Abstract
Purpose:
To describe retinal changes during Spectral Domain Optical Coherence Tomography (SD-OCT) guided bevacizumab treatment for neovascular age- related macular degeneration (AMD).
Settings and Design:
Single center observational study.
Materials and Methods:
We confirmed wet AMD in 47 eyes of 45 patients by fluorescein angiography and SD-OCT. After bevacizumab injection, we examined the patients at 4-week intervals. During each follow-up control, we performed SD-OCT and a complete ophthalmic examination. Criteria for reinjection were visual acuity loss of more than five ETDRS letters, and/or increase of central retinal thickness, sub-retinal fluid, intra-retinal fluid, pigment epithelium detachment. If reinjection criteria were not met, we advised the patient to return in 4 weeks’ time for the next scheduled follow-up. We used 3-dimensional SD-OCT to measure photoreceptor defects and sub-retinal fibrosis. The main efficacy endpoints were the SD-OCT measurements of the size of photoreceptor defects, the size of external membrane defects and the central retinal thickness.
Results:
Over the 12 months study period, the percentage of scans in 3-D imaging mode showing visible defects of the junction between inner and outer segments of photoreceptors increased from 38.96 to 53.8%. The percentage of scans in 3-D imaging mode with visible sub-retinal fibrosis increased from 33 to 52% and mean central retinal thickness decreased from 333 μm (96-900 μm) to 272 μm (P = 0.011).
Conclusion:
In long-term anti- Vascular endothelial growth factor (VEGF) treatment for neovascular AMD, photoreceptor defects and fibrosis progress despite a decrease in central retinal thickness and improvements in visual acuity. We would encourage further discussion as to whether this is the natural course of the disease or a result of the treatment.
Keywords: Age- related macular degeneration, anti-Vascular endothelial growth factor, Avastin, bevacizumab, neovascular age-related macular degeneration, spectral domain optical coherence tomography, spectral domain optical coherence tomography, wet age-related macular degeneration
Antiangiogenic therapies, such as ranibizumab (Lucentis®, Genentech Inc., South San Francisco, CA) and bevacizumab (Avastin®, Genentech Inc.), have revolutionized the management of age-related macular degeneration (AMD).[1,2,3,4,5]
The CATT (The Comparison of Age-related macular degeneration Treatment Trials) study and the IVAN (The alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation randomized trial) study confirmed that both drugs might be regarded as parallel concerning visual acuity gain and safety issues. To date, there have been no reports of toxicity of any anti-VEGF agent on retinal tissue in vivo or in vitro.[6,7] However, a slight anti-proliferative effect on retinal pigment epithelium (RPE) cells was noted.[6,8,9] Additionally, a cumulative effect and metabolic changes on the RPE cells have been observed.[10,11] Improvement in visual acuity is impressive in most patients; however, sub-retinal fluid persists in some cases despite treatment. Additionally, geographic atrophy progression has been observed in a number of treated eyes.[12,13]
Discussion on many proposed treatment schemes continues. The CATT study presented slightly better results in the study arm treated with monthly ranibizumab when compared to treatment as-needed after 24 months follow-up. Minding cutting costs for the patient and the health system recently the “treat and extend” scheme was proposed. This dosing regimen additionally allows increasing the time between injections with stabilization of visual acuity in long term follow up.[14]
In parallel with the advances in drug therapies, optical coherence tomography has evolved and became an important diagnostic tool in AMD. Thanks to its high-speed data acquisition, high resolution, 3-dimensional imaging mode and the additional eye tracking available in some commercial devices, spectral-domain optical coherence tomography (SD-OCT) may enable more accurate diagnosis and follow-up.
Currently, there are no published long-term studies presenting SD-OCT documented changes in retinal morphology after anti-VEGF injections.
The aim of this study is to report on long-term SD-OCT documented results after SD-OCT guided treatment with bevacizumab.
Materials and Methods
This is a single center observational study designed to investigate the efficacy and influence on retinal morphology of intra-vitreal bevacizumab treatment on an as needed basis guided by SD-OCT findings.
The main efficacy endpoints were the size of photoreceptor defects, sub-retinal fibrosis and central retinal thickness in SD-OCT measurements. The secondary endpoint was change in visual acuity and the number of bevacizumab injections over 12 months.
We obtained informed consent from patients and approval of the Institutional Ethics Committee Board. Only treatment naοve patients were included. Consecutive patients with any type of active choroidal neovascularization (CNV) in the fovea due to age related macular degeneration and a 12 months follow- up were included. Confirmation of CNV required leakage documented on fluorescein angiography and any of the following changes found in SD-OCT examination: Increased retinal thickness without sub-retinal and/or intra-retinal fluid of more than 100μm, sub-retinal and/or intraretinal fluid, pigment epithelium detachment [Fig. 1].
