Abstract
Background:
Though the use of prostaglandin analogues (PGA) for reduction of intraocular pressure (IOP) has shown a marked increase, studies evaluating the contralateral effects of PGA are limited.
Aims:
To evaluate if PGA treatment in one eye has an effect on the IOP of the untreated fellow eye.
Design:
Retrospective study.
Materials and Methods:
Thirty patients of open-angle glaucoma with no previous antiglaucoma treatment underwent 24-hour diurnal IOP phasing. They subsequently were started on a uniocular trial with PGA, and had office diurnal IOP measurements 6 weeks later. Twenty-four hour diurnal consisted of 8 IOP readings over 24 hours and office diurnal consisted of 4 IOP readings between 8 AM and 6 PM at 3 hourly intervals.
Statistical Analysis:
IOPs of the fellow eye during the office diurnal were compared with IOPs at similar time points during the 24-hour diurnal using paired t-tests.
Results:
Mean (± standard deviation) IOP in the treated eye reduced (P < 0.001) from 17.17 ± 3.2 mm Hg at baseline to 13.7 ± 2.4 mm Hg at 6 weeks, while that in the untreated eye reduced from 16.4 ± 3.1 mm Hg to 14.8 ± 2.7 mm Hg (P = 0.01). The decrease in IOP in the untreated fellow eye was statistically significant at 8 AM (2.7 mm Hg, P = 0.003) and 11 AM (2.3 mm Hg, P = 0.01) but not so at 2 PM (1.2 mm Hg, P = 0.10) and 5 PM (0.9 mm Hg, P = 0.19). The amount of IOP reduction in the untreated eye was significantly associated with the magnitude of IOP reduction in the treated eye (β = 0.69, P = 0.008).
Conclusion:
Uniocular PGA treatment tends to reduce the IOP of the untreated fellow eye.
Keywords: Contralateral effect, intraocular pressure reduction, prostaglandin analogue
The uniocular therapeutic trial helps to differentiate between the therapeutic effect of a topical antiglaucoma medication on intraocular pressure (IOP) and the background fluctuations of IOP.[1] In this procedure, antiglaucoma medication is started in one eye, and the response to the medication is evaluated after 4-6 weeks. Untreated fellow eye serves as a control for the natural fluctuations of IOP. Therapeutic response to the medication in the treated eye is determined by subtracting the change in IOP of the untreated fellow eye from the change in IOP of the treated eye between the two visits;[2] one of the assumptions here is that there is no contralateral response of the drug in the fellow eye. The presence of a significant contralateral effect therefore underestimates the true effect of the antiglaucoma medication.
Various studies have shown that topical beta adrenergic blockers have a definite IOP lowering effect in the contralateral eye. The magnitude of IOP reduction seen in the contralateral eye with topical beta-blocker treatment has been reported to be between 1 and 5 mm Hg.[3,4,5,6] Topical alpha-adrenergic agonists have also been reported to have a contralateral effect.[7,8,9] Similar studies evaluating the contralateral effects of prostaglandin analogues (PGA) are sparse,[5,10] despite PGA showing a marked increase in their use for reduction of IOP over the past decade.[11] The purpose of this study was to evaluate if PGA treatment in one eye has an effect on the IOP of the untreated fellow eye.
Materials and Methods
This was a retrospective analysis of all newly diagnosed primary open-angle glaucoma (POAG) patients who underwent a 24-hour diurnal IOP phasing in a tertiary eye care center in South India between 2004 and 2009. Glaucoma was diagnosed if the optic disk showed signs of glaucomatous damage (focal or diffuse neuroretinal rim thinning, localized notching or nerve fiber layer defects) and reliable standard automated perimetry (SAP) results showed corresponding visual field defects. Twenty four-hour diurnal IOP phasing was advised only if the IOP at presentation was <21 mm Hg in both eyes. None of these patients were on any antiglaucoma medications previously. The purpose of a 24-hour diurnal IOP phasing was to determine the peak and trough IOPs, the time at which the peak IOP occurred, and the range of IOP fluctuation. The institutional review board of L V Prasad Eye Institute approved the study and all methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects. The current study included only those patients who were started on a PGA in one eye following the 24-hour diurnal IOP phasing and subsequently had an office diurnal IOP recording after 6 weeks.
