Abstract
Purpose:
To study the neuroprotective effect of topical citicoline.
Materials and Methods:
Experimental phase to evaluate the ability of citicoline eye drops to reach the vitreous and the retina: The right eyes of 5 mice CD1 were treated with two drops per day for three days of citicoline 1% and 2% (OMK1, Omikron Italia s.r.l.), and then the vitreous was analyzed with the liquid chromatography and spectrometry mass (LC-MS/MS). Clinical phase to determine if topical citicoline is able to delay glaucoma progression, considering perimetric parameters and electro functional tests. Patients were randomized in two groups, OMK1 and OAG. The first group was treated with OMK1 three times per day, plus hypotensive therapy for two months and one month of wash out. The second group was treated only with hypotensive treatment for three months.
Results:
LC-MS/MS detected the molecule very well, and only OMK1 showed systemic absorption. Thirty-four patients were enrolled, 16 in the OMK1 and 18 in the OAG group. Perimetric parameters showed a positive trend in individual eyes of patients in OMK1 group, but these values were not statistically significant in the whole group. Retinal ganglion cells function improved as shown by reduced P50 latency (P = 0.04) and increased P50-N95 amplitude (P < 0.0001) of pattern electroretinogram, up to 30 days after the washout (P = 0.01; P = 0.002). Visual evoked potential and retino-cortical time improvement regressed after 30 days of washout. In OAG group, there was any change during the follow-up. No adverse reactions were reported in both groups.
Conclusions:
Topical citicoline seems to have a neuroprotective action.
Keywords: Citicoline, glaucoma, neuroprotection, progression
Glaucoma is a chronic degenerative disease that affects the optic nerve, characterized by a progressive loss of retinal ganglion cells (RGCs) and their axons.[1,2]
Many studies have paid attention to RGCs and the mechanisms of susceptibility and degeneration in the retina, optic nerve, and brain leading to generate new approaches to glaucoma treatment focused on neuroprotection.[3,4]
Molecules such as brimonidine, memantine, calcium antagonists, and citidin difosfocolina-5-(citicoline) have been investigated due to their neuroprotective effect. The latter is the most cited in the scientific literature.[5]
Citicoline is a mononucleotide formed by ribose, cytosine, pyrophosphate, and choline, the chemical structure of which corresponds to the 2-oxo-4 aminopirimidina. It is a natural precursor of phosphatidylcholine (PDC), the main component of neuronal and mitochondrial membranes. Citicoline is a water-soluble compound with greater than 90% bioavailability.
Citicoline in the brain acts primarily as a substrate for the formation of PDC and as an inhibitor of Phospholipase A2 (PLP-A2), thus having a direct action on damaged neuron membrane still viable. Moreover, this molecule has neuromodulatory action mainly dependent on the dopamine system, which provides the rational for use of citicoline in the treatment of Parkinson's disease, as well as in glaucoma, as a major neurotransmitter involved in both retinal and post-retinal signal vision transmission.[5]
Citicoline is currently widely used in addition to the hypotensive therapy for the treatment of glaucoma. It has a neuroprotective effect on RGCs from dying over the long term and a neuroenhancing effect on RGC function in the short term.[6]
A series of clinical trials have demonstrated improvement on visual field testing, visual evoked potential, and pattern electroretinogram in patients with glaucoma. Furthermore, a very recent study seems to indicate that supplementation with 500 mg of citicoline in oral solution might significantly slow down glaucomatous rates of progression.[7]
In all these studies, it was administered orally or intramuscularly.[8,9,10,11,12,13]
The purpose of this study is to evaluate the neuroprotective effect of citicoline eye drops and the benefit of the topical administration.
Materials and Methods
This study adhered to the Declaration of Helsinki, and it was approved by local ethics committee.
