantly decreased during the 2nd round of escape testing on Day 6 due to some rats becoming resilient and learning to escape shock. Asterisk indicates p < 0.001. Error bars represent SEM.
Conversely, when helpless rats were treated with E2, only 62.5% were helpless while the other 37.5% (6 of 16 rats) were resilient (Fig. 5C). Furthermore, mean escape latency in the newly resilient rats was significantly decreased compared to their original escape latency (Fig. 5C, 12.12 ± 2.3 s vs. 29.1 ± 0.4 s; p < 0.001). Thus E2 replacement is capable of reversing helplessness and increasing learning during a 2nd round of escape testing in over one-third of the cohort.
4. Discussion
Here we report that E2 replacement at near proestrous levels in adult OVX female rats significantly increases resilience to inescapable shock in the learned helplessness model of depression. Learned helplessness is acquired in about half of all males, untreated OVX, and vehicle-treated OVX rats experiencing inescapable shock, while only about one-fifth of E2-treated rats become helpless. Furthermore, we find that subsequent E2 replacement can reverse previously established helplessness in over one-third of OVX rats displaying the helpless phenotype.
A major strength of our study is that we determined which rats met criteria for helplessness versus resilience before investigating synaptic function and dendritic spine density in hippocampal area CA1. This is in contrast to other previously published reports where there was no attempt to distinguish between rats that acquired helplessness from those that did not before any further analysis was performed (Hajszan et al., 2010; Ryan et al., 2010). Importantly, the differences in the amount of shock experienced by rats reaching criteria for helplessness versus resilience during escape testing are not causing the behavioral phenotype. Rather, the significant difference in escape latency between helpless and resilient rats (with the exception of the E2-treated group) revealed during the first 5 of 30 trials strongly indicates that the molecular changes dictating behavior occur during the two exposures to inescapable shock and are not a consequence of the shock experienced during escape testing. However, we cannot completely rule out that the increased amount of shock during escape testing on Day 3 experienced by rats reaching criteria for helplessness might also contribute. Because in the human population a particular stressor may trigger a depressive episode in some individuals but not others, separately investigating synaptic function/dysfunction in resilient versus helpless rats provides a more precise model for mechanistic investigation of the effects of stress that lead to cognitive deficits associated with depression.
4.1. Learned helplessness in females
Conflicting reports exist from the handful of learned helplessness studies using female rats. Two previous studies reported that inescapable shock does not induce helplessness in ovary intact female rats (Steenbergen et al., 1989; Dalla et al., 2008). However variations in ovarian plasma E2 during behavior potentially masked the beneficial effects of elevated plasma E2. In contrast, Jenkins and coworkers (2001) reported that female rats are more likely to show helplessness during diestrus II when E2 levels are low, and are more resilient to inescapable shock during estrus, when E2 levels are elevated. Differences in the timing of shock exposure in ovary-intact female rats could account for the discrepancies between these previous reports. Although published studies are inconclusive, it is important to note that sensitivity to certain types of pain may vary as plasma E2 levels vary and could impact the outcome (Ryan and Maier, 1988; Leuner et al., 2004; Sherman and LeResche, 2006). An important strength of the present study is that OVX rats were treated with an equal dose of E2 that was aimed to raise plasma E2 levels similar to those of proestrus rats. Thus, our findings, which are consistent with those of Jenkins et al. (2001), help to establish a beneficial role of E2 in the modulation of aversive experiences in adult OVX female rats, which ultimately decreases acquisition of helplessness and increases resilience.
4.2. LTP in helplessness versus resilience
By separately investigating synaptic plasticity in helpless versus resilient rats, we discovered that inescapable shock only leads to a complete loss of LTP at CA3-CA1 synapses in vehicle-treated helpless OVX rats. Given the several published reports showing that acute inescapable stress inhibits LTP (Foy et al., 1987; Shors et al., 1989; Baker and Kim, 2002), the finding that CA3-CA1 synapses in resilient rats are capable of expressing potentiation, despite exposure to the same inescapable shock that causes deficits or complete loss of LTP in helpless rats, is unexpected. This critical difference in LTP magnitude between the two behavioral phenotypes was likely revealed only because we waited 24 h following escape testing to measure LTP to avoid the negative effects of acute stress that could obscure possible differences in plasticity between helpless and resilient rats. Finally, observing significant LTP at CA3-CA1 synapses from E2-treated, but not vehicle-treated, helpless rats suggests that E2 is beneficial for hippocampal function.
