Abstract
Purpose of review
The purpose of this review is to consider the recent literature pertaining to the neurobiology, genetics and treatment of Tourette syndrome (TS).
Recent findings
Over the last several years, both neuropathological and genetic findings have further focused attention on long-standing hypotheses regarding the role of the basal ganglia in the etiology of tics and TS. Moreover, while the field awaits the results the first large-scale genetic studies, recent findings have already mirrored developments in the neuropsychiatric genetics literature more broadly, highlighting the value of the study of rare variation and the overlap of risks among seemingly disparate diagnostic categories. Finally, treatment studies have underscored the importance of cognitive-behavioral as well as pharmacological interventions for the treatment of tic disorders.
Summary
Recent findings have led to novel, testable hypotheses regarding the molecular and cellular mechanisms underlying TS. These, in turn, have begun to provide new avenues to conceptualizing treatment strategies. While the development of additional medication options is a pressing need, recent data has demonstrated both the safety and efficacy of non-pharmacological approaches.
Keywords: Tourette syndrome, striatal interneurons, histaminergic neurotransmission, Habit Reversal Therapy
Introduction
Tourette syndrome (TS) is a potentially disabling developmental neuropsychiatric disorder defined by the combination of persistent brief, repetitive, non-rhythmic motor movements and vocalizations. Despite a longstanding interest in the neurobiology and genetics of the disorder, knowledge regarding specific cellular and molecular mechanisms has remained limited. Compared with other neuropsychiatric syndromes of similar prevalence, the volume of research into TS over the past decade has been relatively modest. Nonetheless, recent findings with regards to the neurobiology and genetics of the syndrome have begun to clarify etiological mechanisms and have pointed to unexpected opportunities for the development of novel therapeutics. In addition, recent data regarding non-pharmacological interventions are offering considerable hope to patients and their families.
Phenomenology
The typical age of onset for TS is in early childhood, with motor symptoms most often preceding vocal tics by several years. Tics wax and wane; tend to occur in bouts; fluctuate in frequency and intensity, and crescendo and then diminish through late adolescence. In fact, only ~20% of affected individuals endorse functional impairment as a consequence of tics at age 20 [1]. There is also aconsiderable population of individuals who suffer either from chronic motor or chronic vocal tics, but not both, and these individuals are considered as being on a TS spectrum of disorders.
The prevalence of TS is estimated at between 0.3 and 1% [2,3]. However, the epidemiological literature is limited, and characterizations of the natural history tend to reflect a strong ascertainment bias. Many individuals with vocal and or motor tics do not seek medical attention. The vast majority of individuals who do, also suffer from other neuropsychiatric symptoms. Upwards of 50% of TS probands seen in clinical settings have attention deficit hyperactivity disorder (ADHD) or obsessive-compulsive disorder (OCD)[4-10]. Learning disabilities, mood and anxiety disorders are also quite common [11-13]. In fact these co-morbid conditions often dominate the clinical picture, and their occurrence along with tics, rather than tics in isolation, can present the most pressing challenges for clinical management
Recent Advances in Neurobiology
Several lines of data provide compelling evidence that the basal ganglia, especially the striatum, are a locus of pathology in TS [14]. The caudate and putamen are reduced in size in medication-naïve patients with TS, relative to matched controls [15]; smaller volumes in children predict increased symptom severity in adulthood [16]. Functional neuroimaging studies have demonstrated an increase in baseline activity in ventral and midline striatum, which is thought to correlate with tics, as well as increased activity in orbitofrontal and other cortical regions, which may better correlate with symptomatology in impulse control and attentional domains [17]. Activity in striatum, as well as in prefrontal and premotor cortex, correlates with the frequency and intensity of tics [18] and voluntary tic suppression leads to activation of the basal ganglia, as well as of afferent cortical areas. The magnitude of this activation correlates inversely with tic severity [19].
Despite a long-standing interest in the contribution of the basal ganglia to TS, it is only relatively recently that reproducible cellular abnormalities in these regions have been identified in post mortem studies. Using unbiased stereological counts in brains from adult TS patients and matched controls, Flora Vaccarino's lab at Yale [20] found a reduction in parvalbumin-expressing fast-spiking interneurons in the dorsal striatum of affected individuals. In contrast, the number and density of these neurons in the globus pallidus, where they constitute the principal cell type, was increased in patients. This may indicate a developmental abnormality in cell migration, whereby an approximately normal number of parvalbumin-expressing neurons is generated, but become abnormally distributed between striatum and globus pallidus.
