Abstract
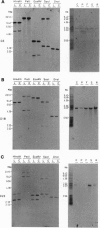
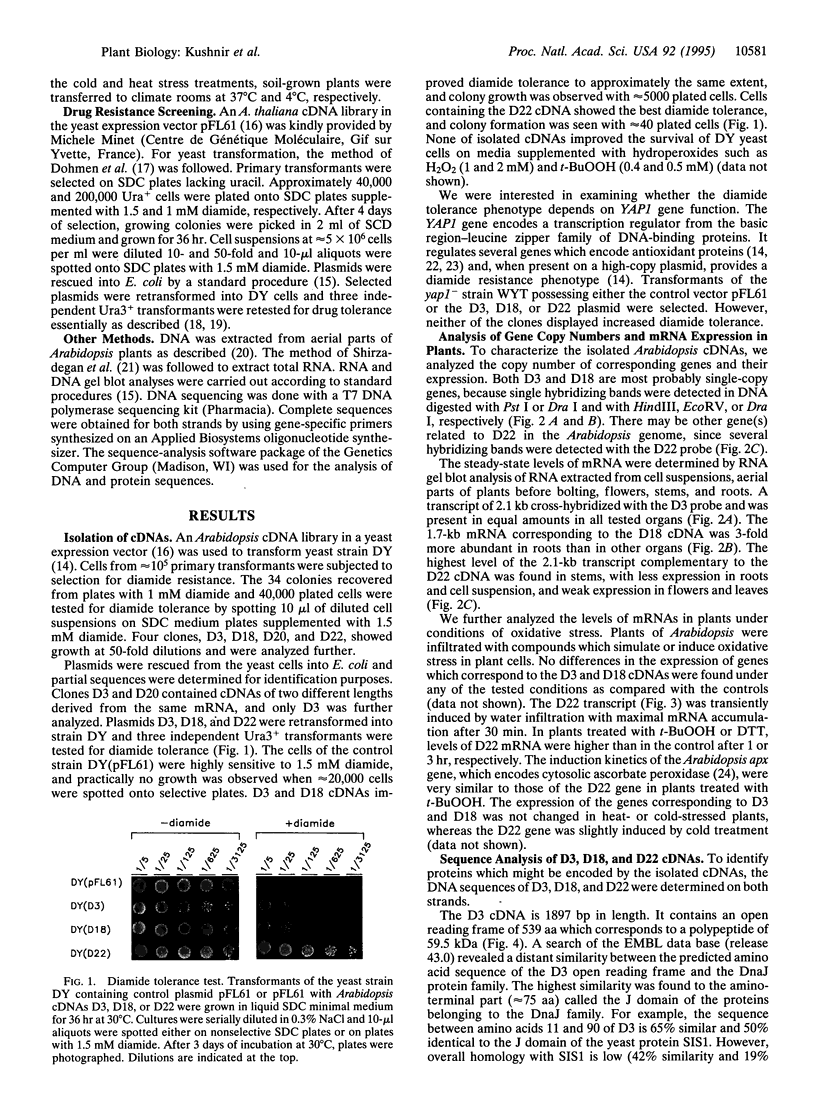
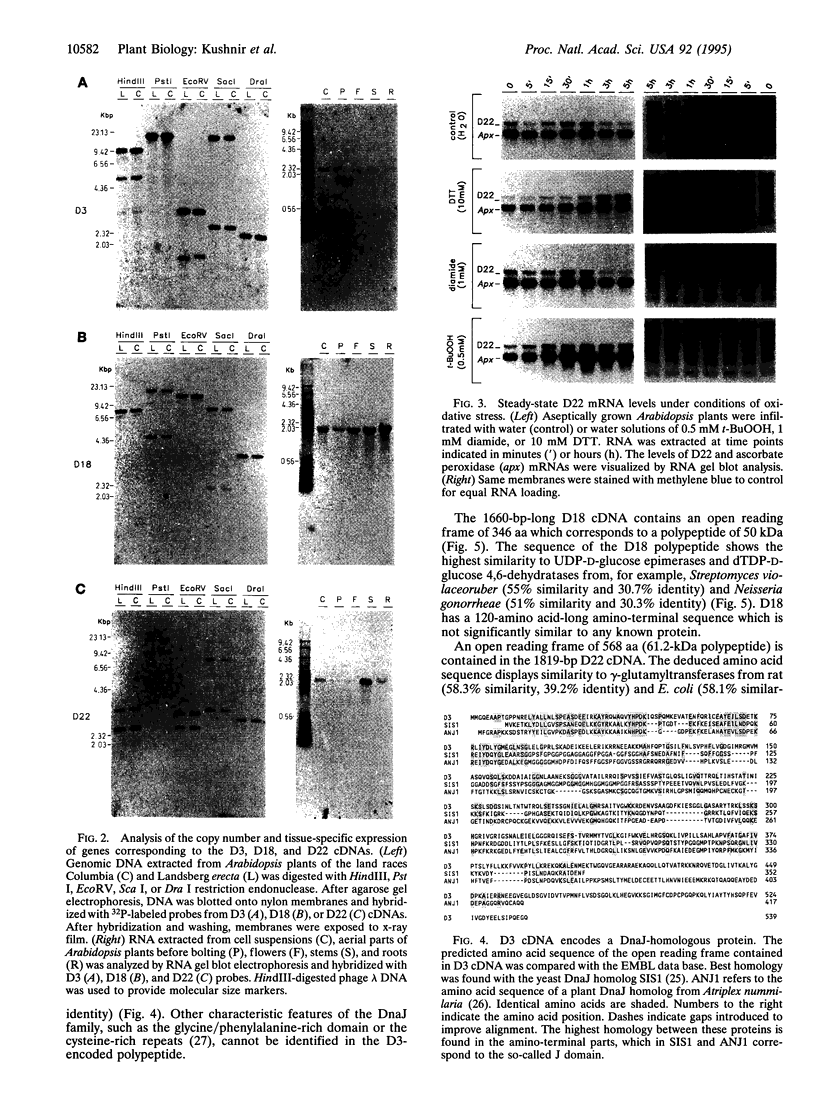
Diamide oxidizes cellular thiols and induces oxidative stress. To isolate plant genes which may, when overexpressed, increase tolerance of plants toward oxidative damage, an in vivo diamide tolerance screening in yeasts was used. An Arabidopsis cDNA library in a yeast expression vector was used to transform a yeast strain with intact antioxidant defense. Cells from approximately 10(5) primary transformants were selected for resistance to diamide. Three Arabidopsis cDNAs which confer diamide tolerance were isolated. This drug tolerance was specific and no cross tolerance toward hydroperoxides was found. One cDNA (D3) encodes a polypeptide which has an amino-terminal J domain characteristic of a divergent family of DnaJ chaperones. Another (D18) encodes a putative dTDP-D-glucose 4,6-dehydratase. Surprisingly, the third cDNA (D22) encodes a plant homolog of gamma-glutamyltransferases. It would have been difficult to predict that the expression of those genes would lead to an improved survival under conditions of depletion of cellular thiols. Hence, we suggest that this cloning approach may be a useful contribution to the isolation of plant genes that can help to cope with oxidative stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee H., Bhaduri A. Distinct functional roles of two active site thiols in UDPglucose 4-epimerase from Kluyveromyces fragilis. J Biol Chem. 1992 Jun 15;267(17):11714–11720. [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993 Jun;4(6):555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Dhillon N., Hoekstra M. F. Characterization of two protein kinases from Schizosaccharomyces pombe involved in the regulation of DNA repair. EMBO J. 1994 Jun 15;13(12):2777–2788. doi: 10.1002/j.1460-2075.1994.tb06571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen R. J., Strasser A. W., Höner C. B., Hollenberg C. P. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast. 1991 Oct;7(7):691–692. doi: 10.1002/yea.320070704. [DOI] [PubMed] [Google Scholar]
