Abstract
Context:
Wolff-Parkinson-White (WPW) is a cardiac conduction system disorder characterized by abnormal accessory conduction pathways between the atria and the ventricles. Symptomatic patients classically present with palpitations, presyncope, or syncope that results from supraventricular tachycardia. While rare, sudden cardiac death may be the first manifestation of underlying disease and occurs more frequently in exercising individuals.
Evidence Acquisition:
Medline and PubMed databases were evaluated through 2012, with the following keywords: WPW, Wolff-Parkinson-White, pre-excitation, sudden cardiac death, risk stratification, and athletes. Selected articles identified through the primary search, along with relevant references from those articles, were reviewed for pertinent clinical information regarding the identification, evaluation, risk stratification, and management of WPW as they pertained to the care of athletes.
Study Design:
Systematic review.
Level of Evidence:
Level 1.
Results:
Diagnosis of WPW is confirmed by characteristic electrocardiogram changes, which include a delta wave, short PR interval, and widened QRS complex. Utilization of the electrocardiogram as part of the preparticipation physical evaluation may allow for early identification of asymptomatic individuals with a WPW pattern. Risk stratification techniques identify individuals at risk for malignant arrhythmias who may be candidates for curative therapy through transcatheter ablation.
Conclusion:
WPW accounts for at least 1% of sudden death in athletes and has a prevalence of at least 1 to 4.5 per 1000 children and adults. The risk of lethal arrhythmia appears to be higher in asymptomatic children than in adults, and sudden cardiac death is often the sentinel event. The athlete with WPW should be evaluated for symptoms and the presence of intermittent or persistent pre-excitation, which dictates further consultation, treatment, and monitoring strategies as well as return to play.
Keywords: Wolff-Parkinson-White, pre-excitation, risk stratification, athletic participation, sudden cardiac death, athlete
Wolff-Parkinson-White (WPW) syndrome was first described in 1930 in a landmark article in the American Heart Journal, in which the authors reported a case series of 11 otherwise healthy patients with electrocardiogram (ECG) findings of a short PR interval and “bundle branch block” morphology who also suffered from paroxysmal supraventricular tachycardia (SVT) or atrial fibrillation (AF).50 Shortly after the description, electrophysiologists were able to elucidate the relationship between accessory pathways and reentrant SVT.35,36 However, it was not until nearly 40 years later that rapid conduction of AF was identified as the mechanism of sudden death.15
Multiple studies of children and adults report a WPW prevalence of 1 to 4.5 per 1000 individuals.1,11,17,18,20,27,31,43 The majority of individuals with WPW demonstrate normal cardiac anatomy. However, WPW has been associated with structural heart disease, notably in Ebstein anomaly and hypertrophic cardiomyopathy. There is a greater prevalence of WPW in newborns and infants compared with adults, suggesting that accessory pathways may represent embryologic remnants.24,34 Within the first year of life, accessory pathways lose anterograde conduction in up to 40% of patients, with a similar decrease in the rate of SVT, suggesting loss of retrograde conduction through these accessory pathways.2,12 Young patients with accessory pathways may be “presymptomatic,” as they have not had time to develop symptoms or a sentinel event.7 Conversely, individuals surviving to adulthood without symptoms may harbor lower risk pathways.7 While prediction of risk for sudden cardiac death (SCD) is difficult, high risk factors include the following: male sex, age <30 years, history of AF, prior syncope, familial WPW, and associated congenital heart disease.7
Risk of Sudden Cardiac Death
WPW accounts for at least 1% of deaths in a long-term registry of SCD in athletes, though it may account for a fraction of a larger group of cases of autopsy-negative sudden unexplained death due to challenges in making a postmortem diagnosis.28,40 Numerous retrospective analyses have suggested that the risk of life-threatening arrhythmias is higher in asymptomatic children than in adults, with as many as 10% to 48% of pediatric cases of WPW presenting with SCD as the initial event.12,22,30,38,45 Several prospective studies have evaluated the risk of SCD in asymptomatic adults and children with WPW, demonstrating a 0.1% to 0.45% risk of SCD per year. In 2 prospective studies following a group of 386 adult and pediatric patients over 10 years, 4 cases of SCD were identified, yielding a 0.1% annual risk of SCD.16,37 Another study reported the incidence of SCD to be 0.45% per year in asymptomatic adults with WPW, with a mean follow-up of 38 months.31 Pappone et al followed 212 asymptomatic adults prospectively for 5 years, with 3 patients (0.2% per year) experiencing VF.31 A similar 5-year prospective study of 184 asymptomatic pediatric patients with WPW reported 3 cases of VF, or a 0.3% per year risk of SCD.39
Mechanism of WPW
Within the normal heart, the atria and ventricles are electrically isolated from each other by nonconductive fibrous atrioventricular (AV) rings except at the AV node and bundle of His. Impulses are typically initiated from within the sinoatrial node, and conduction propagates to the ventricles via the His-Purkinje system.
