Abstract
Glaucoma is a progressive optic neuropathy that causes characteristic changes of the optic nerve and visual field in relation to intraocular pressure (IOP). It is now known that glaucoma can occur at statistically normal IOPs and prevalence studies have shown that normal tension glaucoma (NTG) is more common than previously thought. While IOP is believed to be the predominant risk factor in primary open angle glaucoma (POAG), IOP-independent risk factors, such as vascular dysregulation, are believed to play an important part in the pathogenesis of NTG. Though certain distinguishing phenotypic features of NTG have been reported, such as an increased frequency of disc hemorrhages, acquired pits of the optic nerve and characteristic patterns of disc cupping and visual field loss, there is much overlap of the clinical findings in NTG with POAG, suggesting that NTG is likely part of a continuum of open angle glaucomas. However, IOP modification is still the mainstay of treatment in NTG. As in traditional POAG, reduction of IOP can be achieved with the use of medications, laser trabeculoplasty or surgery. Studies now show that the choice of medication may also be important in determining the outcomes of these patients. Though it is likely that future treatment of NTG will involve modification of both IOP and IOP-independent risk factors, current efforts to develop IOP-independent neuroprotective treatments have not yet proven to be effective in humans.
Keywords: Intraocular pressure, low tension glaucoma, neuroprotection, normal tension glaucoma, risk factors
Glaucoma is a progressive optic neuropathy with characteristic clinical changes of the optic nerve and functional visual field deficits, which are in part, related to intraocular pressure (IOP). Historically, glaucoma was believed to be a disease of elevated IOP, that could lead to blindness if left untreated.[1] Though von Graefe first described “amaurosis with excavation” and postulated that glaucoma could occur in the absence of elevated IOP as early as 1857,[2] it was not until Schnabel later confirmed these findings in 1908 that this concept started to gain public acceptance.[3] However, the notion of “normal tension” glaucoma did not become commonplace until the latter part of the 20th century.[4] The Baltimore Eye Survey has since revealed that about half of all patients diagnosed with primary open angle glaucoma (POAG) in their study population had an initial IOP of less than 21mmHg at the time of diagnosis and approximately 20% had an IOP of less than 21mmHg on each of their first three visits suggesting that normotensive eye disease may be more common than previously thought.[5,6]
Subsequent population-based studies have shown that the prevalence of glaucoma in the presence of an IOP in the statistically normal range is much more common than once believed. On average, these studies show that normal tension glaucoma (NTG) occurs in roughly 30 to 40% of all patients diagnosed with a glaucomatous visual field defect.[5,7,8] For reasons that are unclear, Asian populations appear to be especially susceptible to NTG, with the Japanese Tajimi study showing the prevalence of POAG to be 3.9% in their study population, of which 92% had an IOP of 21 mmHg or less.[9] Similar findings were shown in Korea, where a recent prevalence study in the rural Korean town of Sangju showed that an IOP of less than 21 mmHg was found in 94.4% of cases with open angle glaucoma (OAG).[10] Early diagnosis in these cases can be particularly challenging, since tension-based screening modalities are less helpful in this setting and delayed diagnosis can have significant individual and public health consequences as a result.
Differences with primary open angle glaucoma
By definition, NTG differs from POAG only in that the IOP is consistently less than 21 mmHg.[11] It has been postulated that POAG and NTG represent a continuum of open angle glaucomas on opposite ends of a spectrum, with IOP being the primary causative risk factor in the former and IOP-independent risk factors being more important in the latter.[11] Clinically, it is important to distinguish pure forms of POAG and NTG on each end from “mixed” disease in the middle of the spectrum. In the case of POAG, IOP reduction remains the focus of treatment. However, IOP reduction alone may be less effective in treating “mixed” or “normal tension” disease.
Aside from IOP, subtle clinical differences can help distinguish POAG from NTG. Examination of the optic disc in NTG tends to reveal a narrower neuroretinal rim for a given amount of visual field loss, particularly inferiorly and inferotemporally.[12] Disc hemorrhages and beta zone peripapillary atrophy are also more frequent findings in NTG, with the presence of disc hemorrhages being a significant poor prognostic indicator.[13,14] At the disc margin, peripapillary crescents and halos are seen more commonly as areas of absent retinal pigment epithelium with localized cupping sometimes occurring in this region.[15] These focal areas of cupping may represent acquired pits of the optic nerve (APON), which are more prevalent in the setting of “low” or “normal” tension glaucoma.[16,17] APON tend to occur in the inferior optic disc and have been associated with a greater frequency of disc hemorrhages as well as an increased risk of glaucoma progression.[17,18] Visual field deficits associated with APON tend to occur near fixation, much like the paracentral scotomas that often accompany NTG patients [Figs. 1 and 2].[17] Studies have shown that visual field defects in NTG tend to be closer to fixation, deeper and more focal when compared to those that occur in high tension POAG.[19,20] Despite these differences, reported clinical observations of POAG and NTG are conflicting, suggesting that there is likely some overlap of the underlying mechanism of glaucomatous optic neuropathy in the spectrum between these two entities.[11]
Figure 1.
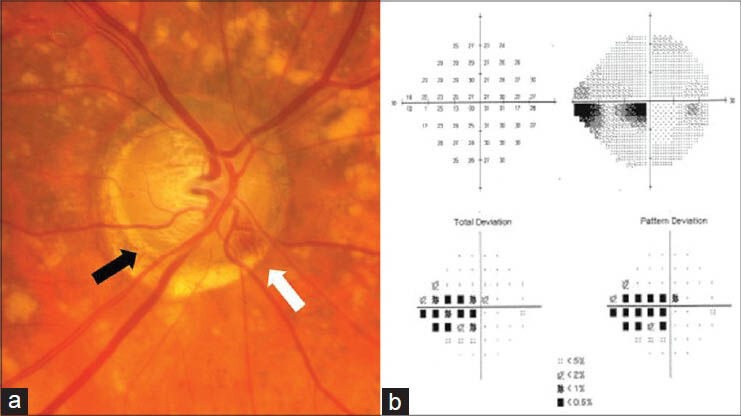
(a) Right optic nerve with an inferotemporal acquired pit of the optic nerve indicated by the black arrow. A disc hemorrhage is also seen in the inferonasal region (white arrow). (b) Standard automatedperimetry shows a corresponding superior paracentral arcuate defect with an inferior paracentral arcuate secondary to superior rim narrowing
Figure 2.
