Abstract
Context:
Congenital glaucoma is a potentially blinding ocular disease of the childhood. Identification of the possible associated risk factors and may be helpful for prevention or early detection of this public health problem.
Aims:
To demonstrate the demographic features of congenital glaucoma subjects.
Setting and Design:
The charts of congenital glaucoma patients referred to Tamcelik Glaucoma Center were retrospectively reviewed through the dates of 2000 and 2013.
Materials and Methods:
Analyzed data included diagnosis, age at first presentation, symptoms at first presentation, laterality of the disease, sex, presence of consanguinity, family history of congenital glaucoma, maturity of the fetus at delivery, and maternal age at conception.
Statistical Analysis Used:
Statistical Package for Social Sciences (SPSS) version 19.0 by IBM (SPSS Inc, Chicago, Illinois, USA) was used to compare the mean of continuous variables with Student's t-test and analysis of variance (ANOVA) and χ2 test was used to test differences in proportions of categorical variables.
Results:
The data of 600 eyes of 311 patients were analyzed. The distribution of primary and secondary congenital glaucoma among the patients were 63.3% (n = 197) and 36.7% (n = 114), respectively. Of the 311 patients, 57.2% (n = 178) were male and 42.8% (n = 133) were female. The overall frequency of bilateral disease was 92.3% (n = 287). Overall rate of consanguinity and positive family history was 45.3% (n = 141) and 21.2% (n = 66), respectively.
Conclusions:
Bilateral disease in this study was more common than previously reported studies. Positive family history was more frequent in primary congenital glaucoma although not statistically significant.
Keywords: Congenital glaucoma, consanguinity, demographics, positive family history
Congenital glaucoma is a potentially blinding ocular disease of the childhood which is more often observed in the developing world due to higher frequencies of consanguinity.[1,2] By definition, congenital glaucoma is a developmental disorder of the childhood that is associated with elevated intraocular pressure, globe enlargement (buphthalmos), corneal edema, Haab striae, optic nerve cupping, and atrophy.[3]
Various classification methods of congenital glaucomas have been proposed with respect to ocular and systemic associations or the primary ocular anatomic site involved in the disease.[1] In the former classification method, congenital glaucoma is classified into primary or secondary congenital glaucoma. Primary congenital glaucoma, also known as isolated trabeculodysgenesis, is typically an isolated idiopathic developmental abnormality of the trabecular meshwork in the absence of other ocular and systemic conditions. In secondary congenital glaucoma, a contributory ocular or systemic pathology is present as a cause. Primary congenital glaucoma has previously been reported as the most common type,[4,5,6] however, studies which demonstrate the frequency of specific entities is limited.
Congenital glaucoma is also one of the very few conditions that requires strict follow-up with the need for more than one specialist monitoring the patient throughout his/her life. Identification of the possible associated risk factors, thus, may be helpful for prevention or early detection of this public health problem. The purpose of this study is to demonstrate the distribution of primary and secondary congenital glaucoma and to identify possible associated risk factors.
Materials and Methods
The registry of Tamcelik Glaucoma Center was browsed for patients diagnosed as congenital glaucoma through the dates of 2000 and 2013. The charts of the patients were retrospectively reviewed. Demographic and clinical information of the subjects were obtained. Congenital glaucoma was defined as the presence of optic neuropathy caused by elevated intraocular pressure (>20 mmHg) with associated clinical signs of disc cupping (>0.3), disc asymmetry (>0.2), enlarged corneal diameter (>11 mm in the newborn, >12 mm in a child of any age), corneal edema, Haab striae, and progressive myopia. All of the patients in this study underwent examination under general anesthesia in preoperative evaluation and in the follow-up period after surgical intervention at necessary intervals. The examination under general anesthesia included intraocular pressure measurement, biomicroscopic examination, gonioscopic examination, fundoscopic examination, central corneal thickness measurement, and corneal diameter measurement. Patients were classified as primary or secondary congenital glaucoma according to their findings in examination under general anesthesia (in preoperative evaluation, confirmed later in the follow-up) and systemic work-up done by the pediatrics consultant. Primary congenital glaucoma was defined as a glaucoma that presents from birth to 3 years of age caused by the maldevelopment of the trabecular meshwork in the absence of any other ocular or systemic pathology. Meanwhile, secondary congenital glaucoma was defined as glaucoma with associated systemic and/or ocular pathologies. Children with acquired glaucomas including aphakic glaucoma, uveitic glaucoma, and traumatic glaucoma were excluded.