Figure 1.
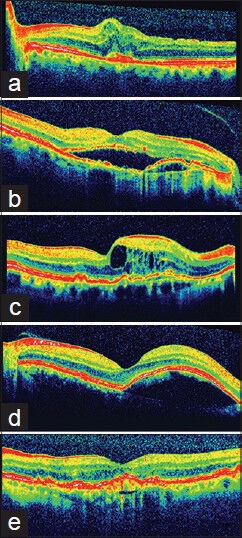
Spectral domain optical coherence tomography features of choroidal neovascularization, by which the authors qualified patients for anti-VEGF treatment. (a) Increased retinal thickness without subretinal and/or intraretinal fluid (b) Subretinal fluid (c) Intraretinal fluid (d) Pigment epithelium detachment (e) Fibro vascular pigment epithelium detachment
Exclusion criteria were diabetic retinopathy, advanced glaucoma, any cardiovascular events in the last year before injection, visual acuity below 0.05 with fibrovascular scars in SD-OCT scans.
The mean interval between initial diagnosis and treatment initiation was 16 days (1-30 days).
We used Spectralis® high-resolution OCT (Heidelberg Engineering, Germany) to obtain all images. This device, because of the simultaneous cooperation of high-resolution OCT and scanning laser ophthalmoscopy beam, allows follow-up scans in almost the same location with a high reproducibility rate. To ensure appropriate image registration from time point to time point we used the follow-up mode enabled by the machine software. We selected the 3-dimensional scanning mode, with 19 images on an area of 4.5 mm × 6 mm, and no averaging was used in order to ensure reduction of observed artifacts. Two experienced examiners (JM, BI) analyzed all B-scans. If any disagreement was noted, the senior author was asked to make the decision on accuracy of data.
We performed intra-vitreal bevacizumab (Avastin®, Roche, Reinach, Switzerland) injections in all subjects as one-day surgery in an operating theatre and advised a scheme of three days pre-injection and five days post-injection antibiotic prophylaxis. We applied topical anesthesia, put an eyelid speculum in place and then cleaned the conjunctival sac with 5% povidone-iodine solution. After repositioning of the conjunctiva, we administered an intra-vitreal injection of 1.25 mg bevacizumab via an oblique scleral tunnel through pars plana 3.5 mm from the limbus in pseudophakic eyes and 4 mm from the limbus in phakic eyes. We applied a drop of ointment containing Hydrocortisone, Oxytetracycline and Polymyxin to the eye. Finally, we measured intraocular pressure with Goldmann applanation tonometry 15 min after the injection.
Follow-up took place on a four-week basis for the next 12 months. During each follow-up visit, we performed an SD-OCT examination taking 19 B-scans at the same site each time by using the eye tracking and follow-up mode included in the commercial software. We analyzed the following morphologic and morphometric data: Central retinal thickness, the extent of fibrosis and photoreceptor layer defects [Fig. 2]. A photoreceptor defect was recognized when the IS/OS junction line became invisible at any spot of a single B-scan. To make comparison more straightforward we converted the number of B-scans in the 3-dimensional imaging mode in which the feature was present to a percentage i.e. if the feature was present in one B-scan the percentage was 1/19 = 5.26% and if a feature was present in 19/19 scans it was = 100% [Fig. 3]. We employed the commercially available Spectralis software to measure automatically central retinal thickness. The boundaries of the measurements were the internal limiting membrane and the outermost reflective band seen in SD- OCT.
Figure 2.
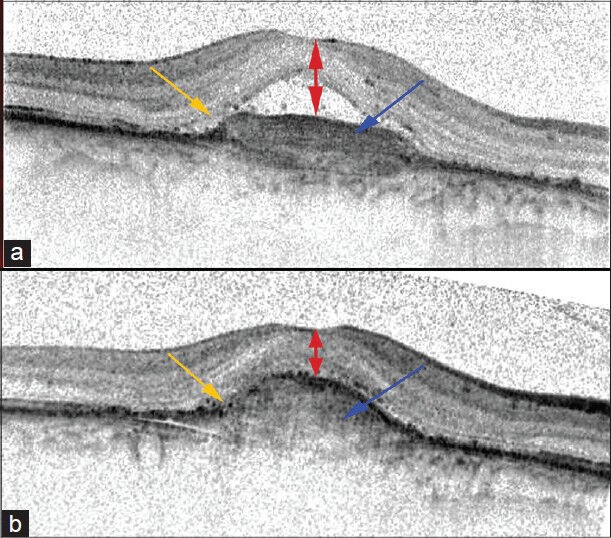
Morphologic and morphometric data that we analyzed before and after anti-VEGF treatment: central retinal thickness (double-head red arrows), fibrosis (blue arrow) and photoreceptor layer defects (orange arrow). “A” shows the pre-treatment B-scan, and “B” is the B-scan of the same area 12 months after treatment began
Figure 3.