- Donovan J. W., Milne G. T., Weaver D. T. Homotypic and heterotypic protein associations control Rad51 function in double-strand break repair. Genes Dev. 1994 Nov 1;8(21):2552–2562. doi: 10.1101/gad.8.21.2552. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gupta A. S., Heinen J. L., Holaday A. S., Burke J. J., Allen R. D. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1629–1633. doi: 10.1073/pnas.90.4.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Fujii J., Taniguchi N., Meister A. Expression of an active glycosylated human gamma-glutamyl transpeptidase mutant that lacks a membrane anchor domain. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):126–130. doi: 10.1073/pnas.92.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Kubo A., Saji H., Tanaka K., Tanaka K., Kondo N. Cloning and sequencing of a cDNA encoding ascorbate peroxidase from Arabidopsis thaliana. Plant Mol Biol. 1992 Feb;18(4):691–701. doi: 10.1007/BF00020011. [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke M. M., Sutton A., Arndt K. T. Characterization of SIS1, a Saccharomyces cerevisiae homologue of bacterial dnaJ proteins. J Cell Biol. 1991 Aug;114(4):623–638. doi: 10.1083/jcb.114.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Chen Y., de Beus M., Bowley S. R., Bowler C., Inzé D., D'Halluin K., Botterman J. Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol. 1993 Dec;103(4):1155–1163. doi: 10.1104/pp.103.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies L. A., Cundliffe E. Analysis of five tylosin biosynthetic genes from the tyllBA region of the Streptomyces fradiae genome. Mol Microbiol. 1994 Jul;13(2):349–355. doi: 10.1111/j.1365-2958.1994.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Meuwly P., Thibault P., Schwan A. L., Rauser W. E. Three families of thiol peptides are induced by cadmium in maize. Plant J. 1995 Mar;7(3):391–400. doi: 10.1046/j.1365-313x.1995.7030391.x. [DOI] [PubMed] [Google Scholar]
- Minet M., Dufour M. E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992 May;2(3):417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Penninckx M. J., Elskens M. T. Metabolism and functions of glutathione in micro-organisms. Adv Microb Physiol. 1993;34:239–301. doi: 10.1016/s0065-2911(08)60031-4. [DOI] [PubMed] [Google Scholar]