4.3. Spine density in helplessness versus resilience
Dendritic spine density is often used as an indirect measure of synaptic function. Duman and coworkers (2000) reported that inescapable shock leads to decreased dendritic spine synapses on CA1 pyramidal cells of male and female rats measured using electron microscopy, although animals were not tested for behavioral helplessness versus resilience (Hajszan et al., 2010). These authors suggested that the decrease in spine synapses contributes to altered synaptic function that dictates behavior. Our finding that vehicle-treated helpless rats have significantly fewer spines and an LTP deficit compared to resilient rats supports this interpretation. However, the dissociation we find between spine density and LTP magnitude in E2-treated resilient versus helpless rats clearly indicates that spine density does not predict synaptic function. If this were the case, spine density should be decreased in accord with the decreased LTP magnitude observed in E2-treated helpless rats. Or alternatively, there should be no deficit in LTP because spine density is not different between E2-treated helpless and resilient rats. This lack of association between spine density and LTP magnitude in E2-treated rats is reminiscent of our previous findings that the time course of the increase in CA1 dendritic spine density does not mirror the time course of the increase in LTP magnitude or enhanced novel object recognition (Smith and McMahon, 2005; Vedder et al., 2013). It is important to point out that our analysis was limited to spine density rather than synapse density. However, our past and current findings suggest that caution is needed when using alterations in dendritic spine density to infer changes circuit function and behavior.
4.4. The role of E2 in behavioral resilience
The cellular mechanisms stimulated by E2 replacement that increase resilience are currently unknown. The precise role of hippocampus in acquisition and/or expression of helplessness is also unknown. However, a role for hippocampus in depression is beginning to be appreciated due to the hippocampal atrophy that occurs in many patients with MDD (Campbell et al., 2004; Vythilingam et al., 2004) and because antidepressants increase hippocampal neurogenesis and reverse depression-like behavior in rodent models (Malberg and Duman, 2003; Holderbach et al., 2007; Vollmayr et al., 2007; Gass and Henn, 2009). In addition, helpless animals fail to learn context discrimination (Seligman and Beagley, 1975; Jackson and Minor, 1988) suggesting that the hippocampus is a likely target. The beneficial effect of E2 on hippocampal neurogenesis, hippocampal plasticity, and hippocampal learning suggests that the capacity of E2 to increase resilience is due in part to its ability to enhance hippocampal function (Smith and McMahon, 2005; Frye et al., 2007; Luine and Frankfurt, 2013; Vedder et al., 2013). Importantly, E2 has antidepressant effects in other preclinical models (Shors and Leuner, 2003; Walf et al., 2004; Walf and Frye, 2005; Sell et al., 2008), which is confirmed by our finding that E2 replacement reverses helplessness in some OVX rats. Despite the beneficial effects of E2 in hippocampus, a small fraction (23%) of E2-treated rats still acquire helplessness, but it is important to note that behaviors may not always correlate with plasma hormone levels. Thus, further investigation is needed to determine whether other brain regions contribute to the development of helpless behavior and the downstream mechanisms that are activated by E2 to induce resilience.