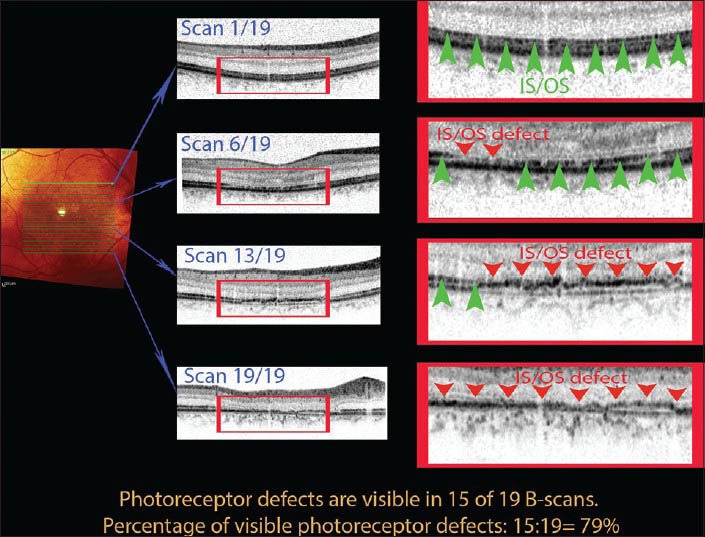
For statistical comparisons between patients, we separately analyzed each of the 19 B-scans in each 3D raster and scored them as follows: Any scan containing a photoreceptor defect (a disturbance of IS/OS line shown here by red arrowheads) scored 1 point. If no defect was present (as indicated by the green arrowheads), the score was 0. We then divided the point total for the raster by 19 and converted the sum to a percentage value. We used the same method to create comparable percentages when studying sub-retinal fibrosis
In addition, we performed complete ophthalmic examinations and tested tonometry and visual acuity initially and during each follow-up control. Visual acuity was measured on ETDRS charts in decimal scale than it was converted to logarithm of the minimum angle of resolution (LogMAR) and presented in LogMAR scale throughout the paper.
After the first injection, we performed reinjections in cases where SD-OCT revealed any of the following indications: Presence of sub-retinal or intra-retinal fluid, presence of serous pigment epithelium detachment (PED) or increased central retinal thickness and in cases of loss of more than 5 ETDRS letters. When visual loss could not be explained by SD-OCT examination, we also performed fluorescein angiography.
We used Sigma Stat 3.5 for Windows to carry out statistical analysis.
Results
We included 47 eyes of 45 patients and the mean age of those 24 men and 21 women was 73.6 years.
25 patients qualified for reinjection after the first control visit. Overall, we administered a mean of 4.15 injections (from 2 to 8) during the 12-month study period, giving a mean time between injections of 2.9 m.
Visual acuity
Initial mean visual acuity was 0.85 LogMAR (2.0 to 0.22 LogMAR). Four weeks after the first bevacizumab injection mean visual acuity improved to 0.79 LogMAR. After 12 months mean visual acuity improvement was statistically significant (from mean 0.85 LogMAR to mean 0.63 LogMAR, P = 0.001). The most significant visual acuity improvement occurred up to month three. We recorded the best mean visual acuity of 0.58 LogMAR in treatment month seven. In the following months (7 to 12 month) visual acuity slightly decreased, but the difference between months seven and 12 was not statistically significant [Fig. 4].
Figure 4.