Individuals with WPW have at least 1 additional accessory electrical pathway between the atria and the ventricles that bypasses the AV node, allowing for the delivery of premature electrical impulses and ventricular pre-excitation. The presence of an accessory pathway also permits impulses to propagate in a retrograde manner. Depending on the conduction characteristics, this accessory connection can be associated with reentrant SVT and sudden death.7
“Reentrant” SVT typically results when an impulse travels down the AV node and His-Purkinje system and returns in a retrograde manner to the atrium via the accessory pathway. However, SCD is triggered when AF is conducted rapidly to the ventricle by the accessory pathway with degeneration to VF. Exercise may enhance accessory pathway conduction, but it is unclear whether exercise has a consistent effect in promoting conversion of SVT to AF, though AF is more common in trained athletes compared with the general population.5,9 Despite a clear mechanistic trigger, most sudden deaths associated with WPW appear to occur during exercise.40,45,49
ECG Criteria and Diagnosis
The diagnosis of WPW typically occurs via ECG. The pathognomonic ECG findings in WPW are the delta wave, characterized by a slurred upstroke in the QRS complex and a short PR interval <120 ms (Figure 1).42 Depolarization of the ventricles via the accessory pathway contributes to QRS durations longer than 120 ms. The location and refractory period of the accessory pathway may diminish the prominence of the delta wave, making the diagnosis more challenging in some cases. ECG findings associated with a subtle WPW pattern include left-axis deviation, abnormal Q waves in leads V5 and V6, ST-segment depression, and T-wave changes. An intermittent WPW pattern on ECG (ie, a delta wave present on every other QRS complex) is considered low risk for ventricular arrhythmia.7
Figure 1.
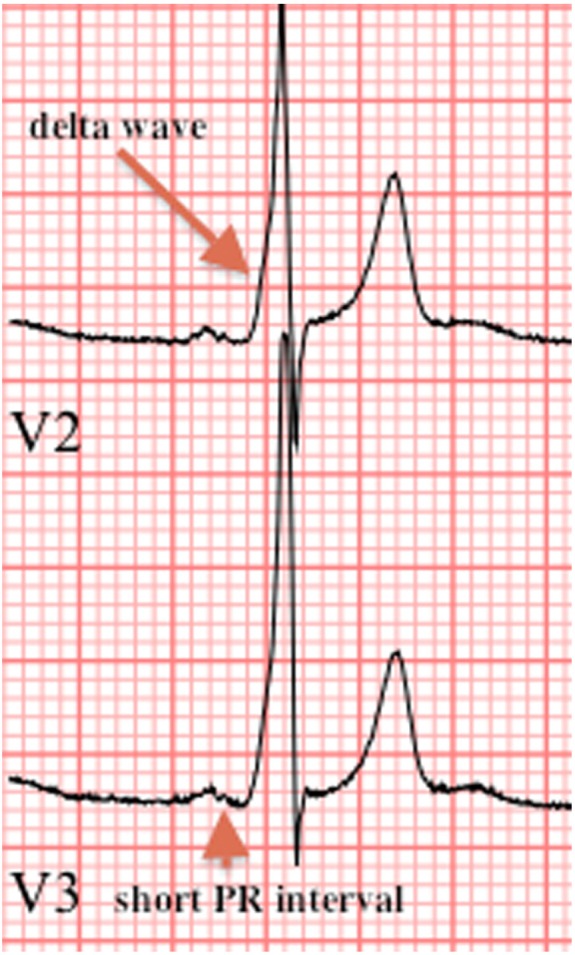
Characteristic ECG findings in WPW. Note the presence of a short PR interval (<120 ms) and delta wave (slurred upstroke of the QRS complex). ECG, electrocardiogram.