Before the 24-hour diurnal IOP phasing, all patients underwent a comprehensive ophthalmic examination including review of medical history, visual acuity testing, slit-lamp biomicroscopy, IOP measurement using Goldmann applanation tonometry (GAT), gonioscopy, central corneal thickness (CCT) measurement, dilated fundoscopic examination, and SAP with Swedish Interactive Threshold Algorithm, either 24-2 or 30-2 (Carl Zeiss Meditec Inc. Dublin, CA).
CCT was measured using ultrasound pachymetry (AL - 1000, Tomey Corporation, Noritake Shinmachi, Nishi-Ku, Nagoya). The ultrasonic velocity was set to 1640 m/s for the CCT measurements. A drop of topical anesthetic was instilled into the lower fornix and the CCT measurement was taken by placing the probe perpendicular to the corneal surface on the center of the cornea. One measurement was taken for each eye. The single measurement mode used calculates the average of 10 sets of pulses and provides the mean and the standard deviation of each from the mean. If the standard deviation was more than 5 μm, it was deleted and a new reading taken.
Twenty-four-hour diurnal IOP phasing consisted of eight IOP recordings during the day and night at 3 hourly intervals, measured by an ophthalmology fellow using GAT at 11 AM, 2 PM, 5 PM, 8 PM, and 11 PM on day 1 and at 2 AM, 5 AM and 8 AM the following day. All IOP measurements were recorded within 30 minutes of the specified time points. The same ophthalmology fellow recorded all IOP measurements of a particular patient, on the same slit lamp in a sitting position. The order of measurement (right eye first or left eye first) was not fixed and was at the discretion of the examiner. Based on the diurnal IOP recordings, uniocular trial with PGA was started in the eye either with higher IOP or with more advanced optic nerve/visual field damage depending on physician's discretion. The choice of the PGA was also physician's choice. All patients were advised to instill the medication at bedtime.
The office diurnal IOP checkup was carried out 6 weeks after starting uniocular drug trial to assess the efficacy of therapy. Before the office diurnal IOP checkup, compliance to treatment was specifically asked for and the test carried out only if the patient was found to be compliant. This consisted of IOP recordings using GAT at 8 AM, 11 AM, 2 PM, and 5 PM on the same day by a trained optometrist in the clinic. All IOP measurements were recorded within 30 minutes of the specified time points. All IOP measurements of a particular patient were recorded by the same optometrist, on the same slit lamp and in a sitting position.
To investigate if PGAs had a contralateral IOP lowering effect, the IOP measurements of the untreated fellow eye recorded at the office diurnal visit were compared with the IOP measurements at similar time points during 24-hour diurnal visit.
Statistical analysis
Descriptive statistics included mean and standard deviation for normally distributed variables and median, first quartile, and third quartile values for non-normally distributed variables. IOP changes in the treated and the fellow eye between the 24-hour diurnal and the post-treatment office diurnal visits were compared using the Student t-test for paired comparisons of continuous variables. The effects of age, gender, laterality, baseline IOP, amount of IOP change in the treated eye, and the type of PGA used in the treated eye on the magnitude of IOP change in the untreated fellow eye were evaluated using the multivariate regression modeling approach.
Statistical analyses were performed using commercial software (Stata ver. 10.0; StataCorp, College Station, TX). A P value of ≤0.05 was considered statistically significant.
Results
Between 2004 and 2009, 187 patients of open-angle glaucoma underwent 24-hour diurnal IOP recordings. Thirty of these patients were started on unilateral PGA therapy and underwent office diurnal IOP recordings 6 weeks later. Demographic and clinical features of these patients are shown in Table 1. Mean deviation (P = 0.03) and pattern standard deviation (P = 0.01) were worse in the eyes which were treated compared to the untreated fellow eye. IOP (P = 0.44), best corrected visual acuity (P = 0.78), and central corneal thickness (P = 0.72) were comparable between the eyes. Eleven of these patients were started on latanoprost (Xalatan, Pfizer, New York, NY), 11 on bimatoprost (Lumigan, Allergan, Irvine, CA) and 8 on travoprost (Travatan, Alcon, Fort Worth, TX).
Table 1.