The study was divided into two phases: The first, experimental, demonstrating the presence of the molecule into the vitreous cavity following topical administration in laboratory animals, the second, clinical (prospective, randomized, controlled, double-blind) to test the effectiveness of this new route of administration in patients with glaucoma. In particular, the primary objective of the clinical phase was the assessment of the potential action of topical citicoline, in addition to hypotensive eye drops, in the maintenance and stabilization of the visual field defects in glaucomatous patients in progression. Secondary objectives included the evaluation of retinal ganglion cell function by pattern electroretinogram (PERG) and optic nerve through visual evoked potential (VEP), and tolerability to the eye drops.
To exclude any interference of citicoline treatment on animal wellbeing, basic hematological parameters of all animals were investigated and compared with experimental controls and with data already available in the literature. Treatment and experimental handling of animals were done also according with the Italian directive (Decree 116/92). A veterinary surgeon controlled the health status of the animals in order to avoid physical injury, suffering, and distress.
The experiment was performed on five CD1 mice (weight of each mouse approximately 30 g) so divided:
Group A) Two mice treated with a solution of 1% citicoline, 0.2% high molecular weight hyaluronic acid (HA), and 0.01% benzalkonium chloride (BAK)
Group B) Two mice treated with a solution of 2% citicoline, 0.2% HA, and 0.01% BAK (OMK1, Omikron Italia s.r.l.)
Group C) One control mouse.
The right eye (RE) of each mouse in group A and B was treated with two drops twice-a-day for three days. On the fourth day after sacrificing the animal, the eyes were taken, rinsed in saline, and stored in ice. The RE of control mouse was damaged and not available for further analysis. After aspirating the vitreous with a SGE Analytical Science 100 μL syringe, it was diluted with 20 μL of distilled water. Subsequently, the vitreous was centrifuged and the 50 μL of solution 50% water and 50% methanol were added to the collected supernatant and passed by vortex.
For the sample analysis, liquid chromatography and mass spectrometry (LC-MS/MS) was used. Instrumentation employed consisted of triple-quadruple mass spectrometer (Applied Biosystems API 3000) equipped with a turbo ion-spray and coupled to an UPLC (Dionex Ultimate 3000 RS). In a preliminary stage, chromatographic separation and mass spectrometry identification has been optimized through the use of a pure grade citicoline standard.
Once set all instrumental and the analytical parameters to get the best conditions, the results were evaluated respect to the therapeutic range expected. This was achieved by mean of a calibration curve built by deuterated citicoline standard solutions made at fixed concentrations.
The vitreous was added with an equal volume of water/methanol solution, containing deuterated-citicoline. Then, the sample obtained was processed though the previously described LC-MS/MS system.
Between tests, the machine had always done white readings to avoid contamination.
Clinical phase
Inclusion criteria to take part in the study were: Age between 20 and 60 years old, best corrected visual acuity ≥5/10, and diagnosis of progressive open-angle glaucoma (loss of 1 db/year). Glaucoma was defined by glaucomatous visual field defect (Humphrey 24-2 SITA Standard, Carl Zeiss Meditec, Inc) with mean deviation (MD) up to 12 dB, glaucomatous optic disc, and IOP less than 21 mmHg on hypotensive treatment.
Exclusion criteria were considered as follows: Ocular surgery in the last three months, argon laser trabeculoplasty in the last six months, cataract or maculopathy, hypersensitivity to citicoline, secondary ocular hypertension (i.e. due to steroids), history of ocular disease, changes in systemic therapy affecting IOP, use of statin, pregnancy or nursing, failure to use contraceptive, diabetes, arthritis, use of anticoagulant and lithium.
The study is supposed to last 24 months. As these patients were progressing, we decided to perform an interim analysis after three months. During this period, patients underwent three visits, at baseline, after 60 days, and after 90 days.
An additional analysis will be performed after six months.
Patients were randomized in two groups, OMK1, treated with OMK1 one drop three times a day plus hypotensive drops for two months and one month of wash out, and OAG, treated only with hypotensive drops for three months.