A recent follow-up study from the same lab replicated this finding in a somewhat larger series of patients [21]. In addition, a second population of striatal interneurons, the cholinergic tonically active neurons (TANs), was reduced in the dorsal striatum (both caudate and putamen). In contrast, neither medium spiny neurons (MSNs), the predominant cell type in the striatum, nor medium-sized calretinin-positive interneurons were decreased.
These post-mortem findings provide a useful framework for conceptualizing the genesis of tics and their neuro-developmental etiology of TS. Understanding the origins of these defined populations of interneurons may lead to new insights into regarding the manner in which genetic, toxic, infectious, hypoxic, or other environmental insults disrupt their division, migration, differentiation, or integration into local circuits and thus lead to the types of circuit-level abnormalities thought to underlie TS. While certainly not yet conclusive, the combination of the characteristic ontogenic trajectory of symptoms [22] and the considerable evidence for a genetic contribution (see below) suggests that developmental disruption plays a key role.
The interaction of environmental factors and disrupted neurodevelopment is suggested by the finding that neonatal hypoxia is a predictor of tic severity in TS [22,23], though it is worth noting that this finding has not been uniformly observed across all studies [24]. Maternal smoking, which can lead to a chronic hypoxic state, has similarly been associated with severity of TS in offspring. In experimental animals, developmental hypoxia appears to produce particular damage to interneurons, at least in cortex [25] though this phenomenon has not yet been documented in detail in the striatum. Moreover, these studies have not shown hypoxia to have a preferential effect on parvalbumin-expressing or cholinergic interneurons. It may be that environmental insults interact with genetic or other risk factors to engender the specificity suggested by neuropathological findings [21].
The observed inter-neuronal pathology is consistent with a long-standing conceptualization of TS, at least in part, as involving excessive dopamine. This notion is supported by model systems data, neuro-imaging studies (though these have not been entirely consistent in this regard) and the observation that neuroleptic medications, which block the D2 dopamine receptor, remain the most effective pharmacotherapy for TS [26]. Acetylcholine (ACh), which in the striatum derives exclusively from the TANs, is thought to exist in an antagonistic balance with striatal dopamine (DA): ACh can reduce dopamine release in the striatum, at least at lower firing frequencies [27]; anticholinergic medications can treat the dystonic effects of D2 antagonism; and ACh and DA are oppositely regulated by reinforcement during certain learning tasks [28]. A reduction in TAN number in TS, therefore, may lead to disinhibited and excessive dopamine tone, producing a hyper-dopaminergic state in TS.
Recent Advances in the genetics of TS
The familial nature of TS has been well appreciated from its initial description by Gilles de la Tourette in 1885. Twin studies, though relatively modest in size, have long supported a high degree of heritability [29]. Nonetheless after two decades of inquiry, there remains considerable uncertainty regarding the specific nature of the genetic contribution.
The notion that TS is a single gene, autosomal dominant disorder is vestigial. Early studies focused on extended, densely affected multigenerational pedigrees that suggested Mendelian inheritance [30-33]. However, no TS locus was identified via studies of these pedigrees, and a single gene hypothesis has been abandoned. More recent segregation analyses [34-36] point to complex inheritance.
It is worth noting here that, in comparison to many other neuropsychiatric syndromes, the volume of data from genetic studies in TS remain quite limited. Given the increasing appreciation of the critical contribution of large sample size to the study of common disorders [37], many fundamental questions remain regarding the overall genomic architecture of TS, including the potential contribution of common polymorphisms, the relevance of transmitted and de novo submicroscopic copy number variation, and whether sporadic and familial forms of TS reflect distinct genetic mechanisms, as has been suggested in other developmental neuropsychiatric disorders [38].
The first phase of a large-scale genome wide association study is reaching completion, promising to begin to en toto illuminate these issues. And while the literature is modest compared to studies of autism, schizophrenia, ADHD or bipolar disorder, very recent findings in TS genetics nonetheless reflect two important general trends in psychiatric genetics: the identification of shared genetic risks across diagnoses that have previously been conceptualized as entirely distinct; and an increasing appreciation of the importance of the study of rare variation in common disease.