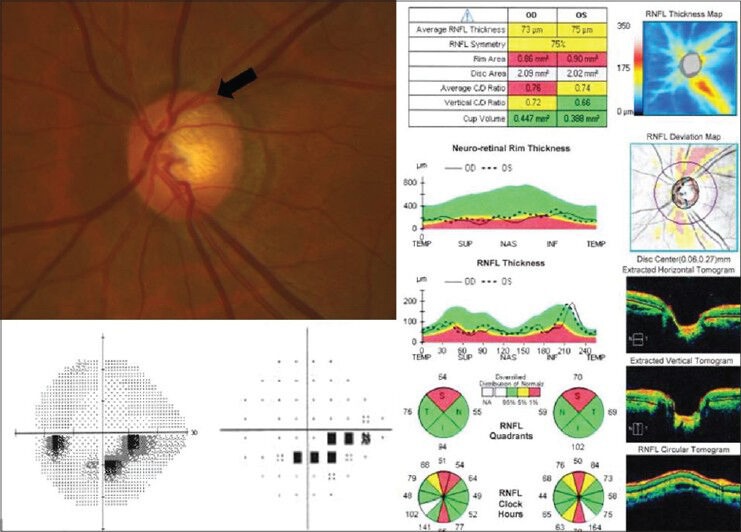
Early acquired pit of the optic nerve occurring in the superotemporal optic nerve head of the left eye (black arrow). Optical coherence tomography of the retinal nerve fiber layer shows thinning corresponding to the region of the APON. The visual field shows an inferior paracentral arcuate defect consistent with these findings
Pathophysiology of normal tension glaucoma
The pathogenesis of NTG remains unclear and it is believed that the interaction of a variety of systemic factors may be involved in the onset and progression of this disease. Harrington in 1959 was the first to suggest that impaired blood flow causes the optic nerve to be susceptible to glaucomatous damage even in the setting of statistically normal IOPs.[21] Shortly thereafter, Haas suggested that there may be reduced resistance of the optic nerve head to IOP in the setting of vascular insufficiency.[22] Subsequently, reduced peripapillary retinal blood flow has also been demonstrated in NTG.[23] More than just a simple lack of perfusion however, damage to the optic nerve head in NTG is now thought to be the result of vascular dysregulation causing repeated reperfusion injury to the optic nerve.[24] In 1968, Johnson and Drance introduced the concept of perfusion pressure suggesting that systemic hypotension could be a contributing etiologic factor in NTG,[25] and this has been supported by more recent studies as well.[26,27,28] Specifically, a diastolic ocular perfusion pressure of less than 55mmHg has been associated with a 2 to 6 fold increase in the prevalence of OAG.[29,30] In addition, the increased frequency of disc hemorrhages in NTG appears to be a strong indicator pointing toward a vascular etiology in the optic nerve damage that occurs in these patients.[11] Mroczkowska et al., recently demonstrated subclinical vascular abnormalities at both the micro- and macro-vascular level using dynamic retinal vessel analysis in newly diagnosed NTG patients.[31] Other observations suggestive of vascular and perfusion abnormalities include the increased prevalence of such systemic conditions like obstructive sleep apnea (OSA), migraine headaches and Raynaud's phenomenon in patients with NTG.[11,32,33,34,35,36] With regard to OSA, it is unclear whether treatment with continuous positive airway pressure (CPAP) decreases the risk of progression of NTG. However, nasal CPAP has been shown to cause an elevation of IOP, though the clinical significance of this finding remains to be seen.[37,38]
More recently, elevated aqueous and plasma levels of endothelin-1 (ET-1) have been linked to glaucoma.[39,40,41,42,43,44] ET-1 is an endothelium-derived vasoregulatory protein which acts as a potent endogenous vasoconstrictor, mainly on small vessels.[43,44,45] In the eye, ET-1 is synthesized and released from the ciliary processes and is believed to be involved in the modulation of ocular blood flow.[43,46] Preclinical studies have shown that intravitreal injections of ET-1 can cause hypoperfusion of the optic nerve head as well as retinal ganglion cell apoptosis in a similar manner to axotomy and other models of glaucoma.[47,48,49] These findings suggest that upregulation of ET-1 may be involved in the pathogenesis of NTG, where vascular dysregulation and other IOP-independent mechanisms seem to contribute. It is not surprising then, that studies have shown a correlation between systemic endothelial vascular cell dysfunction and vascular dysregulation in the setting of NTG and elevated ET-1.[45,50,51] Recent reports have also linked NTG and elevated ET-1 with heart rate variability and subclinical inflammation.[51,52]
Along these lines, the excitotoxic inflammatory cytokine tumor necrosis factor-alpha (TNF-α) has been shown to be upregulated by ischemic and pressure-loaded glial cells resulting in subsequent retinal ganglion cell death and reduction of TNF-α may be neuroprotective.[53,54,55] This finding suggests that there could be an underlying subclinical and highly localized inflammatory process that contributes to glaucomatous optic neuropathy. It is not surprising therefore, that some have postulated a relationship between autoimmune dysfunction and NTG. Cartwright and colleagues found that 30% of NTG patients had one or more immune-related diseases compared to only 8% of controls with ocular hypertension.[56] An increased incidence of autoantibodies and paraproteinemia have also been demonstrated in patients with NTG.[57,58,59] More specifically, an analysis of autoantibody repertoires in the sera of patients with glaucoma by Joachim and colleagues found IgG antibody patterns against retinal antigens in POAG and NTG patients,[60] and these antigen bands were identified in a subsequent study to be αB-crystallin, vimentin and heat shock protein 70.[61]
Of recent interest has been the possible role of intracranial and cerebrospinal fluid (CSF) pressure in NTG. For reasons that are still unclear, patients with NTG have been shown to have a lower CSF pressure than the normal population.[62,63] One possible explanation comes from animal studies demonstrating that the pressure gradient across the lamina cribosa can vary independently of IOP.[64,65] It is believed that lower CSF pressures increase translaminar pressure, that may contribute to glaucomatous optic neuropathy.[66] In addition, a positive linear relationship between CSF pressure and body mass index (BMI) has been reported suggesting that individuals with a lower BMI may be at increased risk of developing glaucoma in part, due to lower CSF pressures.[66] Consistent with this observation, several studies have also shown that increased BMI may be protective for glaucoma.[67,68,69,70]
Diagnosis and evaluation
Diagnostic evaluation of NTG should always begin with a thorough medical history and review of systems. Such a history may be helpful in alerting the clinician to the possibility of nonglaucomatous causes of optic neuropathy, such as ocular trauma or a history of central nervous system (CNS) pathology. It is not uncommon for such patients to communicate a history of cold extremities, migraine headaches, systemic hypotension, or other signs of vascular dysregulation.
A comprehensive eye examination, including gonioscopy, should then be performed to rule out potential secondary causes of glaucoma. Differentiation between high tension and normal tension glaucoma can be difficult based on a single IOP measurement and as a result, a diurnal curve can be helpful to detect episodes of elevated IOP outside of routine clinic visits when the IOP appears to be in the statistically normal range. CNS imaging or neuro-ophthalmic consultation is usually unnecessary in the presence of classic findings, such as APON or optic disc hemorrhages with corresponding visual field defects. As with high-tension glaucoma however, further work-up should be considered in the presence of asymmetric disease or visual field defects suggestive of a compressive CNS lesion or other nonglaucomatous processes.
A systemic evaluation for potentially contributing conditions, such as OSA or Raynaud's phenomenon, is often valuable in cases of disease progression refractory to IOP-lowering therapy. The diagnosis of NTG can even be diagnostic in some patients who were previously unaware of the presence of contributing systemic disease. As IOP-independent risk factors are believed to be significant contributors in the pathogenesis of NTG, coordination with the patient's internist to pursue further evaluation with sleep studies or 24 h ambulatory blood pressure monitoring may be insightful in select cases. Because NTG is a disease entity in which non-ocular systemic abnormalities are believed to play a significant role in disease progression, optimization of potential IOP-independent factors, such as modification of a treatment regimen for systemic hypertension in order to alleviate nocturnal hypotension can be helpful in slowing the progression of their eye disease. When the rate of disease progression remains unchanged despite optimization of both IOP and IOP-independent risk factors, the diagnosis of NTG should be reevaluated and a work-up for nonglaucomatous causes of vision loss considered.