The randomization was masked to the clinicians and the patients. It was made dividing patients into two groups with similar characteristics including age, refraction, and glaucomatous damage. At baseline and at each visit, a comprehensive ophthalmological examination was performed, including corrected visual acuity, biomicroscopy, indirect ophthalmoscopy, IOP measurement with Goldmann applanation tonometer, perimetry white on white (Humphrey 24-2 SITA Standard), recording of PERG and VEP.
The following variables were considered:
Visual field test: MD and pattern standard deviation (PSD)
PERG: Latency P50 and amplitude P50-N95
PEV: Latency N75 and P100, amplitude N75-P100 and P100-N145, and retinocortical time (RTC).
MD and PSD values were analyzed using Wilcoxon sign rank test. PERG and VEP responses between groups A and B were examined by analysis of variance (ANOVA).
The ANOVA was also used for repeated measurements of each parameter in the follow-up compared with baseline.
A P < 0.01 to compensate for multiple comparisons was considered statistically significant.
The safety and tolerability of topical citicoline were analyzed evaluating the anterior segment of the patient at each visit. In each patient, the presence of ocular manifestations was evaluated using a score from 0 to 4 (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) to indicate the presence and intensity of adverse ocular manifestations observed: Conjunctival hyperemia, secretion, inflammation, keratopathy, scleritis, uveitis, cataract, ocular hypertension.
Patients were also asked to express an evaluation using the same score (from 0 to 4) to indicate the presence and intensity of ocular symptoms associated with the instillation of the product: Burning, itching, foreign body sensation, diffuse pain, blurred vision.
Results
Experimental phase
The concentration of citicoline has been calculated for comparison with the calibration curve previously acquired, and the following graph was obtained in which the peak corresponds to the presence of citicoline in the vitreous [Figure 1].
Figure 1.
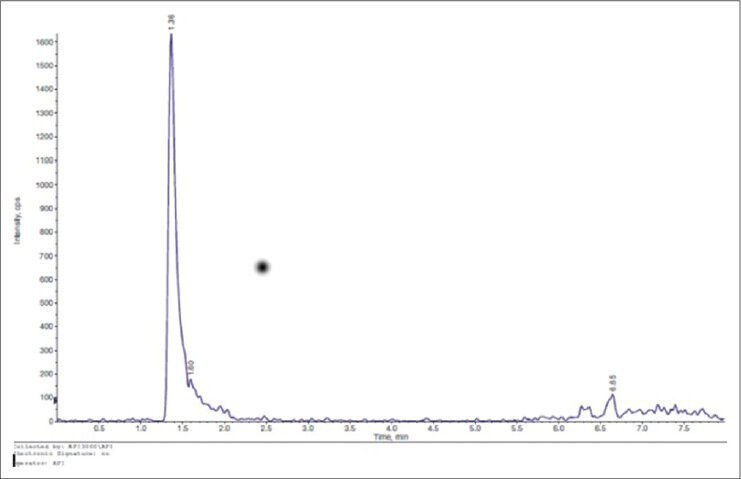
The graph shows the spectrometry result: The peak corresponds to the presence of citicoline in the vitreous.
The machine detected the molecule very well as the signal was present always at the same time and in all four transitions used: The molecule reached the vitreous. There was a difference between the concentrations 1% and 2% regarding the systemic absorption, which was zero for the lower concentration [Figure 2].
Figure 2.
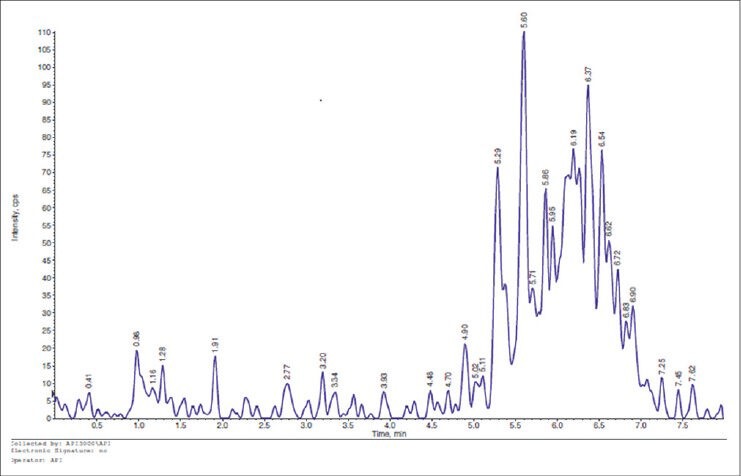
The graph shows the spectrometry result of the left eye of Group A mouse: Citicoline is not in the vitreous.