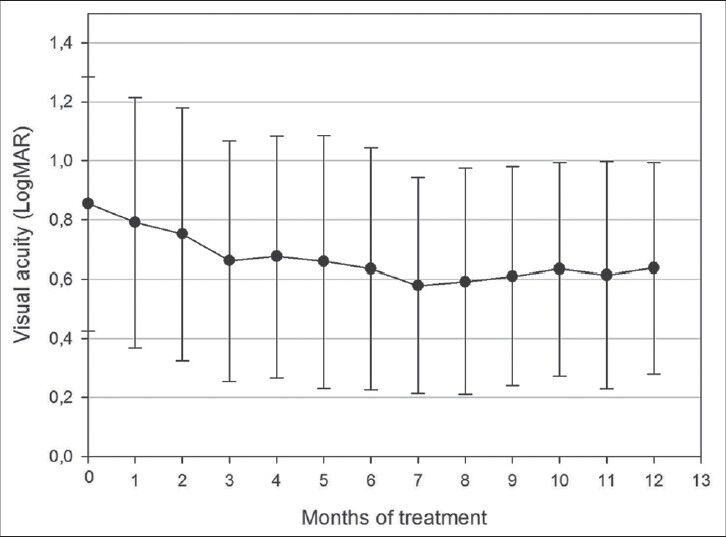
Visual acuity changes presented in LogMAR during 12 m of treatment. Mean VA rapidly improved from 0.85 LogMAR to 0.66 LogMAR in the first 3 m. At month 7 mean VA had improved further to 0.58 LogMAR and remained stable to month 12 (0.63 LogMAR)
Central retinal thickness
Mean central retinal thickness was 333μm at the initial visit (96- 900μm) and decreased to 257 μm four weeks after the initial injection. By the end of the study period mean central retinal thickness had decreased from 333 μm to 272 μm, P = 0.011. The most significant improvement occurred in the third month of treatment and stabilized during the following months [Fig. 5].
Figure 5.
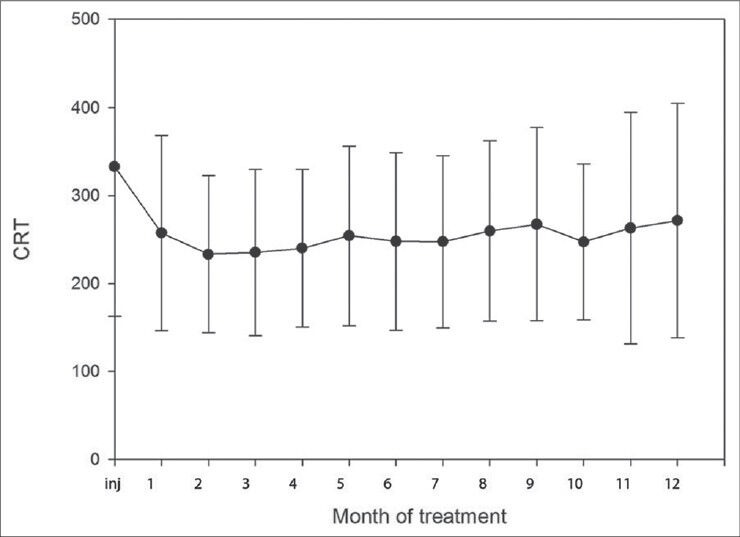
Mean changes in central retinal thickness during the first 12 months of anti-VEGF treatment
Subretinal fibrosis
We initially observed sub-retinal fibrosis on 33% B-scans in the 3-dimensional fovea imaging mode in SD-OCT. This increased to 37.96% four weeks after the initial injection and then increased at about 2% each month until, at the end of the study; we observed sub-retinal fibrosis on 52% of B-scans [Fig. 6].
Figure 6.
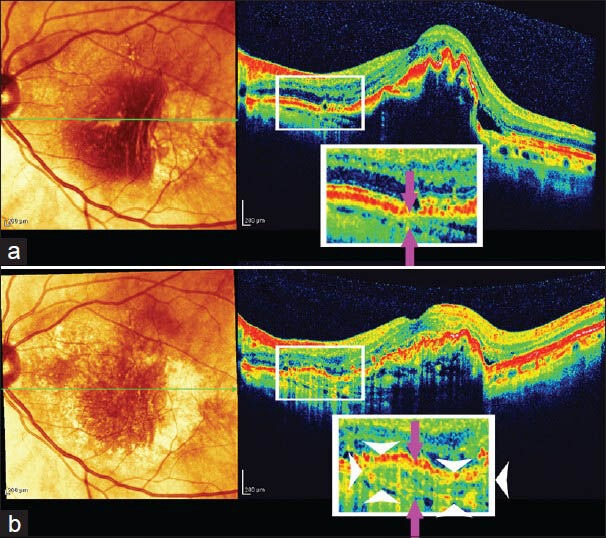
Area of new fibrosis. The corresponding areas where sub-retinal fibrosis enlarged was magnified 2× (white square). White arrowheads limit area of new fibrosis on the lower B-scan. Distance between choroidal vessels and RPE increased due to growth of fibrotic tissue (distance between violet arrows). Follow up B-scan (b) was taken 10 months after initial B-scan (a)
Photoreceptor defects
The initial percentage of scans with photoreceptor layer defects visible in SD-OCT was 38.96%, which increased to 39.98% four weeks after the initial injection. This percentage increased significantly from first month to the third (from 39.98 to 44.8%) and then gradually progressed to reach the mean of 53.8% in 12th month.