Iridotrabeculodysgenesis was defined as the coexistence of trabecular meshwork and iris abnormalities, which included iris hypoplasia/hyperplasia, anomalous iris vessels, iris atrophy, iris coloboma, and iris holes. Patients with aniridia were incorporated into the iridotrabeculodysgenesis/aniridia group. Phakomatoses group included patients with Sturge-Weber and Klippel-Trenaunay syndrome. Patients with severe and multiple anterior segment abnormalities which are clinically difficult to distinguish from some of the known anterior segment dysgenesis syndromes, such as Peters anomaly, were grouped as “anterior segment dysgenesis”.
The demographic and clinical data obtained from the records included age at first presentation, symptoms at first presentation, laterality of the disease, sex, presence of consanguinity, family history of congenital glaucoma, maturity of the fetus at delivery, and maternal age at conception. Consanguinity was defined as history of marriage with a first or second degree cousin. Patients were referred as preterm delivery if child birth occurred before 37 completed weeks of pregnancy. Patients with retinopathy of prematurity (ROP) were separately analyzed as a subgroup of secondary congenital glaucoma.
All data were recorded in a Microsoft Excel datasheet and transferred to Statistical PAckage for Social Sciences (SPSS) version 19.0 by IBM (SPSS Inc, Chicago, Illinois, USA) for statistical analysis. Student's t-test and ANOVA was used to compare the means of continuous variables of the groups. χ2 test was used to test differences in proportions of categorical variables (Fisher exact test or Pearson Chi-square when indicated). P < 0.05 were considered statistically significant. Institutional review board approval was obtained for this study.
Results
The data of 600 eyes of 311 patients were extracted from the registry. The distribution of primary and secondary congenital glaucoma among the patients were 63.3% (n = 197) and 36.7% (n = 114), respectively. Table 1 shows the frequencies of primary congenital glaucoma, secondary congenital glaucoma, and its subgroups. The first three most common pathologies in the secondary congenital glaucoma group were iridotrabeculodysgenesis, Axenfeld-Rieger syndrome, and phakomatoses. Phakomatoses group consisted of 10 patients with Sturge-Weber syndrome and two patients with Klippel-Trenaunay syndrome.
Table 1.
Distribution of primary congenital glaucoma, secondary congenital glaucoma, and its subgroups
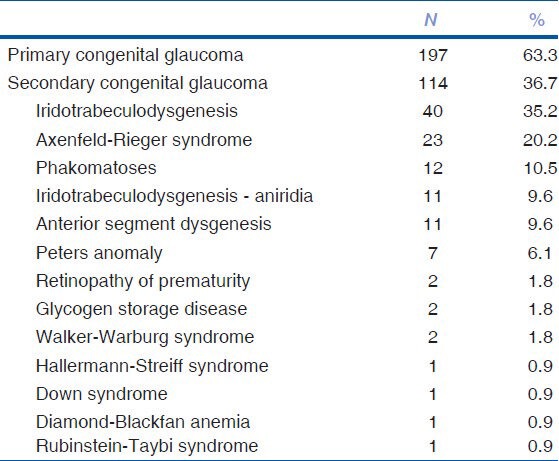
Table 2 shows the distribution of sex and laterality among the groups. Of the 311 patients, 57.2% (n = 178) were male and 42.8% (n = 133) were female. In primary congenital glaucoma, 58.9% (n = 116) of the patients were male and 41.1% (n = 81) of the patients were female. Compared to primary congenital glaucoma, the proportion of female subjects were slightly higher in secondary congenital glaucoma (45.6%, n = 52), but this difference was not statistically significant (P = 0.476).