Clinical phase
We enrolled 34 patients, 16 in the OMK1 and 18 in the OAG group, with mean age of 51 years and male/female ratio of 1:1. The mean IOP in both groups was similar, and it didn’t change significantly during the study. At study inclusion, the mean IOP was 14.3 ± 2.5 mmHg in OMK1 group and 14.6 ± 0.5 mmHg in OAG group, while at the end of the study, it was respectively 15.6 ± 1.5 mmHg (P = 0.55) and 15.3 ± 2.5 mmHg (P = 0.67). In no patients, signs and symptoms indicative of lack of safety and local tolerability were reported.
Perimetric and VEP parameters were highly variable in both groups.
In OMK1 group, MD and PSD values improved [Figures 3 and 4], but this change wasn’t statistically significant (baseline MD -4.92 dB vs. 60th day MD -4.59, P = 0.75; baseline PSD 5.14 dB vs. 60th day PSD 5.01 dB, P = 0.91. At 90th day, MD -4.28 dB, P = 0.55; PSD 4.53 dB, P = 0.62).
Figure 3.
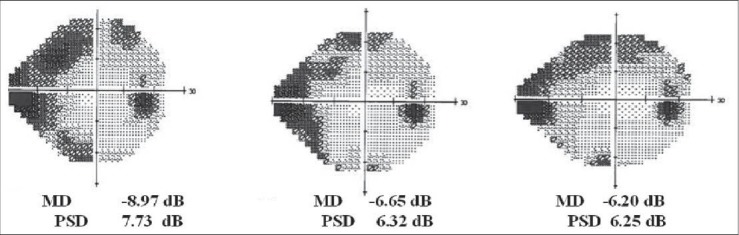
An example of visual field MD improvement after 30 and 60 days
Figure 4.
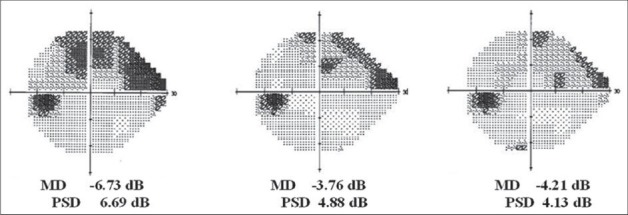
An example of visual field MD improvement after 30 and 60 days
After 60 days of therapy with OMK1, a significant improvement in ganglion cells function was observed, demonstrated by the reduction of the P50 latency and the increase in the N95-P50 amplitude [Tables 1 and 2].
Table 1.
PERG P50 latency (ms=milliseconds) values at baseline and at 60th and 90th day
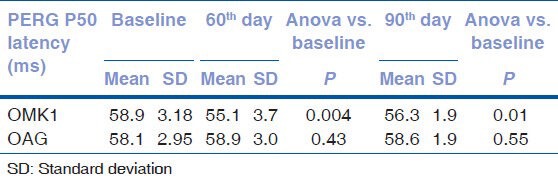
Table 2.
PERG P50-N95 amplitude (μV=microvolt) values at baseline and at 60th and 90th day
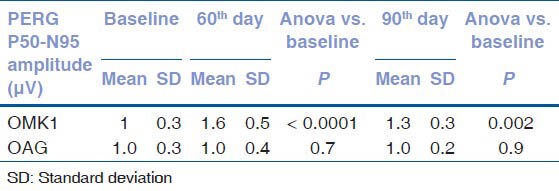
The P100 latency reduction observed after 60 days (127.8 ms vs. 117.9 ms, P = 0.01) regressed after 30 days of washout (127.8 ms vs. 124.4 ms, P = 0.34).