During each monthly control visit there was a statistically significant negative correlation between the percentage of scans with visible photoreceptor defects and visual acuity (P < 0.05 for each month). Additionally each month, we observed a statistically significant positive correlation of the percentage of scans with visible photoreceptor defects with visible fibrosis (P < 0.05 for each month) [Fig. 7].
Figure 7.
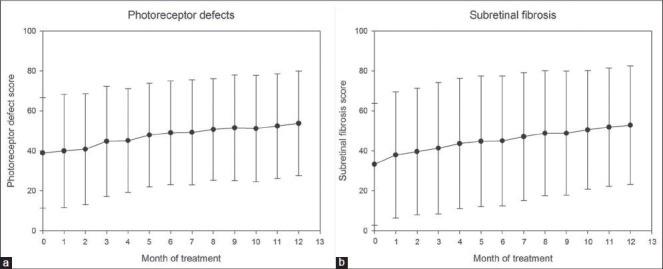
Mean changes in photoreceptor defect score (a) and subretinal fibrosis score (b) during the first 12 months of anti-VEGF treatment
Furthermore, we analyzed the correlation between changes in visual acuity and change in percentage of scans with photoreceptor defects. We calculated changes in visual acuity as the difference between month 3 VA and month 12 VA and similarly calculated change in percentage of scans with visible photoreceptor defects as the difference between their month 3 and month 12 scores. We used the Spearman Rank Order Test and noted that the increase in the extent score of photoreceptor defects did not influence visual acuity.
We also analyzed the area where we noted new photoreceptor defects. We observed that they appeared in most cases in places where sub-retinal fluid was previously present and had been reabsorbed after anti-VEGF treatment [Fig. 8].
Figure 8.
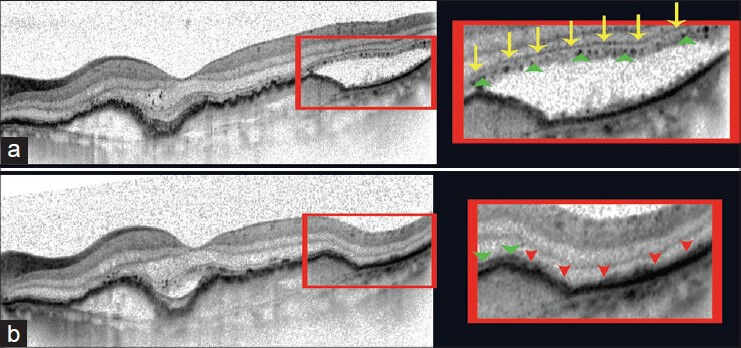
New photoreceptor defects in an area of previously reabsorbed subretinal fluid after anti-VEGF treatment. (a) Pre-treatment SD-OCT B-scan (76-year-old male with subretinal fluid due to CNV in AMD). External limiting membrane (indicated here by yellow arrows) and an interrupted line corresponding with ellipsoid inner segments of photoreceptors (green arrowheads) are clearly visible. The area of subretinal fluid is shown in the enlarged image to the right. (b) B-scan of the same patient taken in the exact same place, using the follow- up mode included in the Spectralis SD-OCT software, four weeks after anti-VEGF treatment showing subretinal fluid has been reabsorbed. External limiting membrane is disrupted and there is no characteristic hyperreflective line to represent the junction between the inner and outer segments of photoreceptors (red arrowheads), the layers are undistinguishable
We noted no adverse effects, such as cardiovascular events or endophthalmitis, during the study.
Discussion
In this series, we used an SD-OCT guided bevacizumab treatment regimen, enabling us to propose a patient-specific treatment guideline. We confirmed that SD-OCT guided treatment on an “as needed” basis enables the administration of fewer bevacizumab injections than in randomized studies (4.15 per 12 months). In this study, for statistical reasons decimal scale was converted to logarithm of minimal angle of resolution. For very low vision that cannot be measured on Snellen chart we used conversion previously described by Holladay.[15] Difference in studied cohorts (CNV types, initial visual acuity) and different way of visual acuity assessment (ETDRS scale vs. LogMAR) makes difficult to compare functional outcomes between different studies. In our study mean visual acuity improvement was 0, 22 LogMAR. In this prospective study, SD-OCT images demonstrated that despite improved visual acuity, the area of photoreceptor damage and fibrovascular scaring increase during the first 12 months.