Electrocardiography is used for cardiovascular screening and in the evaluation of athletes with palpitations, presyncope, or syncope to rule out WPW and other intrinsic cardiac disorders. Athletes identified with a WPW pattern on a screening ECG should undergo additional inquiry about symptoms and familial WPW.7
Risk Stratification
The intent of risk stratification in individuals with WPW is to identify those at risk for lethal arrhythmias and SCD. While some individuals with SCD associated with WPW manifest premonitory symptoms, as many as 50% of younger athletes present with SCD as their sentinel event.12,23 Although historical markers exist to suggest high-risk pathways, history and physical alone are insufficient in assessing risk in the athletic population. Further diagnostic testing should be utilized to better understand the properties of the accessory pathway. Accessory pathways with rapid anterograde conduction properties are more likely to be associated with rapid conduction of AF, which can degenerate to VF.
Noninvasive methods for risk stratification to determine antegrade conduction and the risk of VF include Holter monitoring, exercise treadmill testing, and echocardiography. The Holter monitor records electrical properties of the heart over an extended period, typically 24 to 48 hours. Asymptomatic athletes demonstrating abrupt loss of pre-excitation at physiologic heart rates during this study generally have slower conducting accessory pathways and may be considered lower risk for a ventricular arrhythmia. Intermittent loss of pre-excitation via ambulatory monitoring may occur in as many as 67% of asymptomatic cases.19 Holter monitoring can also identify distinctly different pre-excited morphologies on ECG, raising suspicion for the presence of multiple accessory pathways, which is an independent risk factor for VF.7
Exercise stress testing (EST) adds further value in the noninvasive assessment of WPW pattern. Abrupt disappearance of pre-excitation, as evidenced by loss of the delta wave during exercise testing, has been proposed as a surrogate to more invasive risk stratification measures.4 Only abrupt and complete loss of pre-excitation on EST confirms a long anterograde pathway refractory period and, hence, a low risk profile. Abrupt loss of pre-excitation features during exercise testing may only be demonstrable in 15% of pediatric patients, and confirmation of these findings is further confounded by a lack of interobserver reliability.3
An echocardiogram should be undertaken in all patients with a WPW pattern to rule out structural heart disease associated with WPW, including certain genetic variants of hypertrophic cardiomyopathy as well as Ebstein anomaly.
When noninvasive testing is insufficient in characterizing the anterograde conduction of the accessory pathway, a low-risk pathway cannot be confirmed, or the presence of multiple accessory pathways is suspected, invasive testing should be undertaken. Electrophysiologic (EP) studies, including intracardiac catheterization and transesophageal studies, can elucidate accessory pathway properties.10 The cardiologist can apply pacing techniques as well as medication to induce SVT and AF. By inducing AF, the cardiologist can measure characteristics of the accessory pathway(s), including the shortest pre-excited R-R interval (SPERRI) while in AF. SPERRI measures anterograde conduction through the accessory pathway. Individuals exhibiting a SPERRI of <250 ms are at higher risk of developing malignant arrhythmias.22 SPERRI <250 ms implies that the accessory pathway can conduct faster than 240 beats per minute during AF.