Within the past year, the first study of copy number variation in TS was published by Sundaram and colleagues [39], reporting on 184 individuals (111 probands). The authors used state-of-the-art methods to control for potential confounds and, even in this small sample, identified a overlap of genetic risks among diagnostically distinct syndromes, identifying rare copy number variations (CNVs) previously implicated in autism spectrum disorders and schizophrenia, including the gene NRNX1 and a specific region of chromosome 1q21.
These finding echo several older studies showing a similar convergence. As convincing data has emerged with regard to the overlap of risks for specific CNVs in autism, schizophrenia, epilepsy and intellectual disability [40-42], these findings have been re-cast in a new light. Specifically, in 2003 a 7q35-q36 was found to disrupt the CNTNAP2 chromosomal insertion at locus in three affected individuals from a single family [43]. This was the first suggestion of a role for this gene in neuropsychiatic disorders, and subsequently, highly penetrant recessive mutations have been convincingly demonstrated in epilepsy, intellectual and social disability [44-48]. Similarly, a deletion involving coding segments of the gene NLGN4X (neuroligin 4X) were identified in a family with TS and autism, learning difficulties, anxiety and depression[49]. This gene has been strongly implicated in ID and ASD [50,51].
These studies highlight the increasing likelihood that specific genetic variations disrupting key molecular pathways underlying neuro-developmental processes may manifest in a wide range of behavioral and cognitive phenotypes. They also point to the relevance of studying mutations found in less than 1% of the general population.
The value of the discovery of rare variants carrying large effect sizes has also been demonstrated this past year by our laboratory, through a parametric linkage study of a densely affected family, in which a father and his 8 children were affected by TS and obsessive compulsive disorder [52]. Traditional linkage mapping revealed a single region of the genome reaching the maximum theoretical LOD score (Lod =2.1) for the pedigree and sequencing of all known genes in the linkage interval revealed a single nonsense mutation, in the gene L-histidine decarboxylase (HDC), the rate-limiting enzyme in histamine biosynthesis. While this gene was not initially prioritized in our sequencing efforts, after the identification of the demonstrably functional, extremely rare mutation, its potential impact on dopaminergic function was of great interest, given hypotheses regarding the role of excessive dopaminergic activity in the genesis of tics (figure 1).
Figure 1.
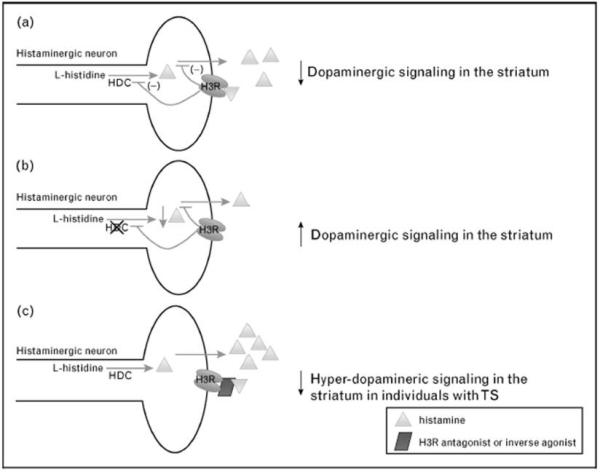
A highly simplified model of the role of l-histidine decarboxylase (HDC) and histaminergic neurotransmission in the genesis or modulation of TS. A) HDC is the rate-limiting enzyme in the biosynthesis of histamine which is known to modulate a variety of neurotransmitters, including dopamine; B) The rare mutation identified in [52] is thought to result in a decrease in histamine synthesis and, consequently, a reduced capacity to regulate dopamine signaling; C) Antagonists and inverse agonists of the pre-synaptic auto-receptor H3R increase both histamine synthesis and release by removing the inhibitory effects of activation of the H3R presynaptic auto-receptor. Based on a model of TS that involves either a sustained or transient hyper-dopaminergic state, H3R compounds would be hypothesized, based on this model, to be an alternative to direct dopamine receptor blockade in the treatment of tics.