Current treatment
IOP remains the only proven modifiable risk factor for the treatment of glaucoma. However, it was not until the Collaborative Normal-Tension Glaucoma Study (CNTGS) that the significance of IOP reduction in the setting of statistically normal IOPs was confirmed. This multicenter, clinical trial demonstrated that a 30% reduction in IOP decreased the long-term risk of progression from 35 to 12%.[71,72] It should be noted that topical beta blockers were not used and over half of all of the patients in CNTGS were able to achieve their goal IOP reduction without the use of surgery or laser trabeculoplasty. However, the study did show a greater risk of needing cataract surgery in the treatment group compared to the control group as well as faster rates of progression in women and in those patients with migraine headaches or disc hemorrhages.[73] In addition, approximately half of untreated patients did not show signs of progression over five to seven years, suggesting that the benefit of IOP reduction may be quite variable among NTG patients.[74]
More recently, the Low-Pressure Glaucoma Treatment Study (LoGTS) has raised questions about the choice of drug therapy in NTG. LoGTS was a prospective, randomized clinical trial which compared the use of timolol with brimonidine as monotherapy for NTG. Not unlike CNTGS, there was a preponderance of females and optic disc hemorrhages in the LoGTS study population.[75] After a mean follow-up of approximately 30 m, brimonidine-treated patients were less likely to have visual field progression compared to timolol-treated patients (9.1 and 39.2%, respectively) despite similar reductions in IOP by both drugs.[76] These results suggest the possibility that either brimonidine is neuroprotective or timolol is neurodestructive when used as monotherapy for NTG. The high rate of brimonidine discontinuance (28.3%) secondary to drug-related adverse events during the course of the study should be noted when interpreting these findings.
The current gold standard for medical glaucoma therapy is the prostaglandin analogues. The prostaglandin analogues are the single most effective agent in reducing IOP with adequate diurnal control.[77] Prior to the introduction of prostaglandin analogues, beta blockers were considered the first-line pressure-lowering agents used in the treatment of POAG. However, topical beta blockers have the potential for significant systemic side effects, such as nocturnal hypotension, that may be of particular concern in NTG. As mentioned, LoGTS suggests that beta blockers may even have a deleterious effect in NTG.[76] Hayreh and colleagues also found topical beta blockers to be an independent risk factor for visual field progression by aggravating nocturnal arterial hypotension in NTG patients.[78] In contrast, alpha agonists, specifically brimonidine, have been shown to be neuroprotective in animal models of optic nerve and retinal injury.[79] With the exception of the prostaglandin analogues, the commonly used classes of anti-glaucoma medications depend largely on reducing aqueous production (as opposed to increasing aqueous outflow) to lower IOP. Because aqueous production naturally decreases while asleep,[80] it is believed that aqueous suppressants have little effect on nocturnal IOP. There is a single report, however, that topical carbonic anhydrase inhibitors may provide better diurnal IOP control than timolol, although these findings have yet to be duplicated.[81] The value of nocturnal IOP reduction remains unclear as there are currently little data to support its clinical significance in glaucoma patients.
Because NTG patients have baseline IOPs in the statistically normal range, it is often difficult to achieve IOPs in the single digits with medications alone. Nonmedical options for treating NTG are the same as those used to treat “high pressure” open angle glaucoma. Laser trabeculoplasty has been found in recent studies to provide better diurnal control than some of the commonly used ocular hypotensive agents.[82,83] However, the Early Manifest Glaucoma Trail (EMGT) found that the combination of the beta blocker, betaxolol and argon laser trabeculoplasty had no significant IOP-lowering effect in NTG patients with a baseline IOP of 15mmHg or less, suggesting that laser trabeculoplasty may have a limited role in the treatment paradigm of NTG.[84]
Glaucoma filtering surgery is indicated when adequate control cannot be achieved with medical therapy or laser trabeculoplasty. Because the target IOP is often lower in NTG than in POAG, NTG patients are at greater risk for ocular hypotony and related complications, such as hypotony maculopathy, post-operatively.[85] Despite the potential complications associated with filtration surgery, trabeculectomy remains the most effective method of achieving low IOPs. In addition, trabeculectomy has been shown to blunt diurnal and nocturnal IOP fluctuations even in the setting of postural changes.[86,87] Because IOP remains relatively constant in the presence of a well-functioning bleb and is not dependent on patient compliance for adequate control, it is the authors’ experience that progression in NTG patients is often reduced even if similar IOP reductions were achieved previously with medications. Aqueous shunts are another surgical option which have become increasingly popular in the past decade and especially so after the recent Tube Versus Trabeculectomy (TVT) study.[88] The TVT study compared the outcomes and complication rates of tube shunt surgery using the Baerveldt-350 implant with trabeculectomy in eyes with a previous history of glaucoma or cataract surgery. After five years, tube shunt surgery was associated with a lower failure rate and fewer complications than trabeculectomy in previously operated eyes.[89,90] It should be noted that the TVT study did not demonstrate superiority of tube shunts over trabeculectomy as primary glaucoma surgery and such a trial is currently in progress. A similar comparison of trabeculectomy with the Ahmed glaucoma valve using more stringent criteria for success (IOP ≤18 mmHg and reduction of IOP ≥20% from baseline) showed that trabeculectomy had a significantly higher 5 year cumulative probability of success compared with Ahmed glaucoma valves when greater reductions in IOP are necessary.[91] As a result, trabeculectomy is likely a more suitable surgical option than glaucoma drainage devices in NTG, since it tends to achieve a lower post-operative IOP. The TVT study also found a lower mean IOP in the trabeculectomy group (12.6 ± 5.9 mmHg) compared to the tube shunt group (14.4 ± 6.9 mmHg) at five years, although this difference was not statistically significant in their study.[89]
More recently, the concept of “non-penetrating” glaucoma surgery has gained interest for its potential to limit some of the complications associated with more invasive procedures to lower IOP. Nonpenetrating deep sclerectomy, is one example of such a procedure in which the internal wall of Schlemm's canal is excised without entering the anterior chamber. Retrospective data by Lachkar et al., showed that nonpenetrating deep sclerectomy was associated with an IOP reduction of 33.73 ± 20.9% after 6 years with few complications.[92] Subsequent prospective studies comparing nonpenetrating deep sclerectomy directly with trabeculectomy have shown similar IOP-lowering results with improved complication rates,[93,94,95,96] suggesting that such less invasive surgical procedures may have an increasing role in the treatment of NTG and other forms of glaucoma moving forward.