Table 2.
Distribution of sex and laterality
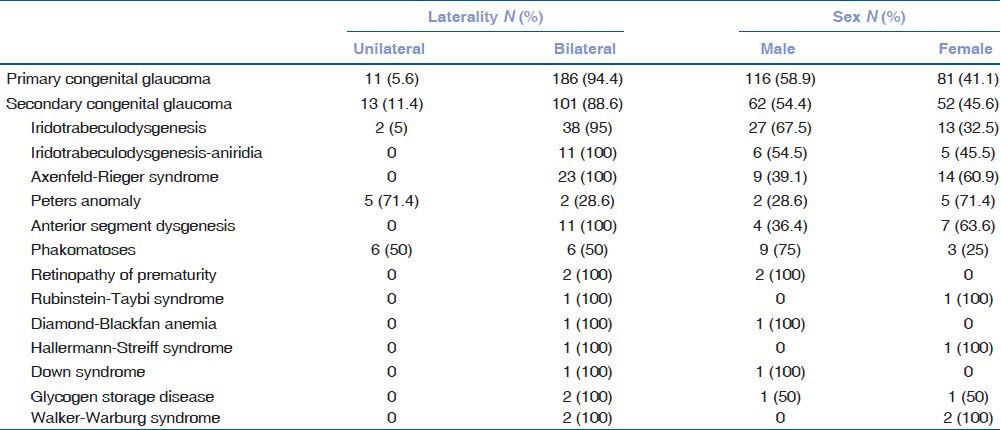
The overall frequency of bilateral disease was 92.3% (n = 287). Bilateral disease was more common in primary congenital glaucoma (94.4%, n = 186) than in secondary congenital glaucoma (88.6%, n = 101); however, this difference was not statistically significant (P = 0.078). The frequency of unilateral disease was significantly higher in phakomatoses (50%, 6/12) and Peters anomaly (71.4%, 5/7) compared to other pathologies in secondary congenital glaucoma group (P = 0.001).
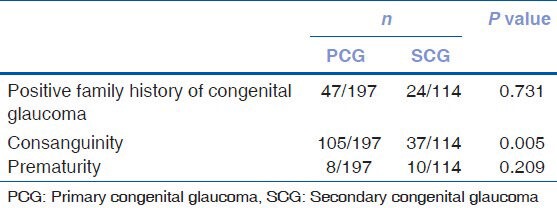
Of the 311 patients, 45.3% (n = 141) had a history of consanguinity. Parents of primary congenital glaucoma patients reported higher rates of consanguinity (53.2%, n = 105) than parents of secondary congenital glaucoma patients (32.4%, n = 37), and this difference was found to be statistically significant (P = 0.005). There was no significant difference between the subgroups of secondary congenital glaucoma in terms of consanguinity (P = 0.321).
Table 3 shows the demographic features of patients with primary and secondary congenital glaucoma. Positive family history was noted in 21.2% (n = 66) of all patients. Although the proportion of positive family history in primary congenital glaucoma (23.8%, n = 47) was higher than in secondary congenital glaucoma (16.6%, n = 19), this difference was not statistically significant (P = 0.283). Contrary to these findings, positive family history was significantly higher in patients with iridotrabeculodysgenesis-aniridia (54.4%, n = 6), Axenfeld-Rieger syndrome (47.8%, n = 11) and Walker-Warburg syndrome (100%, n = 2) in the secondary congenital glaucoma group (P = 0.004). Furthermore, family history was negative in all of the patients with phakomatoses.
Table 3.