Using TD- OCT in their study protocol, the CATT study confirmed comparable results at month 12 between ranibizumab and bevacizumab injected each month or “as needed”. The mean number of injections in the CATT study group treated as needed with bevacizumab was 7.7 and mean gain in visual acuity was 5.9 ETDRS letters.[16] Also using TD-OCT to identify patients qualified for reinjection, the IVAN study reported a mean of 7 injections during the first 12 months for patients on the “as needed” protocol and a mean visual acuity increase of 5,5 ETDRS letters.[17] To date, no studies (Pubmed Medline) have analyzed changes in retinal morphology viewed with SD-OCT after ranibizumab or bevacizumab injections over 12 months. We present that using SD-OCT may enable satisfactory visual acuity results with fewer injections. The “as needed” treatment protocol is based on the idea to retreat first when functional or morphological deterioration appears. Dadgostar et al., reported that in patients with exudative ARMD treated with ranibizumab on “as needed” basis fewer injections per year were needed.[18] The higher resolution of SD-OCT may increase the sensitivity of diagnosis and may help to identify small spots of subretinal or intraretinal fluid that might not show up when using TD-OCT.[19] Detecting early recurrence of activity in CNV may improve the “as needed” decision timing for reinjection of anti-VEGF drugs and reduce the percentage of patients with persistent sub-retinal fluid. Better-timed and more patient-specific intervention may have a role in minimizing the number of injections needed per year.
A previous Time Domain OCT study reported different features such as central retinal thickness and increased retinal volume correlate with decreased visual acuity.[20] We confirmed that also in spectral domain OCT such a correlation exists.
An unexpected finding was the progression of photoreceptor defects and sub-retinal fibrosis despite treatment and improvement in visual acuity. One possible theory is that the ‘as-needed’ treatment regimen does not fully prevent disease progression. Another explanation is that these findings may reflect the natural disease history and that even if anti-VEGF drugs are capable of stopping leakage they may be incapable of completely preventing photoreceptor loss and fibrosis progression. A third hypothesis is that anti-VEGF drugs may promote the formation and growth of geographic atrophy. Most studies agree that anti- VEGF treatment is effective and that visual acuity improves or stabilizes during at least first 24 months of treatment. Oishi managed to confirm this data, showing on a single central SD-OCT B-scan that the IS/OS junction has a tendency to normalize during anti- VEGF treatment.[21] Latest research shows, on the contrary that geographic atrophy progresses during the course of treatment. The 3-dimensional scanning mode with the follow- up mode enables to explain both above mentioned findings. As the IS/OS line may improve on a single B-scan it may become disrupted on the next B-scans only few um away at the same time. Thus, only 3-dimensional presentation of the whole foveal area enables us to have a detailed view on all changes.
Witkin et al., presented retrospective results of 12 patients examined with High Speed Ultrahigh Resolution Optical Coherence Tomography (hsUHR- OCT) one month after ranibizumab injections. In their Group, fibrovascular lesions did not change significantly and photoreceptor abnormalities remained in all cases.[22] Recently, Jaffe and coworkers showed that thickness of sub-retinal tissue complex on a single scan (sub-retinal fibrosis and sub-retinal blood) decreases in the first four months of treatment and then remains stable until month 52.[23] Most SD-OCT studies confirm that even if central macular thickness decreases with treatment the area of the neovascular membrane remains the same either after bevacizumab or ranibizumab treatment.[24,25]
In this current study, we report on a prospective series of 47 eyes followed up for 12 months with Spectral Domain OCT examination. During the first follow-up visit, four weeks after treatment, percentage of B-scans with visible photoreceptor defects increased slightly but without statistical significance. However, the extent of subretinal fibrosis did show a statistically significant increase. The differences between previous studies and our study might be a result of the OCT systems used. Although hsUHR- OCT has a similar resolution to SD-OCT devices, it does not allow 3-dimensional imaging without which it is not possible to present the exact extent or area of a defect. Witkin et al., also present changes on particular scans, not in the entire 3-dimensional foveal area.[22] Furthermore, our final conclusions are based on a much longer follow-up period.
During the 12-month follow-up with SD-OCT, we documented photoreceptor defects increased continuously despite bevacizumab treatment. Initially we observed IS/OS defects in 38.96% of the analyzed scans and this figure rose to 53.8% at 12th month.
Each month, we analyzed the extent of photoreceptor defects to see if they correlated with visual acuity. We proved that patients with greater extent of photoreceptor abnormalities have lower visual acuity. Although, the percentage of visible photoreceptor defects increased, this did not correlate with changes in visual acuity.