Other proposed therapies
The literature clearly demonstrates that IOP modification is effective in treating NTG and that attempts to lower IOP in these patients are certainly justified. Since a significant proportion of NTG patients continue to worsen after IOP reduction, attention in recent years has shifted toward IOP-independent neuroprotective therapies. Glutamate antagonists in particular were once believed to hold promise as potential neuroprotective agents independent of IOP. Glutamate is an excitatory neurotransmitter in the CNS and retina, which is excitotoxic at high extracellular levels. Experimental models of glaucoma have shown glutamate excitotoxicity to be associated with retinal ganglion cell death,[97,98] and the inhibition of excess glutamate by blocking its receptor, N-methyl-D-aspartate (NMDA), has been proposed as a potential therapeutic target for neuroprotection in glaucoma as a result.[99] Memantine is a clinically useful drug in neurologic disorders, namely Alzheimer's dementia, whose principle mechanism of action is blockade of flow through the NMDA receptor.[100] Though multiple preclinical studies demonstrated that memantine had a protective effect in animal models of glaucoma,[101,102,103,104,105] a subsequent multicenter, randomized clinical trial failed to show the same effect in human glaucoma.[106]
Unoprostone is another drug with potential neuroprotective properties in preclinical studies. Unoprostone is a prostanoid and synthetic docosanoid that is approved by the United States Food and Drug Administration for IOP reduction in OAG and ocular hypertension through increased aqueous outflow via the trabecular meshwork.[107] Recent studies suggest that unoprostone may prolong neuronal survival independent of its ability to lower IOP, in part due to improved ocular blood flow via antagonism of ET-1.[108,109] However, further studies are needed to determine the clinical significance of these data in humans.
Calcium channel blockers (CCBs) are a class of drugs that have been widely for the treatment of systemic hypertension and other cardiovascular diseases since their introduction in the 1960s.[110] Their primary mechanism of action involves inhibition of voltage-gated calcium channels in vascular smooth muscle, which in turn leads to decreased contractility and vasodilation. Consequently, early work by Gasser and Flammer investigated the role of peripheral vasospasm in NTG as well as the potential therapeutic application of CCBs to improve ocular perfusion.[111,112,113] Subsequent reports found that centrally acting CCBs, such as nimodipine and nifedipine, improve ocular blood flow and color sensitivity in glaucoma patients.[114,115,116,117] Low-dose nifedipine has also been shown to reverse ET-1 induced ocular hemodynamic effects.[117] However, these effects on visual field and function are mixed, as some studies have reported functional improvement in these patients,[118,119] while others found no effect.[120,121] When used topically, CCBs have been shown in some animal studies to reduce IOP, although the mechanism of this action is not completely understood.[122,123,124,125] Follow-up studies examining the effect of topical verapamil have shown only a modest reduction of IOP in normal human eyes.[126,127,128,129] However, the potential benefits of CCBs have yet to be validated in randomized clinical trials and the side effects of CCBs, namely those related to peripheral edema, limit their tolerability in some patients. In addition, there is concern that systemic hypotension, caused by peripheral vasodilation in the case of CCBs, may exacerbate glaucomatous damage by decreasing diastolic ocular perfusion pressure to the optic nerve.
More recently, the widely used class of anti-cholesterol medications, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, more commonly known as “statins,” have gained interest as a potential treatment for glaucoma. In addition to lowering lipids, statins have been shown to possess an antiapoptotic and neuroprotective effect suggesting that long-term use may reduce the risk of glaucoma.[130,131,132] However, studies have demonstrated mixed results with regard to the protective effect of statins suggesting that an interventional prospective study may be helpful in elucidating the potential benefit of statins for treating NTG and other forms of glaucoma.[132,133,134]
Many investigators are now examining the potential use of nutritional supplements and phytochemicals as adjunctive therapies for glaucoma. One of the more commonly studied phytochemicals is ginkgo biloba, a unique species of trees indigenous to Korea, Japan and China. Extracts from ginkgo leaves, consisting mainly of flavonoids and terpenoids, have long been used for therapeutic purposes, particularly for its purported nootropic effects on cognition and memory through a mechanism that is believed to be related to its vasorelaxant properties.[135] Other studies have shown that ginkgo extracts may have anti-oxidative properties as well.[136,137] With regard to glaucoma, a recent study found improved peripapillary blood flow in NTG patients consuming ginkgo biloba extract when compared to control patients.[138] The ginkgo extract EGb761 has also been found to be neuroprotective in animal models of ischemic CNS injury,[139,140] while human studies have reported improved visual field indices associated with consumption of ginkgo extracts in NTG patients.[141,142,143] Another phytochemical currently under investigation is resveratrol - a naturally-occurring polyphenol commonly found in berries, nuts and the skin of red grapes with reported anti-oxidative and anti-inflammatory properties that may be beneficial in various age-related conditions, including glaucoma.[144,145] One of the ways in which resveratrol is believed to be vasoprotective is by inhibiting ET-1 synthesis.[146] Resveratrol has also been shown to be helpful in preventing damage of trabecular meshwork cells in the setting of chronic oxidative stress.[147] As with other potential therapies under investigation, these findings have yet to be validated in human studies, although the initial data are encouraging.
Conclusion
NTG represents a subtype of the open angle glaucomas with unique diagnostic characteristics and management challenges. While these patients present with statistically normal IOPs, it is clear that IOP modification is currently the best established target for treatment. Many theories have been proposed for the pathogenesis of NTG and there is mounting evidence that IOP-independent risk factors, such as vascular dysregulation, play an important contributing role. Recent research has shown that the choice of medications may be important in the overall prognosis of these patients as well. When medications are unable to achieve adequate stability of visual field progression and optic nerve damage, glaucoma filtering surgery remains the most proven option in the treatment paradigm of NTG. Ideally, future treatment of NTG will target both IOP and IOP-independent risk factors. Further research is still needed to develop clinically effective and useful IOP-independent neuroprotective agents for NTG.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Obstbaum SA, Cioffi GA, Krieglstein GK, Fennerty MB, Alm A, Araie M, et al. Gold standard medical therapy for glaucoma: Defining the criteria identifying measures for an evidence-based analysis. Clin Ther. 2004;26:2102–20. doi: 10.1016/j.clintera.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Von Graefe A. Amaurose mit sehnervenexcavation. Graefes Arch Clin Exp Ophthalmol. 1857;3:484. [Google Scholar]