Histaminergic (HA) neurotransmission in the brain is mediated by three of the four known G-protein coupled histamine receptors (H1-H4). Both histamine 2 (H2R) and histamine 3 (H3R) receptors are significantly enriched in the human and rodent striatum [53], and H3R is acts as a pre-synaptic auto-receptor on HA projection neurons, as a pre-synaptic receptor on non-HA containing neurons that regulates a variety of neurotransmitters, including dopamine and serotonin, and as a post-synaptic receptor, particularly enriched in the striatum and co-localizing both D1 and D2 receptors. HDC null mice show decreased brain HA and increased sensitivity to the hyper-locomotor effects of DA agonists [54].
While the identification of a very rare mutation in a single family may shed little light on the population genetics of TS, it nonetheless sets the stage for a number of interesting focused investigations of the histaminergic pathway, its role in striatal dopamine regulation and its relationship to the genesis or mediation of tics. These opportunities may be particularly timely given widespread interest in the pharmaceutical industry in the development of H3R compounds [55] for a variety of other neuropsychiatric indications.
Recent Advances in Treatment
Antipsychotic medications are the most-effective anti-tic medication currently available[56]. Medium to large effects in the treatment of tics, ranging from 0.4-1.2, have been identified in several randomized, placebo-controlled trials[56]. However, significant side effects [57,58], including weight gain and an increased risk of metabolic syndrome and diabetes, relegate these agents to second-line options, especially in pediatric populations.
Alpha-2 agonists are currently considered first-line treatment [56]. While these show only modest efficacy in tic reduction (effect sizes from 0.1-0.5), their side-effect profile is considerably more benign than anti-psychotics . They have also been shown to be quite effective in the treatment of comorbid ADHD symptoms [59]. In particular, clonidine has similar efficacy to methylphenidate in the treatment of ADHD in children with tics [60]. Psychostimulants, although generally considered the fastest acting and most effective treatment for ADHD in isolation, are often not prescribed in individuals with TS because of an FDA warning listing tics or a family history of Tourette syndrome as a contraindication. This was based on a series of case reports published in the 1980s and 1990s highlighting the emergence or exacerbation of tics with psychostimulant use. Recent evidence from randomized controlled trials suggests that psychostimulant medications are an effective treatment for ADHD in children with tics and are generally very well tolerated [59]. A recent meta-analysis of 4 trials involving 193 children with ADHD and tics found methylphenidate effective in reducing ADHD symptoms (effect size=0.8) and had neutral-to-beneficial effects on tic symptoms (effect size=0.3) [59].
Several randomized, controlled pilot studies over the past decade have pointed to emerging pharmacological agents in the treatment of tic disorders, including pergolide, tetrabenazine and topiramate. Pergolide, a dopamine agonist that acts via D1 and D2 receptors, is hypothesized to improve tics by inhibiting presynaptic dopamine release. It has shown efficacy in reducing tic severity in children and adults [61,62]. Tetrabenazine, inhibits the central vesicular monoamine transporter type 2, and case series have suggested it may be an effective treatment for tics [63]. Topiramate is a broad-spectrum antiepileptic that acts by increasing central GABA. A recent trial demonstrated a significant benefit of topiramate compared to placebo over 10-weeks in 29 patients with TS [63].
In contrast to the relatively modest progress in pharmacological treatments of tic disorders in recent years, there have been quite significant advances in behavioral treatments for tics. Habit reversal training (HRT) is the first psychotherapeutic intervention that has shown promise in reducing tic severity in patients with Tourette syndrome. HRT consists of awareness training and competing response practice. The former consists of 4 components designed to increase an individual's awareness of tics and the latter involves teaching the individuas to produce an incompatible physical response (i.e. isometric contraction of tic-opposing muscles) contingent upon the urge to perform a tic. In recent, multi-site trials, using blinded-raters HRT demonstrated efficacy compared to supportive therapy in both adults and children [64]. The effect size of HRT was similar to that of antipsychotics.
Despite this very encouraging data, key challenges remain to the widespread adoption of HRT for the treatment of tics. Dissemination remains a significant issue as there are few experienced HRT therapists. Further research into identifying the critical components within HRT and the use of internet or telepsychiatry to provide more widespread access to treatment may be particularly helpful. At the same time, the development of more effective and safer pharmacological treatments is a pressing need and this effort will undoubtedly be aided by a progressively deeper understanding of the neurobiology and genetics of TS.