Demographic and clinical characteristics of the study patients (n=30)
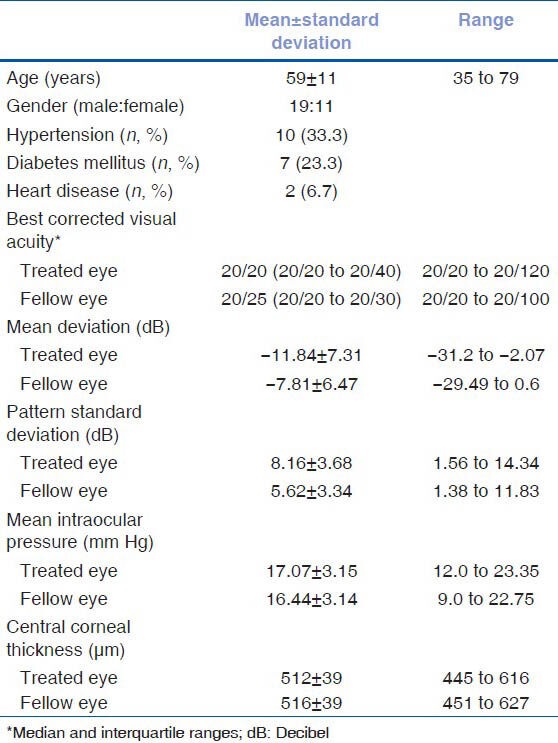
Table 2 and Figure 1 show the IOPs recorded during the 24-hour diurnal in the treated eyes as well as during the office diurnal visit, 6 weeks post-treatment. The IOP decreased significantly at all time points. Table 2 and Figure 2 show the IOPs recorded in the untreated fellow eyes during the 24-hour diurnal visit and the office diurnal visit, 6 weeks after the other eye treatment. The mean as well as the range of IOP in the untreated fellow eye reduced significantly during the office diurnal recording. The IOP decreased significantly at 8 AM and 11 AM time points. Though the IOPs at 2 and 5 PM time points also showed a decrease, these were not statistically significant. The distribution of the amount of IOP change in the treated and the untreated fellow eyes is shown in Figure 3. Five treated eyes (17%) showed either no change or an increase in the IOP. Analyzing the amount of contralateral effect in individual patients, 20 patients showed a decrease in the IOP of the untreated fellow eye. Of these, 13 patients showed a mean IOP decrease of more than 2 mm Hg, 11 showed a decrease of more than 3 mm Hg and 7 showed a decrease of more than 5 mm Hg. Of the 10 patients who showed no change or an increase in the IOP of the untreated fellow eye, 5 showed no change or an increase in the IOP of the treated eye. Mean (± standard deviation) IOP change in eyes treated with latanoprost (-4.4 ± 2.9), bimatoprost (-3.3 ± 3.5), and travoprost (-1.8 ± 4.1) were not statistically significantly different (P = 0.30) from each other. The mean (± standard deviation) IOP change in untreated fellow eyes of patients whose other eye was treated with latanoprost (-2.6 ± 3.0), bimatoprost (-1.5 ± 3.1), and travoprost (-0.47 ± 4.4) was also not statistically significantly different (P = 0.41).
Table 2.
Intraocular pressure (IOP, in mm Hg) in the treated and untreated eyes
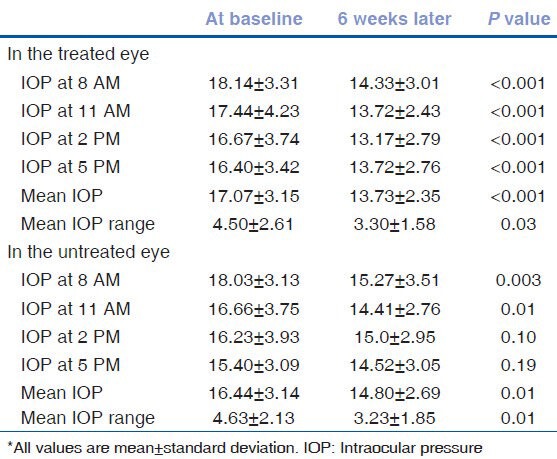
Figure 1.