- 3.Schnabel WJ. Klinische daten zur entwicklungsgeschichte der glaucomatosen. Zeitschr Augenheilkd. 1908;19:335. [Google Scholar]
- 4.Levine RZ. Low-tension glaucoma: A critical review and new material. Surv Ophthalmol. 1980;24:621–63. doi: 10.1016/0039-6257(80)90123-x. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–5. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 6.Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, Javitt J, et al. A population based evaluation of glaucoma screening: The Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–10. doi: 10.1093/oxfordjournals.aje.a116013. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–9. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 8.Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population based study in the Netherlands. The Rotterdam Study. Ophthalmology. 1994;101:1851–5. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 9.Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology. 2004;111:1641–8. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Kang SY, Kim NR, Lee ES, Hong S, Seong GJ, et al. Prevalence and characteristics of glaucoma among Korean adults. Korean J Ophthalmol. 2011;25:110–15. doi: 10.3341/kjo.2011.25.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields MB. Normal tension glaucoma: Is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–8. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- 12.Caprioli J, Spaeth GL. Comparison of the optic nerve head in high- and low-tension glaucoma. Arch Ophthalmol. 1985;103:1145–9. doi: 10.1001/archopht.1985.01050080057020. [DOI] [PubMed] [Google Scholar]
- 13.Tezel G, Kass MA, Kolker AE, Wax MB. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma and ocular hypertension. Ophthalmology. 1996;103:2105–13. doi: 10.1016/s0161-6420(96)30382-5. [DOI] [PubMed] [Google Scholar]
- 14.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707–14. doi: 10.1016/s0002-9394(00)00441-4. [DOI] [PubMed] [Google Scholar]
- 15.Buus DR, anderson DR. Peripapillary crescents and halos in normal-tension glaucoma and ocular hypertension. Ophthalmology. 1989;96:16–9. doi: 10.1016/s0161-6420(89)32930-7. [DOI] [PubMed] [Google Scholar]
- 16.Javitt JC, Spaeth GL, Katz LJ, Poryzees E, Addiego R. Acquired pits of the optic nerve: Increased prevalence in patients with low-tension glaucoma. Ophthalmology. 1990;97:1038–43. doi: 10.1016/s0161-6420(90)32466-1. [DOI] [PubMed] [Google Scholar]
- 17.Nduaguba C, Ugurlu S, Caprioli J. Acquired pits of the optic nerve in glaucoma: Prevalence and associated visual field loss. Acta Ophthalmol Scand. 1998;76:273–7. doi: 10.1034/j.1600-0420.1998.760304.x. [DOI] [PubMed] [Google Scholar]
- 18.Ugurlu S, Weitzman M, Nduaguba C, Caprioli J. Acquired pit of the optic nerve: A risk factor for progression of glaucoma. Am J Ophthalmol. 1998;125:457–64. doi: 10.1016/s0002-9394(99)80185-8. [DOI] [PubMed] [Google Scholar]
- 19.Caprioli J, Spaeth GL. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol. 1984;97:730–7. doi: 10.1016/0002-9394(84)90505-1. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan BC, Drance SM, Douglas GR, Johnson CA. Visual field damage in normal-tension and high-tension glaucoma. Am J Ophthalmol. 1989;108:636–42. doi: 10.1016/0002-9394(89)90854-4. [DOI] [PubMed] [Google Scholar]
- 21.Harrington DO. The pathogenesis of the glaucoma field: Clinical evidence that circulatory insufficiency in the optic nerve is the primary cause of visual field loss in glaucoma. Am J Ophthalmol. 1959;47:177–85. [PubMed] [Google Scholar]
- 22.Haas JS. Low tension glaucoma. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1962;43:153–60. [PubMed] [Google Scholar]
- 23.Chung HS, Harris A, Kagemann L, Martin B. Peripapillary retinal blood flow in normal tension glaucoma. Br J Ophthalmol. 1999;83:466–9. doi: 10.1136/bjo.83.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DR. Glaucoma, capillaries and pericytes: 1. Blood flow regulation. Ophthalmologica. 1996;210:257–62. doi: 10.1159/000310722. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DG, Drance SM. Some studies on the circulation in patients with advanced open angle glaucoma. Can J Ophthalmol. 1968;3:149–53. [PubMed] [Google Scholar]
- 26.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113:216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 27.Leske MC, Wu SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954–9. doi: 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- 28.Choi J, Jeong J, Cho H, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: A risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47:831–36. doi: 10.1167/iovs.05-1053. [DOI] [PubMed] [Google Scholar]
- 29.Leske MC. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiological findings. Curr Opin Ophthalmol. 2009;20:73–8. doi: 10.1097/ICU.0b013e32831eef82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z Early Manifest Glaucoma Trial Group. Predictors of long term progression in the early manifest glaucoma trial. Ophthalmology. 2007;11:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Mroczkowska S, Ekart A, Sung V, Negi A, Qin L, Patel SR, et al. Coexistence of macro- and micro-vascular abnormalities in newly diagnosed normal tension glaucoma patients. Acta Ophthalmol. 2012;90:e553–9. doi: 10.1111/j.1755-3768.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 32.Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106:1009–12. doi: 10.1016/S0161-6420(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 33.Mojon DS, Hess CW, Goldblum D, Boehnke M, Koerner F, Gugger M, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2002;216:180–4. doi: 10.1159/000059625. [DOI] [PubMed] [Google Scholar]
- 34.Sergi M, Salerno DE, Rizzi M, Blini M, andreoli A, Messenio D, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16:42–6. doi: 10.1097/01.ijg.0000243472.51461.24. [DOI] [PubMed] [Google Scholar]
- 35.Drance S, anderson DR, Schulzer M Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 36.Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci. 1985;26:1105–8. [PubMed] [Google Scholar]
- 37.Alvarez-Sala R, Diaz S, Prados C. Increase of intraocular pressure during nasal CPAP. Chest. 1992;101:1477. doi: 10.1378/chest.101.5.1477-a. [DOI] [PubMed] [Google Scholar]
- 38.Kiekens S, Groot VD, Coecklbergh T, Tassignon MJ, van de HP, Backer WD, et al. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49:934–40. doi: 10.1167/iovs.06-1418. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser HJ, Flammer J, Wenk M, Luscher T. Endothelin-1 plasma levels in normal-tension glaucoma: Abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol. 1995;233:484–8. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- 40.Tezel G, Kass MA, Kolker AE, Becker B, Wax MB. Plasma and aqueous endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6:83–9. [PubMed] [Google Scholar]