Conclusions
Recent advances in the neurobiology and genetics of TS have, in many respects, reinforced conventional wisdom by focusing attention on cortical-striatal circuits and dopaminergic neurotransmission. However, these studies have simultaneously offered potentially novel and important insights, with regard to specific deficits in subsets of straital interneurons; convergent genetic risks underlying a range of developmental neuropsychiatric outcomes; and a possible role for histaminergic neurotransmission in the etiology or regulation of tics. Recent controlled trials of habit reversal therapy support efficacy on par with the best current pharmacological alternatives. However dissemination presents an important ongoing challenge. Further studies of these non-medication approaches as well as the development of safer and more effective pharmacological agents remains a pressing need.
Key points.
Recent neuropathological studies point to a relative reduction in parvalbumin containing and cholinergic striatal interneurons in individuals with tics compared to controls;
Tourette syndrome has long been known to have a strong genetic influence but investigations are just now reporting on cohorts of sufficient size to plausibly illuminate the contribution of common or rare genetic variation to tics and TS.
Studies of rare copy number variation have highlighted the possibility of shared risks among TS and distinct diagnostic entities including autism and schizophrenia.
Over the past year, the study of a Mendelian form of TS has pointed to a previously unappreciated role for histaminergic neurotransmission in the genesis or modulation of tics.
Dopamine receptor antagonists, the most effective current medications for treating tics, have increasingly been appreciated to carry significant side effects, particularly in the pediatric population. Notably, recent controlled trials have supported the use of non-medication approaches to tic management, but broad dissemination of these specialized psychotherapies poses an ongoing challenge.
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health through grants 1K23MH091240-01(MB), K08MH081190 (CP); UL1 RR024139 (MB); R01MH091861 (CP) and R01 NS056276 (MS); the AACAP/ Eli Lilly Junior Investigator Award (MB), the Trichotillomania Learning Center (MB), NARSAD (MB), and the State of Connecticut, through its support of the Abraham Ribicoff Research Facilities (CP and MB).
References
- 1.Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, Leckman JF. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160:65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevalence of diagnosed Tourette syndrome in persons aged 6-17 years - United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:581–585. [PubMed] [Google Scholar]
- 3.Robertson MM, Eapen V, Cavanna AE. The international prevalence, epidemiology, and clinical phenomenology of Tourette syndrome: a cross-cultural perspective. J Psychosom Res. 2009;67:475–483. doi: 10.1016/j.jpsychores.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK, Pakstis AJ, Alsobrook JP, Robertson MM, McMahon WM, et al. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:60–68. doi: 10.1002/ajmg.b.10001. [DOI] [PubMed] [Google Scholar]
- 5.Burd L, Freeman RD, Klug MG, Kerbeshian J. Tourette Syndrome and learning disabilities. BMC Pediatr. 2005;5:34. doi: 10.1186/1471-2431-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart SE, Illmann C, Geller DA, Leckman JF, King R, Pauls DL. A controlled family study of attention-deficit/hyperactivity disorder and Tourette's disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:1354–1362. doi: 10.1097/01.chi.0000251211.36868.fe. [DOI] [PubMed] [Google Scholar]
- 7.Roessner V, Becker A, Banaschewski T, Freeman RD, Rothenberger A. Developmental psychopathology of children and adolescents with Tourette syndrome--impact of ADHD. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):24–35. doi: 10.1007/s00787-007-1004-6. [DOI] [PubMed] [Google Scholar]
- 8.Freeman RD. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):15–23. doi: 10.1007/s00787-007-1003-7. [DOI] [PubMed] [Google Scholar]
- 9.Ghanizadeh A, Mosallaei S. Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain Dev. 2009;31:15–19. doi: 10.1016/j.braindev.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.do Rosario MC, Miguel Filho EC. Obsessive-compulsive disorder and Tourette syndrome: is there a relationship? Sao Paulo Med J. 1997;115:1410–1411. doi: 10.1590/s1516-31801997000200008. [DOI] [PubMed] [Google Scholar]