Demographic information of primary and secondary congenital glaucoma patients
The majority of the patients (94.2%, n = 293) were term at delivery. 95.9% of patients in primary congenital glaucoma (n = 189) as opposed to 91.2% of patients in secondary congenital glaucoma (n = 104) were term at delivery, but this difference was not statistically significant. There was no statistically significant difference in the distribution of term/preterm deliveries among the secondary congenital glaucoma subgroups, when ROP-associated cases were excluded from statistical analysis.
Table 4 presents the mean age of the patients at first presentation and mean maternal age at conception. The mean age at first presentation of all patients was 110.22 ± 160.35 days (range: day of delivery to 3 years). Although secondary congenital glaucoma patients (96.92 ± 179.63 days) presented slightly earlier than primary congenital glaucoma patients (119.47 ± 145.55 days), this difference was not statistically significant (P = 0.335). The mean maternal age at conception was 27.13 ± 5.61 years in all patients. The mean maternal age at conception of primary and secondary congenital glaucoma patients were 27.36 ± 5.76 and 26.81 ± 5.42 years, respectively. (P = 0.533). There was no statistically significant difference in the mean age at first presentation and mean maternal age at conception in subgroups of secondary congenital glaucoma; however, statistical power was low due to small sample size of some subgroups. Table 5 shows the frequency of symptoms at first presentation.
Table 4.
Mean age at first presentation and mean maternal age at conception among the groups
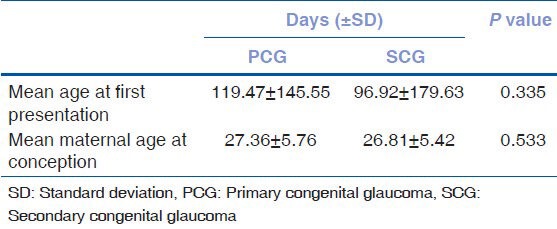
Table 5.
Frequencies of symptoms at first presentation

Discussion
Congenital glaucoma is a rare yet a preventable cause of blindness which is more frequent in populations where consanguinity is common. Its incidence ranges from one in 1,250 in Slovakian Roms (Gypsies), and one in 2,500 in Saudi Arabia to one in 10,000-12,500 in the western world.[6,7] It is also more common in certain regions of Turkey, but epidemiologic data regarding its incidence is lacking. This study demonstrates the demographic features of congenital glaucoma patients, in a referral center, in Istanbul, Turkey. The sex distribution of the subjects in this study (57.2% male, 42.8% female) was consistent with the previous literature which suggests a 3:2 male to female ratio.[4,6,7] The only conflicting study regarding sex distribution was from Japan, which reported a predominance of female subjects over male subjects in patients with primary infantile glaucoma.[7]
Many classification methods have been employed for congenital glaucoma, some of which are more useful for prognostic implications. In this study, isolated abnormality of the trabecular meshwork in the absence of any other ocular and/or systemic association was defined as primary congenital glaucoma and patients who have associated conditions were grouped into secondary congenital glaucoma. The reason to use this classification method was to reveal any differences of demographic and clinical features between these two groups of disease which possibly have different underlying mechanisms and genetic backgrounds. Primary congenital glaucoma accounted for the majority (63.3%) of all congenital glaucoma patients in this study, which is a consistent finding with previous literature.[5,8] Different frequencies were reported as causes of secondary congenital glaucoma in several studies. The BIG eye study reported lens-related congenital glaucomas, phakomatoses, uveitic glaucomas, and anterior segment dysgenesis as the more frequent causes of secondary pediatric glaucomas.[4] Another study reported Peters anomaly, anterior segment dysgenesis, and aniridia/Rieger syndrome as the more common causes of secondary congenital glaucoma.[8] An epidemiologic study of 306 patients revealed aphakic glaucoma, Sturge-Weber syndrome, anterior segment dysgenesis, trauma, and aniridia as the major causes of secondary glaucoma.[5] Anterior segment dysgenesis group, in the mentioned study, also included patients with Axenfeld-Rieger syndrome and Peters anomaly along with unclassified developmental disorders of the anterior segment. By having excluded acquired glaucomas; iridotrabeculodysgenesis, Axenfeld-Rieger syndrome, and phakomatoses were the more frequent causes of secondary congenital glaucoma according to this study. These results may more accurately resemble the actual distribution in this group of disease, as the total sample size in this study is larger than previously reported.