The incidence of lethal WPW phenotypes is relatively low in asymptomatic individuals with WPW.7 However, consensus guidelines on the management of asymptomatic young patients with WPW recommend a SPERRI of <250 ms as criteria for ablation.7,22 In addition to shorter accessory-pathway refractory periods, malignant arrhythmias are more likely to be associated with multiple accessory pathways and sustained AF induced by AV re-entrant tachycardia.32
Management
Management of symptomatic athletes with WPW syndrome or any athlete with a high-risk WPW pathway should include a discussion of the risks and benefits of noninvasive and invasive treatment strategies, activity participation, and follow-up. In the case of the symptomatic athlete, medication management can be chosen to prevent arrhythmias and slow ventricular response, but these medications pose side effects that may diminish athletic performance. In the case of asymptomatic athletes with WPW pattern on ECG, risk assessment is necessary to determine their preparticipation clearance and is best performed through a referral to cardiology. A discussion of family risk assessment should also be considered, as the prevalence of WPW in family members is 5.5 per 1000 and has the potential for an autosomal-dominant inheritance pattern.48
Transcatheter ablation is considered by many as first-line treatment for WPW and offers the potential for a definitive cure. Transcatheter ablation includes both radiofrequency ablation (RFA) and cryoablation techniques. RFA is considered the gold standard for invasive management due to its higher success rate in extinguishing accessory pathways and lower recurrence rate.7 RFA may be coupled with cryoablation, which offers a higher safety profile, particularly in the ablation of septal accessory pathways and pathways close to small coronary arteries and the coronary sinus. Cryoablation demonstrates a lower risk of inducing AV block than RFA but at the expense of lower success and higher recurrence rates.17 While initial data from the mid-1990s demonstrated an ablation success rate of 91% in adults and children, recurrence rates reached 23% at 3-year follow-up.25 More recent studies in adults have shown increased success in ablation (95.7%), with reductions in complications (from 4.3% to 2.9%) and recurrence (10.7%) at 1-year follow-up.46 Results are similar in pediatric studies, with ablation success rates of 92% to 100% and recurrence rates of 0% to 13%.6,13,26,29,44,47
While complications of EP studies are relatively rare, the potential harm of a catheterization procedure must be considered against the risk of WPW-associated SCD. Two large studies of >1300 adults described various complications of EP procedures, including local venous occlusion and/or formation of an AV fistula (2%), pulmonary embolus (0.3%-1.6%), thrombophlebitis (0.6%), infection (0.8%), and catheter-induced permanent complete AV block (0.1%).14,21 EP studies also carry the risk of induced AF degenerating to VF through a high-risk pathway that requires external defibrillation, and death has been reported as a complication of RFA in 0.22% of cases.41 This mortality risk from an EP study with ablation procedure approximates the annual risk of dying from WPW (0.1%-0.45%). However, the risk of SCD from WPW in an untreated individual accumulates over time, especially in children where the long-term risk may become substantially higher than the risks of the procedure itself. The mortality risk is also lower for an EP study without ablation where risk stratification identifies a low-risk pathway. Patients and families should receive appropriate counseling before any procedure to clarify these relative risks and ensure that the patient and family understand both the immediate and the long-term risks and potential benefits of treatment versus monitoring.
Athlete-Specific Considerations
While some studies suggest that exercise does not alter accessory pathway characteristics, exercise appears to put some athletes with WPW at risk for a lethal arrhythmia.40,42,49 It is unclear whether these athletes are symptomatic before SCD.7 The cardiovascular care of athletes may utilize an ECG for screening or diagnostic purposes. WPW can be readily identified in asymptomatic athletes, and ECG allows early detection of young athletes at risk of SCD that have not manifested symptoms.