- 41.Iwabe S, Lamas M, Vasquez Pelaez CG, Carrasco FG. Aqueous humor endothelin-1 (Et-1), vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2) in Mexican glaucomatous patients. Curr Eye Res. 2010;35:287–94. doi: 10.3109/02713680903545315. [DOI] [PubMed] [Google Scholar]
- 42.Sin BH, Song BJ, Park SP. Aqueous vascular endothelial growth factor and endothelin-1 levels in branch retinal vein occlusion associated with normal tension glaucoma. J Glaucoma. 2013;22:104–9. doi: 10.1097/IJG.0b013e3182312047. [DOI] [PubMed] [Google Scholar]
- 43.Källberg ME, Brooks DE, Garcia-Sanchez GA, Komaromy AM, Szabo NJ, Tian L. Endothelin 1 levels in the aqueous humor of dogs with glaucoma. J Glaucoma. 2002;11:105–9. doi: 10.1097/00061198-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ghanem AA, Elewa AM, Arafa LF. Endothelin-1 and nitric oxide levels in patients with glaucoma. Ophthalmic Res. 2011;46:98–102. doi: 10.1159/000323584. [DOI] [PubMed] [Google Scholar]
- 45.Galassi F, Giambene B, Varriale R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4467–71. doi: 10.1167/iovs.10-6710. [DOI] [PubMed] [Google Scholar]
- 46.Wollensak G, Schaefer HE, Ihling C. An immunohistochemical study of endothelin-1 in the human eye. Curr Eye Res. 1998;17:541–5. doi: 10.1076/ceyr.17.5.541.5187. [DOI] [PubMed] [Google Scholar]
- 47.Sasaoka M, Taniguchi T, Shimazawa M, Ishida N, Shimazaki A, Hara H. Intravitreal injection of endothelin-1 caused optic nerve damage following to ocular hypoperfusion in rabbits. Exp Eye Res. 2006;83:629–37. doi: 10.1016/j.exer.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Lau J, Dang M, Hockmann K, Ball AK. Effects of acute delivery of endothelin-1 on retinal ganglion cell loss in the rat. Exp Eye Res. 2006;82:132–45. doi: 10.1016/j.exer.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi T, Shimazawa M, Sasaoka M, Shimazaki A, Hara H. Endothelin-1 impairs retrograde axonal transport and leads to axonal injury in rat optic nerve. Curr Neurovasc Res. 2006;3:81–8. doi: 10.2174/156720206776875867. [DOI] [PubMed] [Google Scholar]
- 50.Buckley C, Hadoke PW, Henry E, O’Brien C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br J Ophthalmol. 2002;86:227–32. doi: 10.1136/bjo.86.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cellini M, Strobbe E, Gizzi C, Balducci N, Toschi PG, Campos EC. Endothelin-1 plasma levels and vascular endothelial dysfunction in primary open angle glaucoma. Life Sci. 2012;91:699–702. doi: 10.1016/j.lfs.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Lee NY, Park HY, Na KS, Park SH, Park CK. Association between heart rate variability and systemic endothelin-1 concentration in normal-tension glaucoma. Curr Eye Res. 2012;38:516–9. doi: 10.3109/02713683.2012.745881. [DOI] [PubMed] [Google Scholar]
- 53.Tezel G, Wax MB. Increased production of tumor necrosis factor-α by glial cells exposed to stimulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 2010;51:903–6. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- 55.Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cerebr Blood Flow Metab. 1996;16:378–84. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Cartwright MJ, Grajewski AL, Friedberg ML, anderson DR, Richards DW. Immune-related disease and normal-tension glaucoma. A case-control study. Arch Ophthalmol. 1992;110:500–2. doi: 10.1001/archopht.1992.01080160078035. [DOI] [PubMed] [Google Scholar]
- 57.Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1994;117:561–8. doi: 10.1016/s0002-9394(14)70059-5. [DOI] [PubMed] [Google Scholar]
- 58.Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1968–75. [PubMed] [Google Scholar]
- 59.Grus FH, Joachim SC, Hoffman EM, Pfeiffer N. Complex autoantibody repertoires in patients with glaucoma. Mol Vis. 2004;25:132–7. [PubMed] [Google Scholar]
- 60.Joachim SC, Grus FH, Pfeiffer N. Analysis of autoantibody repertoires in sera of patients with glaucoma. Eur J Ophthalmol. 2003;13:752–8. doi: 10.1177/1120672103013009-1003. [DOI] [PubMed] [Google Scholar]
- 61.Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Antibodies to alpha B-crystalllin, vimentin and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr Eye Res. 2007;32:501–9. doi: 10.1080/02713680701375183. [DOI] [PubMed] [Google Scholar]
- 62.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma and ocular hypertension: A case-control study. Invest Ophthalmol Vis Sci. 2008;49:5412–8. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren R, Jonas JB, Tian G. Cerebrospinal fluid pressure in glaucoma: A prospective study. Ophthalmology. 2010;117:259–66. doi: 10.1016/j.ophtha.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 64.Morgan WH, Yu DY, Alder VA, Cringle SJ, Cooper RL, House PH, et al. The correlation between the cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998;39:1419–28. [PubMed] [Google Scholar]
- 65.Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci. 1995;36:1163–72. [PubMed] [Google Scholar]
- 66.Berdahl JP, Fleischman D, Zadylarova J, Stinnett S, Allingham RR, Fautsch MP. Body mass index has a linear relationship with cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2012;53:1422–7. doi: 10.1167/iovs.11-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asrani S, Samuels B, Thakur M, Santiago C, Kuchibhatla M. Clinical profiles of primary open angle glaucoma versus normal tension glaucoma patients: A pilot study. Curr Eye Res. 2011;36:429–35. doi: 10.3109/02713683.2011.559563. [DOI] [PubMed] [Google Scholar]
- 68.Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113:918–24. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 69.Pasquale LP, Willett WC, Rosner BA, Kang JE. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology. 2010;117:1521–9. doi: 10.1016/j.ophtha.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramdas WD, Wolfs RC, Hofman A, de Jong PT, Vingerling JR, Jansonius NM. Lifestyle and risk of developing open-angle glaucoma: The Rotterdam Study. Arch Ophthalmology. 2011;129:767–72. doi: 10.1001/archophthalmol.2010.373. [DOI] [PubMed] [Google Scholar]
- 71.Collaborative Normal-tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 72.The Collaborative Normal-tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 73.Drance S, anderson DR, Schulzer M Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 74.Drance S, anderson DR, Schulzer M Collaborative Normal-Tension Glaucoma Study Group. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–53. doi: 10.1016/s0161-6420(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 75.Krupin T, Liebmann JM, Greenfield DS, Rosenberg LF, Ritch R, Yang JW Low-Pressure Glaucoma Study Group. The low-pressure glaucoma treatment study (LoGTS) study design and baseline characteristics of enrolled patients. Ophthalmology. 2005;112:376–85. doi: 10.1016/j.ophtha.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 76.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–81. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Gulati V, Fan S, Zhao M, Maslonka MA, Gangahar C, Toris CB. Diurnal and nocturnal variations in aqueous humor dynamics of patients with ocular hypertension undergoing medical therapy. Arch Ophthalmol. 2012;130:677–84. doi: 10.1001/archophthalmol.2011.2573. [DOI] [PubMed] [Google Scholar]