There was no significant changes of N75-P100 amplitude (7.03 ms vs. 8.63 ms, P = 0.27; 7.03 ms vs. 6.91 ms, P = 0.92).
RCT P100 latency didn’t show statistically improvement after 60 days (68.9 ms vs. 62.8 ms, P = 0.15), and it got worse after 30 days (62.8 ms vs. 68.1 ms, P = 0.83).
OAG group didn’t show any significant change in perimetric and electrofunctional parameters during the follow-up, comparing to baseline data.
Discussion
Citicoline is a natural precursor of the PDC with important neuroprotective features, as demonstrated in the literature.
Many studies demonstrate how citicoline have a positive effect on both visual field and the entire visual way, through the use of PERG and VEP.[6,7,8,9] In particular, Parisi et al. confirmed the same result in patients under both hypotensive medical therapy and citicoline administered intramuscularly (1000 mg/day) or orally (1600 mg/day) compared with patients receiving only hypotensive medical therapy, and the need to repeat the treatment to maintain the positive effects on visual function (total period of the study was 8 years).[10,11,12] In addition, there weren’t reported adverse side effects related to the molecule. Recently, it has been demonstrated that also citicoline oral solution 500 mg seems to have a neuroprotective effect after a cycle of at least 60 days.[7,13]
Other studies have used mice as laboratory animals to test the neuroprotective effect of citicoline. Oshitari et al. showed the results of quantitative analysis of TdT-dUTP terminal sequence (TUNEL) on the retina of mice fixed and incubated with four different concentrations of citicoline (0.01, 0.1, 1, 10 mol/l). The TUNEL sequence was significantly lower in the retina treated with citicoline at all concentrations compared to the retina of controls not incubated with other active agents.[14]
In 2009, Chan et al. have published the results of the use of in vivo magnetic resonance spectroscopy (1H MRS proton magnetic resonance spectroscopy) on models of experimental glaucoma in rats, showing that glaucoma is also characterized by altered metabolism of choline in the visual cortex, reflecting the impairment of structural integrity of neuronal membranes.[15]
To date, there has not been a neuroprotective drug in eye drops since they are very large and lipophilic molecules, which are not able to penetrate the ocular tissues. To increase its penetration, it is necessary to relax the intercellular junctions of the corneal epithelium, which is possible through the use of BAK, used for decades in ophthalmic preparations as “drug penetration enhancer.”[16,17]
The experimental study has demonstrated that the ophthalmic solution OMK1 based on citicoline 2% in combination with high molecular weight hyaluronic acid and BAK at low concentration, 0.01%, guarantees the passage of the molecule in the posterior segment, reaching the retina and the optic nerve head: Once in the anterior chamber, the molecule diffuses in the vitreous through the zonula and through the uveoscleral route. Hyaluronic acid has the ability to convey the active ingredient and to increase the contact time in the anterior chamber, thus increasing the absorption through the retina, conjunctiva, and sclera.
The clinical study showed that treatment with topical citicoline induces, after 60 days of therapy, a significant improvement in the ganglion cell function (increased amplitude and reduced latency of PERG). This improvement was still present after 30 days of discontinuing treatment. In addition, improvements in the ganglion cells function induced a reduction of the delay of post-retinal nerve conduction (although the improvement of RCT was not significant).
The simultaneous significant improvement in the ganglion cells function together with a reduction of the delay of the post-retinal nerve conduction results in an improvement of nerve conduction over the entire optical pathway (reduction of the latency of the VEP).
After 30 days of suspension of the treatment, the residue improvement of ganglion cell function no longer causes the reduction of post-retinal nerve conduction delay, which returns to pre-treatment values (RCT values similar to baseline). The loss of this effect on post-retinal nerve conduction is reflected on the entire visual pathway (latency of VEP unchanged at 90 days).