In future studies it will be important to determine which estrogen receptors mediate the beneficial effects of E2 replacement and the downstream cellular mechanisms that enhance resilience to stress. E2 increases brain derived neurotrophic factor (BDNF) levels (Scharfman and MacLusky, 2006; Harte-Hargrove et al., 2013; Pluchino et al., 2013), which may mediate many of the beneficial effects of estrogen in hippocampus, as well as in the antidepressant effects of E2 (Kiss et al., 2012). Therefore, fully understanding the interactions between E2 and BDNF in depression is of high importance (Duman and Monteggia, 2006; Bath et al., 2012). Dysfunction in the cholinergic system is also implicated in depression (Mineur et al., 2013) and E2-cholinergic interactions are critical in the memory enhancing effects of increased plasma E2 levels (Gibbs, 2002; Gibbs et al., 2011). Thus, future investigations into the potential role of muscarinic and/or nicotinic receptors in the antidepressant effects of E2 are needed. The antidepressant effects of E2 could also be related to its ability to manipulate the serotonergic system (Kiss et al., 2012). Clearly much more work in the area of E2 and depression is necessary. However, the results reported here add to current knowledge by demonstrating the beneficial effects of E2 in the learned helplessness model using female rats and have important implications for the use of E2 replacement therapy in the subgroup of women who are at increased risk of developing depression during periods of ovarian hormonal fluctuations such as during the menopausal transition.
Because helplessness is only one of many symptoms that may occur in depression, studies in women directly linking E2 levels with learned helplessness are not available. However, our results are consistent with the known beneficial effects of E2 treatment during perimenopause (Rubinow and Schmidt, 1987; Schmidt et al., 2000; Soares et al., 2001; Cohen et al., 2005; Schmidt and Rubinow, 2009; Perez-Lopez et al., 2013). Unfortunately, clinical studies in postmenopausal women report conflicting results regarding the efficacy of E2 replacement therapy on mood. The lack of benefit may be explained by variability in estrogen replacement formulations as well as the duration of hormone deprivation prior to initiating treatment (Wharton et al., 2012; Craig, 2013). Future preclinical and clinical studies are needed to resolve these issues.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health NIMH Award MH-082304 to L.L. McMahon, the Brain and Behavior Research Foundation NARSAD award to L.L. McMahon, and the NIH National Center for Research Resources grant #5TL1 RR025775-03 which provided trainee support for T. Brede-mann. We would also like to acknowledge support from the UAB Behavioral Assessment Core (P30 NS47466) for the use of Colbourne Instruments equipment and the technical assistance of Dr. Thomas Van Groen. We would like to acknowledge the UAB High Resolution Imaging Facility for use of the Nikon Eclipse microscope, Metamorph Molecular Devices Software, and the technical assistance of Mr. Shawn Williams.
Role of the funding source
This work was funded by NIH grant award, MH-082304, and will require that the published manuscript is available via PubMed Central per the policies of the NIH.
Footnotes
Conflict of interest
We have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.01.004.
References
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn. Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcon G, Schmidt PJ, Berman KF. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am. J. Psychiatry. 2013;170:305–314. doi: 10.1176/appi.ajp.2012.12030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol. Psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chen X, Guo T, Li B. Influence of prophylactic oophorectomy on mood and sexual function in women of menopausal transition or postmenopausal period. Arch. Gynecol. Obstet. 2013;288:1101–1106. doi: 10.1007/s00404-013-2865-1. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am. J. Med. 2005;118(Suppl. 12B):93–97. doi: 10.1016/j.amjmed.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Craig MC. Should psychiatrists be prescribing oestrogen therapy to their female patients? Br. J. Psychiatry. 2013;202:9–13. doi: 10.1192/bjp.bp.111.102855. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsycho-pharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D'Sa C. Neuronal plasticity and survival in mood disorders. Biol. Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Henn FA. Is there a role for neurogenesis in depression? Biol. Psychiatry. 2009;66:3–4. doi: 10.1016/j.biopsych.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm. Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm. Behav. 2011;59:503–511. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ, Leranth C, Duman RS. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol. Psychiatry. 2010;67:168–174. doi: 10.1016/j.biopsych.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Cantley TC, Galvin JM, Day BN, Anthony RV. Influence of ovarian steroids on relaxin-induced uterine growth in ovariectomized gilts. Endocrinology. 1992;130:3159–3166. doi: 10.1210/endo.130.6.1597136. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor—estrogen interactions in the hippo-campal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biol. Psychiatry. 2007;62:92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Jackson RL, Minor TR. Effects of signaling inescapable shock on subsequent escape learning: implications for theories of coping and “learned helplessness”. J. Exp. Psychol. Anim. Behav. Process. 1988;14:390–400. [PubMed] [Google Scholar]