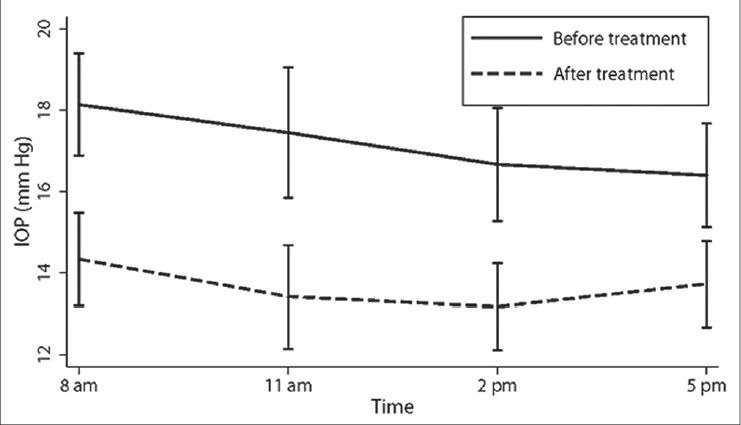
Intraocular pressures in the treated eyes before and 6 weeks after treatment. Vertical lines represent 95% confidence limits
Figure 2.
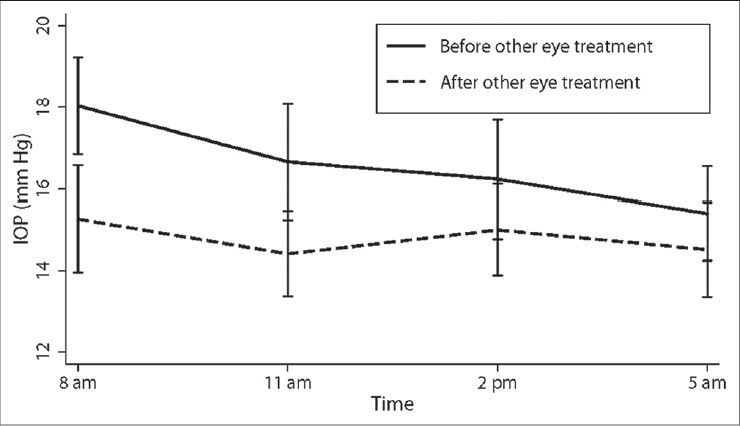
Intraocular pressures in the untreated fellow eyes before and 6 weeks after the other eye treatment. Vertical lines represent 95% confidence limits
Figure 3.
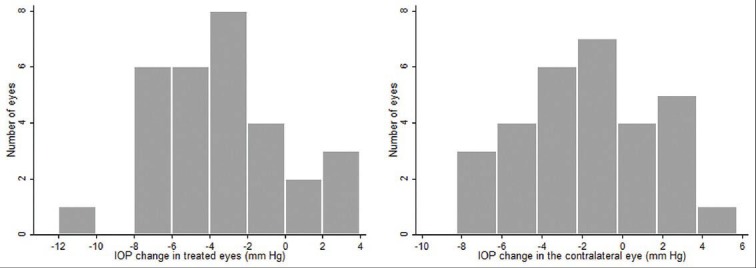
Distribution of the amount of intraocular pressure change in the treated and the untreated fellow eyes
Table 3 shows the percentage reduction of IOP in the treated and fellow eyes as well as the magnitude of contralateral effect in relation to the effect in the treated eye. The magnitude of contralateral effect was highest during the early hours of the day and gradually decreased as the day progressed [Figure 2].
Table 3.
Percentage of intraocular pressure reduction in the treated eye, untreated eye and the magnitude of contralateral effect with respect to the effect in the treated eye
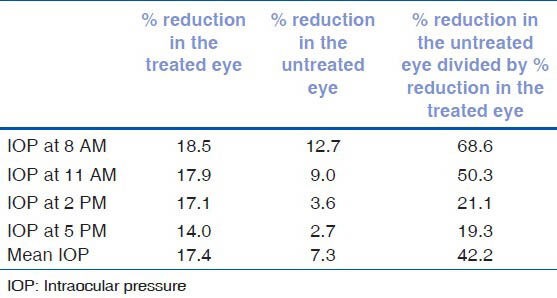
Table 4 shows the factors affecting the magnitude of contralateral effect. The only factor significantly associated with the magnitude of contralateral effect was the amount of IOP reduction in the treated eye. Figure 4 shows the significant association (R2 = 0.59, P < 0.001) between the magnitude of contralateral effect and the amount of IOP reduction in the treated eye.
Table 4.