- Prasad T. K., Anderson M. D., Martin B. A., Stewart C. R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell. 1994 Jan;6(1):65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Voos W., Pfanner N. Partner proteins determine multiple functions of Hsp70. Trends Cell Biol. 1995 May;5(5):207–212. doi: 10.1016/s0962-8924(00)89001-7. [DOI] [PubMed] [Google Scholar]
- Shirzadegan M., Christie P., Seemann J. R. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991 Nov 11;19(21):6055–6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993 Jul 15;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kumagai H., Echigo T., Tochikura T. DNA sequence of the Escherichia coli K-12 gamma-glutamyltranspeptidase gene, ggt. J Bacteriol. 1989 Sep;171(9):5169–5172. doi: 10.1128/jb.171.9.5169-5172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J. S., Lo S. F., Ploux O., He X., Liu H. W. Studies of the biosynthesis of 3,6-dideoxyhexoses: molecular cloning and characterization of the asc (ascarylose) region from Yersinia pseudotuberculosis serogroup VA. J Bacteriol. 1994 Sep;176(17):5483–5493. doi: 10.1128/jb.176.17.5483-5493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie J. A., Szczypka M. S., Thiele D. J., Moye-Rowley W. S. Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J Biol Chem. 1994 Dec 23;269(51):32592–32597. [PubMed] [Google Scholar]
- Wu A. L., Moye-Rowley W. S. GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol Cell Biol. 1994 Sep;14(9):5832–5839. doi: 10.1128/mcb.14.9.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lockshin C., Herbert A., Winter E., Rich A. Zuotin, a putative Z-DNA binding protein in Saccharomyces cerevisiae. EMBO J. 1992 Oct;11(10):3787–3796. doi: 10.1002/j.1460-2075.1992.tb05464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T., Arndt K. T. The yeast SIS1 protein, a DnaJ homolog, is required for the initiation of translation. Cell. 1993 Jun 18;73(6):1175–1186. doi: 10.1016/0092-8674(93)90646-8. [DOI] [PubMed] [Google Scholar]
- Zhu J. K., Shi J., Bressan R. A., Hasegawa P. M. Expression of an Atriplex nummularia gene encoding a protein homologous to the bacterial molecular chaperone DnaJ. Plant Cell. 1993 Mar;5(3):341–349. doi: 10.1105/tpc.5.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]