A striking feature of this study was the distribution of laterality. Virtually, all previously published studies report bilateral disease between 70 and 80% in congenital glaucoma patients.[1,4,5,9] Congenital glaucoma in this study is, however, observed in 92.3% of the patients. The frequency of unilateral disease in secondary congenital glaucoma was higher than in primary congenital glaucoma, probably owing to the more frequent observation of unilaterality in phakomatoses, and Peters anomaly.
As aforementioned, Peters anomaly and phakomatoses patients showed higher rates of unilateral disease and this difference was statistically significant compared to other causes of secondary congenital glaucoma. A recently published review of literature reported unilateral disease in 36.8% of 58 cases with Peters anomaly.[10] The rate of unilateral disease, however, was observed in 71.4% of seven patients in this study, but this difference may be attributed to the relatively smaller sample size. Comparably, half of the phakomatoses patients had unilateral disease in this study and this is consistent with published literature which also demonstrate a relatively more even distribution of laterality.[5,11]
Strong efforts have been spent in identifying gene mutations which might be associated with congenital glaucoma. Examples to these gene mutations include the recessively inherited CYP1B1, LTBP2 mutations, and the dominantly inherited heterozygous MYOC mutation.[12,13,14,15] It is suggested that siblings of children with these mutations should be tested and provided with genetic counseling even if they are clinically unaffected by the disease.[16] Most of these genes are inherited in an autosomal recessive pattern with variable penetrance which explains the familial pattern in 10-40% of the cases.[17] Consanguinity is responsible for clustering of these certain gene profiles that are found to be linked with primary congenital glaucoma.[18,19] In this study, consanguinity was present in 45.3% of all patients. The rate of consanguinity was significantly lower in secondary congenital glaucoma group, probably due to the sporadic nature of some of the diseases in this group. In addition, positive family history was also more frequent in primary congenital glaucoma (23.8%) than in secondary congenital glaucoma (16.6%); however, this difference was not statistically significant. What needs to be mentioned regarding positive family history in this study, is the presence of significantly higher rates in iridotrabeculodysgenesis-aniridia, Axenfeld-Rieger syndrome, Walker-Warburg syndrome, and the total absence in phakomatoses.
Aniridia is an uncommon bilateral ocular disease which affects not only the iris but also the cornea, anterior chamber angle, lens, retina, and the optic nerve. Most of the cases are inherited by autosomal dominant transmission with high penetrance and variable expression. About two-thirds of the cases have an affected parent, but sporadic cases have also been reported.[20] In this study, 54.4% of patients with iridotrabeculodysgenesis-aniridia had a positive family history.
Axenfeld-Rieger syndrome is a spectrum of disease associated with several ocular and/or systemic findings which is inherited in an autosomal dominant pattern.[21,22] Very few studies exist in literature which describe the rate of positive family history. A study with a relatively large number of patients from Germany reported positive family history in 46.2% of 26 Axenfeld-Rieger patients who also showed signs of glaucoma or elevated intraocular pressure.[9] This finding is consistent with the positive family history rate of 47.1% of 23 patients in this study. Meanwhile, Walker-Warburg syndrome is a very rare autosomal recessive congenital muscular dystrophy, associated with cerebellar and ocular abnormalities. Ocular abnormalities include anterior segment anomalies (cataracts, shallow anterior chamber, microcornea and microphthalmia, and lens defects) and a spectrum of posterior segment anomalies (retinal detachment or dysplasia, hypoplasia or atrophy of the optic nerve and macula, and coloboma). Glaucoma or buphthalmos may also be present.[23] Positive family history is also a hallmark of this genetic disease.[24]
Conversely, Sturge-Weber and Klippel-Trenaunay syndromes are neurocutaneous disorders in the phakomatoses group of diseases which are typically sporadic.[25,26] Consistent with this knowledge, family history was not reported in any patient with phakomatoses in this study.