- 78.Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol. 1999;128:301–19. doi: 10.1016/s0002-9394(99)00160-9. [DOI] [PubMed] [Google Scholar]
- 79.Saylor M, McLoon HK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: An evidence-based review. Arch Ophthalmol. 2009;127:402–6. doi: 10.1001/archophthalmol.2009.9. [DOI] [PubMed] [Google Scholar]
- 80.Reiss GR, Lee DA, Topper JE, Brubaker RF. Aqueous humor flow during sleep. Invest Ophthalmol Vis Sci. 1984;25:776–8. [PubMed] [Google Scholar]
- 81.Liu JH, Medeiros FA, Slight JR, Weinreb RH. Comparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapy. Ophthalmology. 2009;116:449–54. doi: 10.1016/j.ophtha.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 82.Lee AC, Mosaed S, Weinreb RN, Kripke DF, Liu JH. Effect of laser trabeculoplasty on nocturnal intraocular pressure in medically treated glaucoma patients. Ophthalmology. 2007;114:666–70. doi: 10.1016/j.ophtha.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 83.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: The effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2009;93:497–501. doi: 10.1136/bjo.2008.148510. [DOI] [PubMed] [Google Scholar]
- 84.Heijl A, Leske MC, Hyman L, Yang Z, Bengtsson B EMGT Group. Intraocular pressure reduction with a fixed treatment protocol in the Early Manifest Glaucoma Trial. Acta Ophtalmol. 2011;89:749–54. doi: 10.1111/j.1755-3768.2009.01852.x. [DOI] [PubMed] [Google Scholar]
- 85.Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993;116:314–26. doi: 10.1016/s0002-9394(14)71349-2. [DOI] [PubMed] [Google Scholar]
- 86.Klink T, Praetorius S, Leippi S, Klink J, Grehn FJ. Diurnal and nocturnal intraocular pressure fluctuations after trabeculectomy. Ophthalmologica. 2012;227:160–5. doi: 10.1159/000333099. [DOI] [PubMed] [Google Scholar]
- 87.Hirooka K, Takenaka H, Baba T, Takagishi M, Mizote M, Shiraga F. Effect of trabeculectomy on intraocular pressure fluctuation with postural change in eyes with open-angle glaucoma. J Glaucoma. 2009;18:689–91. doi: 10.1097/IJG.0b013e31819c49f4. [DOI] [PubMed] [Google Scholar]
- 88.Desai MA, Gedde SJ, Feuer WJ, Shi W, Chen PP, Parrish RK., 2nd Practice preferences for glaucoma surgery: A survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42:202–8. doi: 10.3928/15428877-20110224-04. [DOI] [PubMed] [Google Scholar]
- 89.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy Study after five years of follow-up. Am J Ophthalmol. 153:789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC Tube versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy Study (TVT) during five years of follow-up. Am J Ophthalmol. 2012;153:804–14. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tran DH, Souza C, Ang MJ, Loman J, Law SK, Coleman AL, et al. Comparison of long-term surgical success of Ahmed Valve implant versus trabeculectomy in open-angle glaucoma. Br J Ophthalmol. 2009;93:1504–9. doi: 10.1136/bjo.2008.150870. [DOI] [PubMed] [Google Scholar]
- 92.Lachkar Y, Neverauskiene J, Jeanteur-Lunel MN, Gracies H, Berkani M, Ecoffet M, et al. Nonpenetrating deep sclerectomy: A 6-year retrospective study. Eur J Ophthalmol. 2004;14:26–36. doi: 10.1177/112067210401400105. [DOI] [PubMed] [Google Scholar]
- 93.El Sayyad F, Helal M, El-Kholify H, Khalil M, El-Maghraby A. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107:1671–4. doi: 10.1016/s0161-6420(00)00263-3. [DOI] [PubMed] [Google Scholar]
- 94.Cillino S, Di Pace F, Casuccio A, Lodato G. Deep sclerectomy versus punch trabeculectomy: Effect of low-dosage mitomycin C. Ophthalmologica. 2005;219:281–6. doi: 10.1159/000086112. [DOI] [PubMed] [Google Scholar]
- 95.Russo V, Scott IU, Stella A, Balducci F, Cosma A, Barone A, et al. Nonpenetrating deep sclerectomy with reticulated hyaluronic acid implant versus punch trabeculectomy: A prospective clinical trial. Eur J Ophthalmol. 2008;18:751–7. doi: 10.1177/112067210801800515. [DOI] [PubMed] [Google Scholar]
- 96.Leszczynski R, Forminska-Kapuscik M, Bubula-Stachowicz B, Mrukwa-Kominek E, Filipek E, Pawlicki K. Nonpenetrating very deep sclerectomy with hyaluronic acid implant vs trabeculectomy—A 2-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2012;250:1835–41. doi: 10.1007/s00417-012-1985-9. [DOI] [PubMed] [Google Scholar]
- 97.Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–43. [PubMed] [Google Scholar]
- 98.Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S142–50. doi: 10.1016/s0039-6257(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 99.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–33. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–7. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Kim TW, Kim DM, Park KH, Kim H. Neuroprotective effect of memantine in a rabbit model of optic nerve ischemia. Korean J Ophthalmol. 2002;16:1–7. doi: 10.3341/kjo.2002.16.1.1. [DOI] [PubMed] [Google Scholar]
- 102.Schuettauf F, Quinto K, Naskar R, Zurakowski D. Effects of antiglaucoma medications on ganglion cell survival: The DBA/2J mouse model. Vis Res. 2002;42:2333–7. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- 103.Hare WA, WoldeMussie E, Weinreb RN, Ton H, Ruiz G, Wijono M, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Invest Ophthalmol Vis Sci. 2004;45:2640–51. doi: 10.1167/iovs.03-0567. [DOI] [PubMed] [Google Scholar]
- 104.Hare WA, WoldeMussie E, Lai RK, Ton H, Ruiz G, Chun T, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest Ophthalmol Vis Sci. 2004;45:2625–39. doi: 10.1167/iovs.03-0566. [DOI] [PubMed] [Google Scholar]
- 105.Yucel YH, Gupta N, Zhang Q, Mizisin AP, Kalichman MW, Weinreb RW. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol. 2006;124:217–25. doi: 10.1001/archopht.124.2.217. [DOI] [PubMed] [Google Scholar]
- 106.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009;87:450–4. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 107.Harms NV, Toris CB. Current status of unoprostone for the management of glaucoma and the future of its use in the treatment of retinal disease. Expert Opin Pharmacother. 2013;14:105–13. doi: 10.1517/14656566.2013.748038. [DOI] [PubMed] [Google Scholar]
- 108.Polska E, Doelemeyer A, Luksch A, Ehrlich P, Kaehler N, Percicot CL, et al. Partial antagonism of endothelin 1-induced vasoconstriction in the human choroid by topical unoprostone isopropyl. Arch Ophthalmol. 2002;120:348–52. doi: 10.1001/archopht.120.3.348. [DOI] [PubMed] [Google Scholar]
- 109.Munemasa Y, Kitaoka Y, Hayashi Y, Takeda H, Fujino H, Ohtani-Kaneko R, et al. Effects of unoprostone on phosphorylated extracellular signal-regulated kinase expression in endothelin 1-induced retinal and optic nerve damage. Vis Neurosci. 2008;25:197–208. doi: 10.1017/S095252380808053X. [DOI] [PubMed] [Google Scholar]
- 110.Araie M, Mayama C. Use of calcium channel blockers for glaucoma. Prog Retin Eye Res. 2011;30:54–71. doi: 10.1016/j.preteyeres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Gasser P, Flammer J. Influence of vasospasm on visual function. Doc Ophthalmol. 1987;66:3–18. doi: 10.1007/BF00144735. [DOI] [PubMed] [Google Scholar]
- 112.Gasser P, Flammer J. Short- and long-term effect of nifedipine on the visual field in patients with presumed vasospasm. J Int Med Res. 1990;18:334–9. doi: 10.1177/030006059001800411. [DOI] [PubMed] [Google Scholar]