After detailed analysis, we noted that visual acuity improvement mostly occurred in the first two months. Over the same period, sub-retinal fluid re-absorption and central retinal thickness decrease were also most significant. Our study additionally shows that during the study period, mean best-corrected visual acuity was achieved in the seventh month of treatment in our group. From seventh months of treatment through to 12th month, mean visual acuity seemed to progressively decrease but the VA differences is not statistically significant. Recently, Dunavoelgyi et al., reported the results of 3-year treatment of wet AMD with bevacizumab in accordance with the official European label regimen.[26] In native treated patients visual outcomes after 3 years were unsatisfactory. The final visual acuity in those patients showed a statistically significant decrease when compared to initial VA. Given the 3-year study period, this may be due to a further progression of the continuing photoreceptor damage that we visualized with SD-OCT.
We observed that in most cases new photoreceptor defects occur in areas where, with SD-OCT, we previously found sub-retinal fluid, which was reabsorbed after bevacizumab injection. This finding may be the natural course of the disease, or perhaps SD-OCT or TD-OCT guided treatment, by allowing sub-retinal fluid to reappear before reinjection, leads to worse functional results in the long term in comparison to monthly injections, which do not allow sub-retinal fluid to reappear. The matter requires further study.
The current study reports a continuous increase in sub-retinal fibrosis during the first year of bevacizumab treatment. At the beginning of the study, it was present in approximately 33% of analyzed scans and after 12 months of treatment in 52% of scans.
There is still much discussion regarding the CATT study having demonstrated formation of geographic atrophy in 30% of patients treated with monthly ranibizumab injections. Some patients treated with monthly or as-needed bevacizumab also had signs of progressing geographical atrophy. In both the MARINA and ANCHOR trials, approximately 10% of patients had decreased vision despite treatment.[2,5] Rosenfeld et al., proved that retinal pigment abnormalities indicative for geographic atrophy were associated with this vision loss.[27] In addition, other studies confirm that even if there is an incredible short-term gain in visual acuity after anti-VEGF drugs, this effect diminishes after at least 24 months of treatment.[28] Furthermore, a recent paper suggests that too much VEGF inhibition might have an influence on progression of geographic atrophy.[29]
A possible explanation for the above may be the role of RPE in phagocytosis of the outer segments of photoreceptors. RPE is also responsible for the passage of nutrients and oxygen from the choroid towards the retina and damaged RPE may not be able to fulfill this task, which would lead to disturbance in photoreceptors renewal. Choriocapillaris are dependent on RPE-derived VEGF. Mouse studies showed that aggressively destroying VEGF could unintentionally destroy choriocapillaris and furthermore produce geographic atrophy.[30,31,32] VEGF was also shown to be a survival factor for photoreceptor cells, Müller cells and ganglion cells.[31,33]
Our investigation suggests that anti-VEGF treatment for wet AMD might not prevent photoreceptor damage and sub-retinal fibrosis. The issue of dry AMD progression in patients treated for CNV may be the target for the development of future drug therapies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Wu L, Arevalo FJ, Maia M, Berrocal MH, Sanchez J, Evans T, et al. Comparing outcomes in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration treated with two different doses of primary intravitreal bevacizumab: Results of the Pan-American Collaborative Retina Study Group (PACORES) at the 12-month follow-up. Jpn J Ophthalmol. 2009;53:125–30. doi: 10.1007/s10384-008-0622-y. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A Variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PRONTO study. Am J Ophthalmol. 2009;148:43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Zayit-Soudry S, Zemel E, Loewenstein A, Perlman I. Safety evaluation of repeated intravitreal injections of bevacizumab and ranibizumab in rabbit eyes. Retina. 2010;30:671–81. doi: 10.1097/IAE.0b013e3181c0858c. [DOI] [PubMed] [Google Scholar]
- 7.Thaler S, Fiedorowicz M, Choragiewicz TJ, Bolz S, Tura A, Henke-Fahle S, et al. Toxicity testing of the VEGF inhibitors bevacizumab, ranibizumab and pegaptanib in rats both with and without prior retinal ganglion cell damage. Acta Ophthalmol. 2010;88:170–6. doi: 10.1111/j.1755-3768.2010.01927.x. [DOI] [PubMed] [Google Scholar]
- 8.Spitzer MS, Yoeruek E, Sierra A, Wallenfels-Thilo B, Schraermeyer U, Spitzer B, et al. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:1837–42. doi: 10.1007/s00417-007-0568-7. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RK, Chalam KV. In vitro evaluation of bevacizumab toxicity on a retinal ganglion cell line. Acta Ophthalmol. 2009;87:618–22. doi: 10.1111/j.1755-3768.2008.01410.x. [DOI] [PubMed] [Google Scholar]