Factors associated with the amount of intraocular pressure reduction in the untreated fellow eye
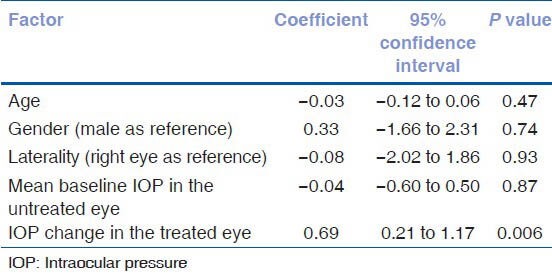
Figure 4.
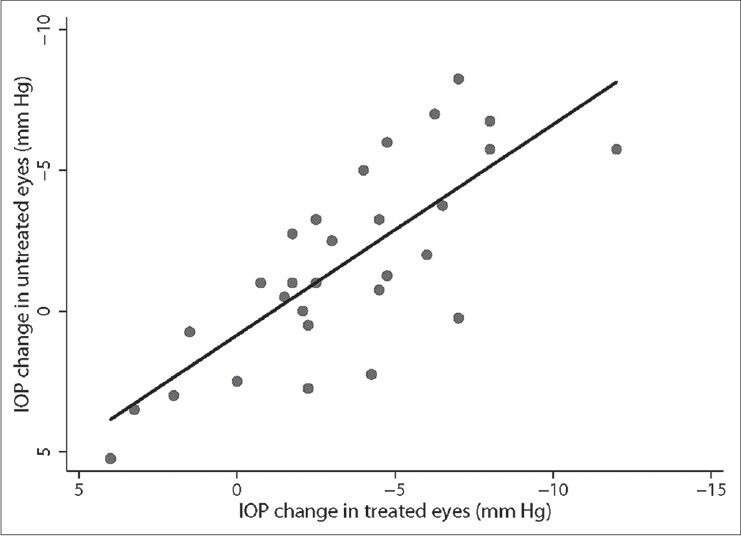
Relationship between the magnitude of contralateral effect and the amount of intraocular pressure reduction in the treated eye
Discussion
In this study, we found that PGA treatment in one eye tends to have an effect on the IOP of the untreated fellow eye. The contralateral IOP lowering effect, however, was significant only during the initial 12 hours after instillation but was insignificant during the latter 12 hours.
The widely quoted study for the absence of contralateral effect of PGA is a randomized study comparing the IOP lowering effect of latanoprost with that of timolol, by Alm et al.[5] published in 1995. Assessing that the contralateral response of PGA was not the primary aim of this study and uniocular PGA was only administered to patients with unilateral glaucoma. Also the authors provided no data on the actual IOPs in the treated and the untreated fellow eyes of patients on uniocular PGA treatment. The only other study on the contralateral IOP lowering effect of PGA was a recent report by Newman et al.[10] published in 2010, which in nine patients treated with uniocular PGA found no contralateral IOP lowering effect. Another recent study by Bhorade et al.[2] while evaluating the utility of uniocular PGA trial found that the amount of IOP reduction with PGA when the baseline IOP of the same eye was used as control (unadjusted method) was equivalent to that when the fellow eye was used as control (adjusted method). This indirectly suggested that PGA had no contralateral IOP lowering effect.
In our study, the average IOP reduction seen in the untreated eye was 40% of that seen in the treated eye (2 mm Hg). The mechanism of contralateral effect is not clear. The most widely accepted mechanism of contralateral effect of topical beta-blockers is systemic absorption of the drug, primarily through the nasolacrimal mucosa and transport of the drug to the fellow eye via the blood stream.[3,4,6,12,13] This may be the mechanism in the case of PGA too. Though the plasma elimination half-life of PGA is short, the clearance high, and the volume of distribution small, a study by Sjöquist and Stjernschantz has shown that PGA does attain a detectable concentration in plasma after topical administration.[14] However the concentration in plasma is only one in thousand times that in the aqueous humor. The other mechanisms proposed for the contralateral effect of topical beta-blockers are a centrally mediated effect of the systemically absorbed drug and a consensual ophthalmotonic reaction in which alterations in the IOP of one eye results in a reflex IOP change in the fellow eye.[15,16,17] IOP reduction in our study was greatest at 8 AM and the effect gradually decreased over the day. This may be related to the peak effect of PGA which is reported to occur 12 hours after instillation.[18]
Analyzing the factors associated with the magnitude of contralateral effect, we found that the amount of IOP reduction in the treated eye correlated significantly with IOP reduction in the fellow eye. Piltz et al.[6] while evaluating the contralateral effect of topical beta-blockers also found that the magnitude of contralateral IOP reduction was significantly associated with the amount of IOP reduction in the treated eye. Ten patients in our study showed no change or an increase in the IOP in the contralateral eye. Five of these showed no change or increase in IOP in the treated eye too. These 5 of 30 patients (17%) likely represent “non-responders” to PGAs.