The mean age at first presentation for all patients in this study was 110.22 ± 160.35 days. Overall, 34.4% of the patients presented to the referring ophthalmologist or congenital glaucoma specialist within the 1st week, 45.1% within the 1st month, and 96.4% within the 1st year of life. Although secondary glaucoma patients presented slightly earlier than primary congenital glaucoma patients, this difference was not statistically significant. Patients with Peters anomaly, iridotrabeculodysgenesis-aniridia, and anterior segment dysgenesis presented earlier than those patients with iridotrabeculodysgenesis, Axenfeld-Rieger syndrome, and phakomatoses in the secondary congenital glaucoma group; however, statistical analysis could not be performed due to small sample sizes in more than one subgroups of secondary congenital glaucoma. These results are comparable to previously reported studies.[4,5,8]
Mean maternal age was 27.13 ± 5.61 years in all patients and it did not differ significantly between primary and secondary congenital glaucoma patients. Overall rate of preterm delivery was 5.8%, a higher rate was observed in the secondary congenital glaucoma group (8.8%) due to the presence of subjects with associated ROP. The difference between primary and secondary congenital glaucoma; however, was not statistically significant when ROP subjects were excluded. A population-based, case-control study of isolated primary congenital glaucoma reported a preterm birth rate of 28.9% in 52 subjects and this result was significantly higher than the matched controls and population controls. However, this difference was explained by the Gypsy demographics of several mothers included in the study. The authors have reported that certain anthropological characteristics of these mothers, which include low body weight and small stature, together with their low socioeconomic status may result in a higher rate of low birth weight and associated prematurity.[27]
Finally, as expected, cloudy cornea and buphthalmos were the most frequent signs that had alerted the parents to seek for a specialist according to this study. Pediatrician concern should not be overlooked as pediatricians should be able to recognize this disease and refer promptly to a specialist, as early intervention is usually curative. It is also important to note that more thorough investigations regarding identification of gene mutations associated with congenital glaucoma are needed. These investigations will improve our knowledge about the genetics of congenital glaucoma and will provide us newer developments in genetic counseling and antenatal detection of this preventable disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol. 2011;59:148–57. doi: 10.4103/0301-4738.73683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur K, Mandal AK, Chakrabarti S. Primary Congenital Glaucoma and the Involvement of CYP1B1. Middle East Afr J Ophthalmol. 2011;18:7–16. doi: 10.4103/0974-9233.75878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharafieh R, Child AH, Sarfarazi M. Molecular genetics of primary congenital glaucoma. In: Traboulsi EI, editor. Genetic Diseases of the Eye. 2nd ed. New York: Oxford University Press; 2012. p. 295. [Google Scholar]
- 4.Papadopoulos M, Cable N, Rahi J, Khaw PT BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4100–6. doi: 10.1167/iovs.06-1350. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RH, Ainsworth JR, Evans AR, Levin AV. The epidemiology of pediatric glaucoma: The Toronto experience. J AAPOS. 1999;3:308–15. doi: 10.1016/s1091-8531(99)70028-5. [DOI] [PubMed] [Google Scholar]
- 6.Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: A population-based study. Arch Ophthalmol. 2010;128:478–82. doi: 10.1001/archophthalmol.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma) Surv Ophthalmol. 1983;28:1–19. doi: 10.1016/0039-6257(83)90174-1. [DOI] [PubMed] [Google Scholar]