- 113.Gasser P, Flammer J, Guthauser U, Mahler F. Do vasospasms provoke ocular diseases? Angiology. 1990;41:213–20. doi: 10.1177/000331979004100306. [DOI] [PubMed] [Google Scholar]
- 114.Bose S, Piltz JR, Breton ME. Nimodipine, a centrally active calcium antagonist, exerts a beneficial effect on contrast sensitivity in patients with normal-tension glaucoma and in control subjects. Ophthalmology. 1995;102:1236–41. doi: 10.1016/s0161-6420(95)30884-6. [DOI] [PubMed] [Google Scholar]
- 115.Luksch A, Rainer G, Koyuncu D, Ehrlich P, Maca T, Gschwandtner ME, et al. Effect of nimodipine on ocular blood flow and colour contrast sensitivity in patients with normal tension glaucoma. Br J Ophthalmol. 2005;89:221–5. doi: 10.1136/bjo.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michalk F, Michelson G, Harazny J, Werner U, Daniel WG, Werner D. Single-dose nimodipine normalizes impaired retinal circulation in normal tension glaucoma. J Glaucoma. 2004;13:158–62. doi: 10.1097/00061198-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 117.Strenn K, Matulla B, Wolzt M, Findl O, Bekes MC, Lamsfuss U, et al. Reversal of endothelin-1 induced ocular hemodynamic effects by low-dose nifedipine in humans. Clin Pharmacol Ther. 1998;63:54–63. doi: 10.1016/S0009-9236(98)90121-7. [DOI] [PubMed] [Google Scholar]
- 118.Piltz JR, Bose S, Lanchoney D. The effect of nimodipine, a centrally active calcium antagonist, on visual function and macular blood flow in patients with normal-tension glaucoma and in control subjects. J Glaucoma. 1998;7:336–42. [PubMed] [Google Scholar]
- 119.Gaspar AZ, Flammer J, Hendrickson P. Influence of nifedipine on the visual fields of patients with optic-nerve-head diseases. Eur J Ophthalmol. 1994;4:24–8. doi: 10.1177/112067219400400105. [DOI] [PubMed] [Google Scholar]
- 120.Harris A, Evans DW, Cantor LB, Martin B. Hemodynamic and visual function effects of oral nifedipine in patients with normal-tension glaucoma. Am J Ophthalmol. 1997;124:296–302. doi: 10.1016/s0002-9394(14)70821-9. [DOI] [PubMed] [Google Scholar]
- 121.Rainer G, Kiss B, Dallinger S, Findl O, Georgopolous M, Vass C, et al. A double masked placebo controlled study on the effect of nifedipine on optic nerve blood flow and visual field function in patients with open angle glaucoma. Br J Clin Pharmacol. 2001;52:210–2. doi: 10.1046/j.0306-5251.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santafe J, De Ibarreta MJ, Segarra J, Melena J. A long-lasting hypotensive effect of topical diltiazem on the intraocular pressure in conscious rabbits. Naunyn-Schmiedebergs Arch Pharmacol. 1997;355:645–50. doi: 10.1007/pl00004996. [DOI] [PubMed] [Google Scholar]
- 123.Santafe J, De Ibarreta MJ, Segarra J, Melena J. The effect of topical diltiazem on hypertension induced by water loading in rabbits. Gen Pharmacol. 1999;32:201–5. doi: 10.1016/s0306-3623(98)00196-7. [DOI] [PubMed] [Google Scholar]
- 124.Melena J, Santafe J, Segarra J. The effect of topical diltiazem on the intraocular pressure in betamethasone-induced ocular hypertensive rabbits. J Pharmacol Exp Ther. 1998;284:278–82. [PubMed] [Google Scholar]
- 125.Siegner SW, Netland PA, Schroeder A, Erickson KA. Effect of calcium channel blockers alone and in combination with antiglaucoma medications on intraocular pressure in the primate eye. J Glaucoma. 2000;9:334–9. doi: 10.1097/00061198-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 126.Segarra J, Santafe J, De Ibarreta MJ. The topical application of verapamil and nifedipine lowers intraocular pressure in conscious rabbits. Gen Pharmacol. 1993;24:1163–71. doi: 10.1016/0306-3623(93)90364-4. [DOI] [PubMed] [Google Scholar]
- 127.Netland PA, Feke GT, Konno S, Goger DG, Fujio N. Optic nerve head circulation after topical calcium channel blocker. J Glaucoma. 1996;5:200–6. [PubMed] [Google Scholar]
- 128.Netland PA, Grosskreutz CL, Feke GT, Hart LJ. Color Doppler ultrasound analysis of ocular circulation after topical calcium channel blocker. Am J Ophthalmol. 1995;119:694–700. doi: 10.1016/s0002-9394(14)72772-2. [DOI] [PubMed] [Google Scholar]
- 129.Abreu MM, Kim YY, Shin DH, Netland PA. Topical verapamil and episcleral venous pressure. Ophthalmology. 1998;105:2251–5. doi: 10.1016/S0161-6420(98)91224-6. [DOI] [PubMed] [Google Scholar]
- 130.Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: General mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24:79–95. [PubMed] [Google Scholar]
- 131.Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurons. J Neurochem. 2005;92:1386–98. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- 132.Ostrowski SM, Wilkinson BL, Golde TE, Landreth G. Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem. 2007;282:26832–44. doi: 10.1074/jbc.M702640200. [DOI] [PubMed] [Google Scholar]
- 133.Owen CG, Carey IM, Shah S, de Wilde S, Wormald R, Whincup PH, et al. Hypotensive medications, statins and the risk of glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3524–30. doi: 10.1167/iovs.09-4821. [DOI] [PubMed] [Google Scholar]
- 134.Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119:2074–81. doi: 10.1016/j.ophtha.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: An adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390–402. [PMC free article] [PubMed] [Google Scholar]
- 136.Ou HC, Lee WJ, Lee IT, Chiu TH, Tsai KL, Lin CY, et al. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J Appl Physiol. 2009;106:1674–85. doi: 10.1152/japplphysiol.91415.2008. [DOI] [PubMed] [Google Scholar]
- 137.Eckert A, Keil U, Scherping I, Hauptmann S, Muller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by ginkgo biloba extract EGb 761. Ann N Y Acad Sci. 2005;1056:474–85. doi: 10.1196/annals.1352.023. [DOI] [PubMed] [Google Scholar]
- 138.Park JW, Kwon HJ, Chung WS, Kim CY, Seong GJ. Short-term effects of gingko biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J Ophthalmol. 2011;25:323–8. doi: 10.3341/kjo.2011.25.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung SY, Cheng FC, Lee MS, Lin JY, Lin MC, Wang MF. Ginkgo biloba leaf extract (EGb761) combined with neuroprotective agents reduces the infarct volumes of gerbil ischemic brain. Am J Chin Med. 2006;34:803–17. doi: 10.1142/S0192415X06004302. [DOI] [PubMed] [Google Scholar]
- 140.Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–55. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110:359–62. doi: 10.1016/S0161-6420(02)01745-1. [DOI] [PubMed] [Google Scholar]
- 142.Lee J, Sohn SW, Kee C. Effect of gingko biloba extract on visual field progression in normal tension glaucoma. J Glaucoma. 2012;22:780–4. doi: 10.1097/IJG.0b013e3182595075. [DOI] [PubMed] [Google Scholar]
- 143.Shim SH, Kim JM, Choi CY, Kim CY, Park KH. Gingko biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J Med Food. 2012;15:818–23. doi: 10.1089/jmf.2012.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 145.Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Ragione FD. Resveratrol: From Basic Science to the Clinic. Cell Cycle. 2007;6:2495–510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- 146.Mozaffarieh M, Grieshaber MC, Orgul S, Flammer J. The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol. 2008;53:479–505. doi: 10.1016/j.survophthal.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 147.Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