Looking at the diurnal curves of IOP during the day, IOP tended to be higher in the mornings and decreased toward the evenings. This is similar to that found in earlier studies.[19,20,21] This pattern was less obvious in eyes on treatment with PGA similar to that reported previously.[19,20]
We found the mean percentage reduction of IOP in the treated eyes to be 17%. This is less than that reported with PGA in other studies, where the average IOP reduction was close to 30%.[22] A multicentric study in Indian patients also reported a mean IOP reduction of 35% (24.9 mm Hg to 16.1 mm Hg) with latanoprost.[23] We also found that eyes with higher mean baseline IOP had significantly more reduction in IOP after treatment. For every 1 mm higher baseline IOP, percentage of IOP reduction was greater by 4% (P < 0.001). Such a relationship between IOP reduction and baseline IOP in eyes treated with PGA as well as beta-blockers has been reported by Camras et al.[24] Thus, the lesser IOP reduction seen in our study may be related to the lower mean baseline IOP in this cohort (17 mm Hg).
The presence of a contralateral effect of PGA has implications on unilateral PGA trials. Our results show that if the contralateral eye is used as a control, the true therapeutic effect of the PGA may be underestimated. Using the IOP of the same eye before treatment as control may be a better way to estimate the therapeutic effect of PGAs.
The limitations to the current study are because of its small sample size and retrospective nature. First is the bias in IOP measurements. Different observers recorded the IOP before and after the treatment. Lack of masking also would have introduced bias to the measurements. Second, “regression to mean” is a well-recognized statistical phenomenon in studies relating to IOP measurements where observations that are high relative to the mean regress toward the mean and have a lower value when measured the second time.[25] The effect of “regression to mean” on the contralateral effect cannot be completely ruled out in our study. Including a control group of patients with no antiglaucoma treatment to either of the eyes would have helped us to evaluate this issue. However delaying treatment would not have been ethical in these eyes. Only one IOP recording was taken at all time points in our study. An average of multiple IOP recordings at each time point might have reduced the “regression to mean” effect. Third, none of these patients had any previous experience of using antiglaucoma medications and they were not monitored during the 6-week period. The possibility of improper use of medication (an anxious patient using medication to both eyes) or medication spilling over to the fellow eye cannot be ruled out.
In conclusion, PGA treatment in one eye tends to have an effect on the IOP of the untreated fellow eye. The contralateral IOP lowering effect, however, was significant only during the initial 12 hours after instillation but was insignificant during the latter 12 hours. Future prospective studies with adequate sample size are needed to conclusively address this issue.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.San Francisco, CA: American Academy of Ophthalmology; 2005. [Last accessed on 2010 Dec 9]. American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern. Primary Open-Angle Glaucoma; p. 13. Available from: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=ca9ec1b5-2567-4e85-96f6-b6540e5ac5a1 . [Google Scholar]
- 2.Bhorade AM, Wilson BS, Gordon MO, Palmberg P, Weinreb RN, Miller E, et al. The utility of the monocular trial: Data from the ocular hypertension treatment study. Ophthalmology. 2010;117:2047–54. doi: 10.1016/j.ophtha.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwitko GM, Shin DH, Ahn BH, Hong YJ. Bilateral effects of long-term monocular timolol therapy. Am J Ophthalmol. 1987;104:591–4. doi: 10.1016/0002-9394(87)90169-3. [DOI] [PubMed] [Google Scholar]
- 4.Dunham CN, Spaide RF, Dunham G. The contralateral reduction of intraocular pressure by timolol. Br J Ophthalmol. 1994;78:38–40. doi: 10.1136/bjo.78.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alm A, Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group Ophthalmology. 1995;102:1743–52. doi: 10.1016/s0161-6420(95)30798-1. [DOI] [PubMed] [Google Scholar]