- Jenkins JA, Williams P, Kramer GL, Davis LL, Petty F. The influence of gender and the estrous cycle on learned helplessness in the rat. Biol. Psychol. 2001;58:147–158. doi: 10.1016/s0301-0511(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J. Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17beta-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav. Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippo-campus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann. N. Y. Acad. Sci. 1997;821:271– 284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm. Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Seligman ME. Depression and learned helplessness in man. J. Abnorm. Psychol. 1975;84:228–238. doi: 10.1037/h0076720. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Parker WH. Bilateral oophorectomy versus ovarian conservation: effects on long-term women's health. J. Minim. Invasive Gynecol. 2010;17:161–166. doi: 10.1016/j.jmig.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Perez-Lopez FR, Perez-Roncero G, Fernandez-Inarrea J, Fernandez-Alonso AM, Chedraui P, Llaneza P, The M.R.G. Resilience, depressed mood, and menopausal symptoms in postmenopausal women. Menopause. 2013;21 doi: 10.1097/GME.0b013e31829479bb. http://dx.doi.org/10.1097/GME.0b013e31829479bb. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271– 279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, Chrousos GP. Hypothalamic—pituitary— adrenal function in patients with the premenstrual syndrome. J. Clin. Endocrinol. Metab. 1990;71:1158–1162. doi: 10.1210/jcem-71-5-1158. [DOI] [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de Andrade M, Melton LJ., III Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- Romano-Torres M, Fernandez-Guasti A. Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress. Behav. Pharmacol. 2010;21:104–111. doi: 10.1097/FBP.0b013e328337bdfc. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roy-Byrne P, Hoban MC, Grover GN, Stambler N, Post RM. Premenstrual mood changes. Characteristic patterns in women with and without premenstrual syndrome. J. Affect. Disord. 1986;10:85–90. doi: 10.1016/0165-0327(86)90030-3. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Mood disorders and the menstrual cycle. J. Reprod. Med. 1987;32:389–394. [PubMed] [Google Scholar]
- Ryan BK, Vollmayr B, Klyubin I, Gass P, Rowan MJ. Persistent inhibition of hippocampal long-term potentiation in vivo by learned helplessness stress. Hippocampus. 2010;20:758–767. doi: 10.1002/hipo.20677. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Maier SF. The estrous cycle and estrogen modulate stress-induced analgesia. Behav. Neurosci. 1988;102:371–380. doi: 10.1037//0735-7044.102.3.371. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone—growth factor interactions in the adult CNS. Front. Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am. J. Obstet. Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann. N. Y. Acad. Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Beagley G. Learned helplessness in the rat. J. Comp. Physiol. Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Sell SL, Craft RM, Seitz PK, Stutz SJ, Cunningham KA, Thomas ML. Estradiol-sertraline synergy in ovariectomized rats. Psychoneuroendocrinology. 2008;33:1051–1060. doi: 10.1016/j.psyneuen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R245–R256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J. Affect. Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J. Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J. Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Steenbergen HL, Heinsbroek RP, Van Haaren F, Van de Poll NE. Sex-dependent effects of inescapable shock administration on behavior and subsequent escape performance in rats. Physiol. Behav. 1989;45:781–787. doi: 10.1016/0031-9384(89)90295-3. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA. Learned helplessness in the rat: improvements in validity and reliability. Brain Res. Brain Res. Protoc. 2001;8:1–7. doi: 10.1016/s1385-299x(01)00067-8. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic—pituitary—adrenal axis activity. Neuropsycho-pharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 2007;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol. Biochem. Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2013 doi: 10.1016/j.jsbmb.2013.06.001. http://dx.doi.org/10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed]
- Wharton W, Gleason CE, Olson SR, Carlsson CM, Asthana S. Neurobiological underpinnings of the estrogen—mood relationship. Curr. Psychiatry Rev. 2012;8:247–256. doi: 10.2174/157340012800792957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.