- 11.Casey MB, Cohen M, Schuerholz LJ, Singer HS, Denckla MB. Language-based cognitive functioning in parents of offspring with ADHD comorbid for Tourette syndrome or learning disabilities. Dev Neuropsychol. 2000;17:85–110. doi: 10.1207/S15326942DN1701_06. [DOI] [PubMed] [Google Scholar]
- 12.Robertson MM, Orth M. Behavioral and affective disorders in Tourette syndrome. Adv Neurol. 2006;99:39–60. [PubMed] [Google Scholar]
- 13.Coffey BJ, Biederman J, Smoller JW, Geller DA, Sarin P, Schwartz S, Kim GS. Anxiety disorders and tic severity in juveniles with Tourette's disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:562–568. doi: 10.1097/00004583-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Frey KA, Albin RL. Neuroimaging of Tourette syndrome. J Child Neurol. 2006;21:672–677. doi: 10.1177/08830738060210080501. [DOI] [PubMed] [Google Scholar]
- 15.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 16.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun AR, Randolph C, Stoetter B, Mohr E, Cox C, Vladar K, Sexton R, Carson RE, Herscovitch P, Chase TN. The functional neuroanatomy of Tourette's syndrome: an FDG-PET Study. II: Relationships between regional cerebral metabolism and associated behavioral and cognitive features of the illness. Neuropsychopharmacology. 1995;13:151–168. doi: 10.1016/0893-133X(95)00052-F. [DOI] [PubMed] [Google Scholar]
- 18.Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, Frith CD, Frackowiak RS, Dolan RJ. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–748. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- 19.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 20.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010518:277–291. doi: 10.1002/cne.22206. [“This postmortem study, a follow-up to Kalanithi et al (2005), confirmed a reduction in fast-spiking interneurons and documented, for the first time, that cholinergic tonically active interneurons are also reduced in number in patients with TS, focusing attention on this key interneuronal population.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leckman JF. Tourette's syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, Shaffer D, Paneth N. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Arch Gen Psychiatry. 1997;54:847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- 24.Mathews CA, Bimson B, Lowe TL, Herrera LD, Budman CL, Erenberg G, Naarden A, Bruun RD, Freimer NB, Reus VI. Association between maternal smoking and increased symptom severity in Tourette's syndrome. Am J Psychiatry. 2006;163:1066–1073. doi: 10.1176/ajp.2006.163.6.1066. [DOI] [PubMed] [Google Scholar]
- 25.Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, Vaccarino FM. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer HS. Tourette's syndrome: from behaviour to biology. Lancet Neurol. 2005;4:149–159. doi: 10.1016/S1474-4422(05)01012-4. [DOI] [PubMed] [Google Scholar]
- 27*.Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [This electrophysiological study dissects the cellular mechanisms by which cholinergic and dopaminergic signaling interact in the striatum.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815–820. doi: 10.1001/archpsyc.1985.01790310077011. [DOI] [PubMed] [Google Scholar]
- 30.Baron M, Shapiro E, Shapiro A, Rainer JD. Genetic analysis of Tourette syndrome suggesting major gene effect. Am J Hum Genet. 1981;33:767–775. [PMC free article] [PubMed] [Google Scholar]
- 31.Kidd KK, Pauls DL. Genetic hypotheses for Tourette syndrome. Adv Neurol. 1982;35:243–249. [PubMed] [Google Scholar]
- 32.Curtis D, Robertson MM, Gurling HM. Autosomal dominant gene transmission in a large kindred with Gilles de la Tourette syndrome. Br J Psychiatry. 1992;160:845–849. doi: 10.1192/bjp.160.6.845. [DOI] [PubMed] [Google Scholar]
- 33.Pauls DL, Leckman JF. The inheritance of Gilles de la Tourette's syndrome and associated behaviors. Evidence for autosomal dominant transmission. N Engl J Med. 1986;315:993–997. doi: 10.1056/NEJM198610163151604. [DOI] [PubMed] [Google Scholar]
- 34.Walkup JT, LaBuda MC, Singer HS, Brown J, Riddle MA, Hurko O. Family study and segregation analysis of Tourette syndrome: evidence for a mixed model of inheritance. Am J Hum Genet. 1996;59:684–693. [PMC free article] [PubMed] [Google Scholar]