For athletes with WPW pattern on ECG, the 36th Bethesda Conference recommends that risk stratification with invasive EP studies is advisable for asymptomatic younger athletes engaged in moderate- to high-intensity competitive sports.33 Symptomatic athletes also should be considered for catheter ablation therapy of the accessory pathway prior to returning to sport.33 The European Society for Cardiology advises a more aggressive approach by recommending that all athletes identified with WPW undergo a comprehensive EP study, regardless of sport.8 While American and European recommendations differ, it is uniformly recommended that the discovery of ventricular pre-excitation in an adolescent, regardless of athletic involvement, should result in prompt referral to a cardiac electrophysiologist familiar with risk stratification.7
Ablation is recommended for high-risk pathways and symptomatic athletes. Competitive athletes with low-risk pathways identified during EP study not undergoing ablation therapy should be monitored for the development of new symptoms. After an athlete has undergone an ablation procedure, return to play is guided by symptoms and follow-up studies.33 Athletes who remain asymptomatic and have normal AV conduction properties on follow-up ECG (ie, normal QRS complex and PR interval) usually may return to sport within 1 week.
Case Study
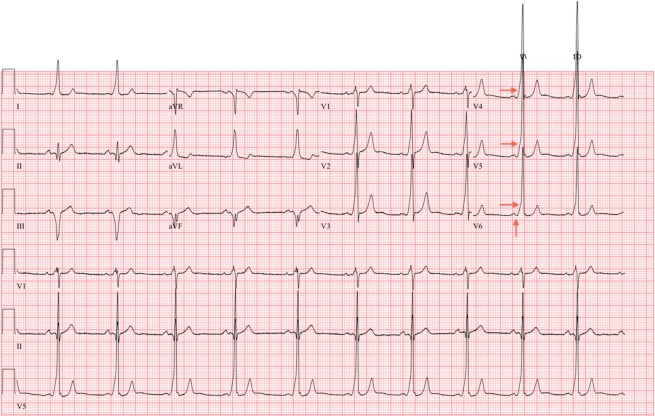
An 18-year-old male National Collegiate Athletic Association Division I baseball athlete presented for his preparticipation physical evaluation before intercollegiate athletic activities. He denied presyncope, syncope, chest pain, palpitations, or a family history of sudden cardiac or unexplained death. His cardiovascular examination yielded normal results. A screening ECG revealed a WPW pattern, with characteristic delta waves, short PR interval, and a wide QRS (Figure 2). The athlete was referred to a cardiac electrophysiologist for further evaluation.
Figure 2.
ECG at initial preparticipation physical evaluation (preablation) demonstrates a WPW pattern. Red arrows identify the characteristic delta wave and short PR interval. ECG, electrocardiogram; WPW, Wolff-Parkinson-White.
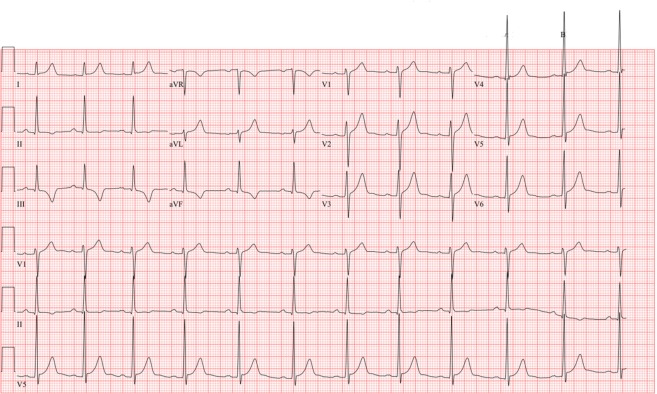
The patient underwent an echocardiogram with no structural abnormalities. For risk stratification, an EST and invasive EP study (with option for ablation for a high-risk accessory pathway if identified) were both offered. The family elected to proceed with intracardiac catheterization. A high-risk, anterograde-conducting, right-sided posteroseptal accessory pathway, with a SPERRI of 230 ms, was identified and successfully ablated by RFA. An immediate postprocedure ECG showed that the WPW pattern was extinguished. The athlete was restricted from participation for 1 week to allow for postprocedure recovery. A follow-up ECG (Figure 3) was obtained at 1 week postprocedure and yielded normal results.
Figure 3.