In individual eyes, we observed an improvement of the visual field parameters (MD and PSD). However, as the baseline data were highly variable to reach significant values, it is necessary to have a greater sample size, which we will reach at the end of the full study period (24 months).
In conclusion, this interim analysis shows that topical citicoline seems to have a neuroprotective effect that is independent of pressure-lowering effect due to any hypotensive drops.
Furthermore, topical administration has several advantages: It's easier to use than other forms (intramuscular and oral), and it contains lower concentration of the molecule because it bypasses the hepatic and renal metabolism; therefore, patients can use it several times throughout the day.
Acknowledgment
The authors acknowledge Omikron S.R.L. for providing OMK1 drops.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Sommer A, Miller NR, Pollack I, Maumenee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 1977;95:2149–56. doi: 10.1001/archopht.1977.04450120055003. [DOI] [PubMed] [Google Scholar]
- 2.Harwerth RS, Carter-Dawson L, Shen F, Smith EL, 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–50. [PubMed] [Google Scholar]
- 3.Sakugawa M, Chihara E. Blockage at two points of axonal transport in glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 1985;223:214–8. doi: 10.1007/BF02174064. [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Secades JJ. Citicoline: Pharmacological and clinical review, 2010 update. Rev Neurol. 2011;52(Suppl 2):S1–62. [PubMed] [Google Scholar]
- 6.Chang EE, Goldberg JL. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–86. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, Rossetti L. Citicoline oral solution in glaucoma: Is there a role in slowing disease progression? Ophthalmologica. 2013;229:219–26. doi: 10.1159/000350496. [DOI] [PubMed] [Google Scholar]
- 8.Pecori Giraldi J, Virno M, Covelli G, Grechi G, De Gregorio F. Therapeutic value of citicoline in the treatment of glaucoma (computerized and automated perimetric investigation) Int Ophthalmol. 1989;13:109–12. doi: 10.1007/BF02028649. [DOI] [PubMed] [Google Scholar]
- 9.Virno M, Pecori-Giraldi J, Liguori A, De Gregorio F. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up) Acta Ophthalmol Scan Suppl. 2000;232:56–7. doi: 10.1111/j.1600-0420.2000.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 10.Rejdak R, Toczo³owski J, Kurkowski J, Kamiñski ML, Rejdak K, Stelmasiak Z, et al. Oral citicoline treatment improve visual pathway function in glaucoma. Med Sci Monit. 2003;9:P124–8. [PubMed] [Google Scholar]
- 11.Parisi V, Manni G, Colacino G, Bucci MG. Cytidine-5’-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology. 1999;106:1126–34. doi: 10.1016/S0161-6420(99)90269-5. [DOI] [PubMed] [Google Scholar]
- 12.Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5’-diphosphocholine (citicoline): A study of 8 years of follow-up. Doc Ophthalmol. 2005;110:91–102. doi: 10.1007/s10633-005-7348-7. [DOI] [PubMed] [Google Scholar]
- 13.Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, et al. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res. 2008;173:541–54. doi: 10.1016/S0079-6123(08)01137-0. [DOI] [PubMed] [Google Scholar]
- 14.Bubella RM, Carità S, Badalamenti R, Bubella DM. Neuroprotezione del paziente con glaucoma cronico ad an golo aperto: Ruolo della citicolina in soluzione orale. Ottica fisiopatologica. 2011;XVI:171–7. [Google Scholar]
- 15.Oshitari T, Fujimoto N, Adachi-Usami E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport. 2002;13:2109–11. doi: 10.1097/00001756-200211150-00023. [DOI] [PubMed] [Google Scholar]
- 16.Chan KC, So KF, Wu EX. Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp Eye Res. 2009;88:65–70. doi: 10.1016/j.exer.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Ashton P, Podder SK, Lee VH. Formulation influence on conjunctival penetration of four beta blockers in the pigmented rabbit: A comparison with corneal penetration. Pharm Res. 1991;8:1166–74. doi: 10.1023/a:1015810619869. [DOI] [PubMed] [Google Scholar]