- 35.Kurlan R, Eapen V, Stern J, McDermott MP, Robertson MM. Bilineal transmission in Tourette's syndrome families. Neurology. 1994;44:2336–2342. doi: 10.1212/wnl.44.12.2336. [DOI] [PubMed] [Google Scholar]
- 36.Hasstedt SJ, Leppert M, Filloux F, van de Wetering BJ, McMahon WM. Intermediate inheritance of Tourette syndrome, assuming assortative mating. Am J Hum Genet. 1995;57:682–689. [PMC free article] [PubMed] [Google Scholar]
- 37.State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron. 2010;68:254–269. doi: 10.1016/j.neuron.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Sundaram SK, Huq AM, Wilson BJ, Chugani HT. Tourette syndrome is associated with recurrent exonic copy number variants. Neurology. 2010;74:1583–1590. doi: 10.1212/WNL.0b013e3181e0f147. [The first report of copy number variation analysis in Tourette syndrome demonstrates a possible overlap of genetic risks with other childhood developmental neuropsychaitric disorders.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mefford HC, Sharp AJ, Baker C, Itsara A, Jiang Z, Buysse K, Huang S, Maloney VK, Crolla JA, Baralle D, et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med. 2008;359:1685–1699. doi: 10.1056/NEJMoa0805384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 43.Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 44.Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 45.Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- 50.Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, Abbott T, Gupta A, King RA, Pauls DL, et al. L-histidine decarboxylase and Tourette's syndrome. N Engl J Med. 2010362:1901–1908. doi: 10.1056/NEJMoa0907006. [A rare mutation in the gene l-histidine decarboxylast, found in a multiply affected pedigree, points to the involvement of histaminergic neurotranmission in TS and tics. The finding suggests a number of testable hypothesis regarding molecular mechanism relevant to more common forms of the disorder as well as potentially novel avenues of treatment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 54.Kubota Y, Ito C, Sakurai E, Watanabe T, Ohtsu H. Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J Neurochem. 2002;83:837–845. doi: 10.1046/j.1471-4159.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- 55.Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Mol Interv. 2006;6:77–88. 59. doi: 10.1124/mi.6.2.5. [DOI] [PubMed] [Google Scholar]
- 56.Scahill L, Erenberg G, Berlin CM, Jr., Budman C, Coffey BJ, Jankovic J, Kiessling L, King RA, Kurlan R, Lang A, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(Suppl 7):22–31. [PubMed] [Google Scholar]
- 58.Martin A, Scahill L, Anderson GM, Aman M, Arnold LE, McCracken J, McDougle CJ, Tierney E, Chuang S, Vitiello B. Weight and leptin changes among risperidone-treated youths with autism: 6-month prospective data. Am J Psychiatry. 2004;161:1125–1127. doi: 10.1176/appi.ajp.161.6.1125. [DOI] [PubMed] [Google Scholar]
- 59*.Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [This meta-analysis demonstrated that psychostimulant medications do not significantly worsen tics in children with comorbid ADHD. Alpha-2 agonists and atomoxetine were demonstrated to be effective in the treatment of both ADHD and tics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 61.Gilbert DL, Dure L, Sethuraman G, Raab D, Lane J, Sallee FR. Tic reduction with pergolide in a randomized controlled trial in children. Neurology. 2003;60:606–611. doi: 10.1212/01.wnl.0000044058.64647.7e. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert DL, Sethuraman G, Sine L, Peters S, Sallee FR. Tourette's syndrome improvement with pergolide in a randomized, double-blind, crossover trial. Neurology. 2000;54:1310–1315. doi: 10.1212/wnl.54.6.1310. [DOI] [PubMed] [Google Scholar]
- 63.Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010;81:70–73. doi: 10.1136/jnnp.2009.185348. [DOI] [PubMed] [Google Scholar]
- 64**.Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010303:1929–1937. doi: 10.1001/jama.2010.607. [This large, multi-site study demonstrated a behavioral therapy had efficacy in reducing tic severity for adults with tourette syndrome. The effect size demonstrated was equal to the best pharmacological treatments available.] [DOI] [PMC free article] [PubMed] [Google Scholar]