- 10.Klettner AK, Kruse ML, Meyer T, Wesch D, Kabelitz D, Roider J. Different properties of VEGF-antagonists: Bevacizumab but not Ranibizumab accumulates in RPE cells. Graefes Arch Clin Exp Ophthalmol. 2009;247:1601–8. doi: 10.1007/s00417-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 11.Klettner A, Möhle F, Roider J. Intracellular bevacizumab reduces phagocytotic uptake in RPE cells. Graefes Arch Clin Exp Ophthalmol. 2010;248:819–24. doi: 10.1007/s00417-010-1317-x. [DOI] [PubMed] [Google Scholar]
- 12.Comparison of age- related macular degeneration treatment trials (CATT) Research Group. Ranibizumab and Bevacizumab for treatment of neovascular age- related macular degeneration. Two- year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi A, Yamashiro K, Tsujikawa A, Ooto S, Tamura H, Nakata I, et al. Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2013;251:1–7. doi: 10.1007/s00417-012-2022-8. [DOI] [PubMed] [Google Scholar]
- 14.Engelbert M, Zweifel SA, Freund KB. Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina. 2010;30:1368–75. doi: 10.1097/IAE.0b013e3181d50cbf. [DOI] [PubMed] [Google Scholar]
- 15.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30:287–90. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;19(364):1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Dadgostar H, Ventura AA, Chung JY, Sharma S, Kaiser PK. Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology. 2009;116:1740–7. doi: 10.1016/j.ophtha.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Sayanagi K, Sharma S, Yamamoto T, Kaiser PK. Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology. 2009;116:947–55. doi: 10.1016/j.ophtha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Keane PA, Liakopoulos S, Chang KT, Wang M, Dustin L, Walsh AC, et al. Relationship between optical coherence tomography retina parameters and visual acuity in age- related macular degeneration. Ophthalmology. 2008;115:2206–14. doi: 10.1016/j.ophtha.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oishi A, Shimozono M, Mandai M, Hata M, Nishida A, Kurimoto Y. Recovery of photoreceptor outer segments after anti-VEGF therapy for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013;25:435–40. doi: 10.1007/s00417-012-2034-4. [DOI] [PubMed] [Google Scholar]
- 22.Witkin AJ, Vuong LN, Srinivasan VJ, Gorczynska I, Reichel E, Baumal CR, et al. High-speed ultrahigh resolution optical coherence tomography before and after ranibizumab for age-related macular degeneration. Ophthalmology. 2009;116:956–63. doi: 10.1016/j.ophtha.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS. Macular morphology and visual acuity in the comparison of age- related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–70. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You JY, Chung H, Kim HC. Evaluation of changes in choroidal neovascularization secondary to age-related macular degeneration after anti-VEGF therapy using spectral domain optical coherence tomography. Curr Eye Res. 2012;37:438–45. doi: 10.3109/02713683.2011.647227. [DOI] [PubMed] [Google Scholar]
- 25.Framme C, Panagakis G, Birngruber R. Effects on choroidal neovascularization after anti-VEGF Upload using intravitreal ranibizumab, as determined by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:1671–6. doi: 10.1167/iovs.09-4496. [DOI] [PubMed] [Google Scholar]
- 26.Dunavoelgyi R, Sacu S, Eibenberger K, Palkovits S, Leydolt C, Pruente C, et al. Retreatment with anti-vascular endothelial growth factor therapy based on changes in visual acuity after initial stabilization of neovascular age-related macular degeneration: 3-year follow-up results. Retina. 2012;32:1471–9. doi: 10.1097/IAE.0b013e318236e805. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the Phase III Ranibizumab clinical trials. Ophthalmology. 2011;118:523–30. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Tao Y, Libondi T, Jonas JB. Long-term follow-up after multiple intravitreal bevacizumab injections for exudative age-related macular degeneration. J Ocul Pharmacol Ther. 2010;26:79–83. doi: 10.1089/jop.2009.0095. [DOI] [PubMed] [Google Scholar]
- 29.Helzner J. Can Anti-VEGF Trigger GA? The issue is spurring a lively debate. Retin Phys. 2012;9:8–10. [Google Scholar]
- 30.Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–9. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, et al. Endogenous VEGF is required for visual function: Evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3:3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford KM, Saint-Geniez M, Walshe T, Zahr A, D’Amore PA. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:9478–87. doi: 10.1167/iovs.11-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishijima K, Ng YS, Zhong L, Schubert W, Jo N, Akita J, et al. VEGF-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–6. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]