- 6.Piltz J, Gross R, Shin DH, Beiser JA, Dorr DA, Kass MA, et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am J Ophthalmol. 2000;130:441–53. doi: 10.1016/s0002-9394(00)00527-4. [DOI] [PubMed] [Google Scholar]
- 7.Toris CB, Gleason ML, Camras CB, Yablonski ME. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995;113:1514–7. doi: 10.1001/archopht.1995.01100120044006. [DOI] [PubMed] [Google Scholar]
- 8.Yuksel N, Karabas L, Altintas O, Yildirim Y, Caglar Y. A comparison of the short-term hypotensive effects and side effects of unilateral brimonidine and apraclonidine in patients with elevated intraocular pressure. Ophthalmologica. 2002;216:45–9. doi: 10.1159/000048296. [DOI] [PubMed] [Google Scholar]
- 9.Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128:8–14. doi: 10.1016/s0002-9394(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 10.Newman H, Kurtz S, David R. Intraocular pressure changes in the contralateral eye after topical treatment: Does an “ophthalmotonic consensual reaction” exist? Isr Med Assoc J. 2010;12:568–71. [PubMed] [Google Scholar]
- 11.De Natale R, Draghi E, Dorigo MT. How prostaglandins have changed the medical approach to glaucoma and its costs: An observational study of 2228 patients treated with glaucoma medications. Acta Ophthalmol Scand. 2004;82:393–6. doi: 10.1111/j.1395-3907.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 12.Saari KM, Ali-Melkkila T, Vuori ML, Kaila T, Iisalo E. Absorption of ocular timolol: Drug concentrations and beta-receptor binding activity in the aqueous humour of the treated and contralateral eye. Acta Ophthalmol (Copenh) 1993;71:671–6. doi: 10.1111/j.1755-3768.1993.tb04659.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin XD, Rabineau PA. Intraocular pressure effects of timolol after unilateral instillation. Ophthalmology. 1988;95:1620–3. doi: 10.1016/s0161-6420(88)32966-0. [DOI] [PubMed] [Google Scholar]
- 14.Sjoquist B, Stjernschantz J. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol. 2002;47(Suppl 1):S6–12. doi: 10.1016/s0039-6257(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 15.Radius RL, Diamond GR, Pollack IP, Langham ME. Timolol. A new drug for management of chronic simple glaucoma. Arch Ophthalmol. 1978;96:1003–8. doi: 10.1001/archopht.1978.03910050527005. [DOI] [PubMed] [Google Scholar]
- 16.Gibbens MV. The consensual ophthalmotonic reaction. Br J Ophthalmol. 1988;72:746–9. doi: 10.1136/bjo.72.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbens MV. Sympathetic influences on the consensual ophthalmotonic reaction. Br J Ophthalmol. 1988;72:750–3. doi: 10.1136/bjo.72.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters TR, DuBiner HB, Carpenter SP, Khan B, VanDenburgh AM. 24-Hour IOP control with once-daily bimatoprost, timolol gel-forming solution, or latanoprost: A 1-month, randomized, comparative clinical trial. Surv Ophthalmol. 2004;49(Suppl 1):S26–35. doi: 10.1016/j.survophthal.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004;138:389–95. doi: 10.1016/j.ajo.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH. Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol. 2006;141:1131–3. doi: 10.1016/j.ajo.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Dinn RB, Zimmerman MB, Shuba LM, Doan AP, Maley MK, Greenlee EC, et al. Concordance of diurnal intraocular pressure between fellow eyes in primary open-angle glaucoma. Ophthalmology. 2007;114:915–20. doi: 10.1016/j.ophtha.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 22.van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: A meta-analysis of randomized clinical trials. Ophthalmology. 2005;112:1177–85. doi: 10.1016/j.ophtha.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Thomas R, Parikh R, Sood D, Vijaya L, Sekhar GC, Sood NN, et al. Efficacy and safety of latanoprost for glaucoma treatment: A three-month multicentric study in India. Indian J Ophthalmol. 2005;53:23–30. doi: 10.4103/0301-4738.15281. [DOI] [PubMed] [Google Scholar]
- 24.Camras CB, Hedman K. Rate of response to latanoprost or timolol in patients with ocular hypertension or glaucoma. J Glaucoma. 2003;12:466–9. doi: 10.1097/00061198-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bhorade AM, Gordon MO, Wilson B, Weinreb RN, Kass MA. Variability of intraocular pressure measurements in observation participants in the ocular hypertension treatment study. Ophthalmology. 2009;116:717–24. doi: 10.1016/j.ophtha.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