- 8.Alanazi FF, Song JC, Mousa A, Morales J, Al Shahwan S, Alodhayb S, et al. Primary and Secondary Congenital Glaucoma: Baseline Features From a Registry at King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia. Am J Ophthalmol. 2013;155:882–9. doi: 10.1016/j.ajo.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Dressler P, Gramer E. [Morphology, family history, and age at diagnosis of 26 patients with Axenfeld-Rieger syndrome and glaucoma or ocular hypertension] Ophthalmologe. 2006;103:393–400. doi: 10.1007/s00347-006-1335-6. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari R, Ferri S, Whittaker B, Liu M, Lazzaro DR. Peters anomaly: Review of the literature. Cornea. 2011;30:939–44. doi: 10.1097/ICO.0b013e31820156a9. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, Viaño J. Sturge-Weber syndrome: Study of 55 patients. Can J Neurol Sci. 2008;35:301–7. doi: 10.1017/s0317167100008878. [DOI] [PubMed] [Google Scholar]
- 12.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–7. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 13.Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, et al. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet. 1998;62:325–33. doi: 10.1086/301725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84:664–71. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur K, Reddy AB, Mukhopadhyay A, Mandal AK, Hasnain SE, Ray K, et al. Myocilin gene implicated in primary congenital glaucoma. Clin Genet. 2005;67:335–40. doi: 10.1111/j.1399-0004.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 16.Khan AO. Genetics of primary glaucoma. Curr Opin Ophthalmol. 2011;22:347–55. doi: 10.1097/ICU.0b013e32834922d2. [DOI] [PubMed] [Google Scholar]
- 17.Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14:422–8. doi: 10.1038/eye.2000.126. [DOI] [PubMed] [Google Scholar]
- 18.Genĉík A. Epidemiology and genetics of primary congenital glaucoma in Slovakia. Description of a form of primarycongenital glaucoma in gypsies with autosomal-recessive inheritance and complete penetrance. Dev Ophthalmol. 1989;16:76–115. [PubMed] [Google Scholar]
- 19.Martin SN, Sutherland J, Levin AV, Klose R, Priston M, Héon E. Molecular characterisation of congenital glaucoma in a consanguineous Canadian community: A step towards preventing glaucoma related blindness. J Med Genet. 2000;37:422–7. doi: 10.1136/jmg.37.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokotas H, Petersen MB. Clinical and molecular aspects of aniridia. Clin Genet. 2010;77:409–20. doi: 10.1111/j.1399-0004.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 21.Chisholm IA, Chudley AE. Autosomal dominant iridogoniodysgenesis with associated somatic anomalies: Four-generation family with Rieger's syndrome. Br J Ophthalmol. 1983;67:529–34. doi: 10.1136/bjo.67.8.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortemousque B, Amati-Bonneau P, Couture F, Graffan R, Dubois S, Colin J, et al. Axenfeld-Rieger anomaly: A novel mutation in the forkhead box C1 (FOXC1) gene in a 4-generation family. Arch Ophthalmol. 2004;122:1527–33. doi: 10.1001/archopht.122.10.1527. [DOI] [PubMed] [Google Scholar]
- 23.Vajsar J, Schachter H. Walker-Warburg syndrome. Orphanet J Rare Dis. 2006;1:29. doi: 10.1186/1750-1172-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bornemann A, Pfeiffer R, Beinder E, Wenkel H, Schlicker U, Meyermann R, et al. Three siblings with Walker-Warburg Syndrome. Gen Diagn Pathol. 1996;141:371–5. [PubMed] [Google Scholar]
- 25.Berry SA, Peterson C, Mize W, Bloom K, Zachary C, Blasco P, et al. Klippel-Trenaunay syndrome. Am J Med Genet. 1998;79:319–26. [PubMed] [Google Scholar]
- 26.Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509–16. doi: 10.1177/08830738030180080701. [DOI] [PubMed] [Google Scholar]
- 27.Vogt G, Horváth-Puhó E, Czeizel AE. A population-based case-control study of isolated primary congenital glaucoma. Am J Med Genet A. 2006;140:1148–55. doi: 10.1002/ajmg.a.31276. [DOI] [PubMed] [Google Scholar]