Postablation ECG. WPW pattern has been extinguished. Note the absence of a delta wave and normalization of the PR interval. New T-wave inversion in the inferior leads is a result of the ablation therapy that typically resolves over time. ECG, electrocardiogram; WPW, Wolff-Parkinson-White.
The athlete was cleared for participation and has since participated in intercollegiate athletics without complication.
Management Algorithm
The algorithm in Figure 4 is presented to help guide physicians who identify WPW in their patients. An athlete with identified WPW pattern on ECG should be evaluated for persistent (every QRS complex) versus intermittent pre-excitation. If pre-excitation is persistent on ECG, consultation with a cardiologist familiar with WPW risk stratification should be undertaken. Typically, a Holter monitor or EST is offered, while an ECG is performed to evaluate for concomitant structural heart disease, such as Ebstein anomaly or hypertrophic cardiomyopathy. Nonathletic patients, as well as athletes competing in low-intensity sports who have a clear loss of pre-excitation during Holter monitoring or EST, can be considered low risk for developing a lethal arrhythmia and may be followed up with periodically and counseled on symptom awareness. However, in moderate- and high-intensity sports, athletes demonstrating intermittent pre-excitation and abrupt loss on noninvasive testing should be considered for further risk stratification via diagnostic invasive EP studies. Athletes without abrupt loss of pre-excitation should be considered for diagnostic invasive EP studies. Transcatheter ablation should be offered as an option should a high-risk pathway, with a SPERRI in AF ≤250 ms, be identified. In cases with a SPERRI in AF >250 ms, counseling can be offered for symptom awareness and monitoring or ablation, depending on both patient characteristics and pathway location.
Figure 4.
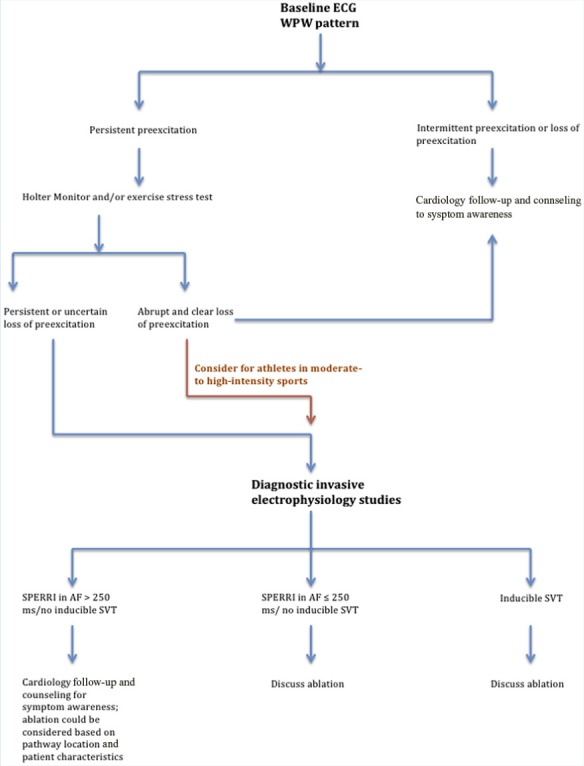
WPW management algorithm. AF, atrial fibrillation; ECG, electrocardiogram; SPERRI, shortest pre-excited R-R interval; SVT, supraventricular tachycardia; WPW, Wolff-Parkinson-White.
Conclusion
WPW syndrome is a disorder of the cardiac conduction system caused by the presence of an accessory AV pathway, and it is responsible for at least 1% of athletic SCD. Once an athlete with WPW pattern is identified, risk stratification should occur through additional studies and EP consultation. Individuals with low-risk characteristics may be monitored closely and counseled for symptom awareness, while those with symptoms or high-risk accessory pathways should be considered for ablation therapy. WPW can be appropriately managed to mitigate the risk of SCD and promote safer athlete participation.
Footnotes
The following author declared potential conflicts of interest: Irfan M. Asif, MD, is employed by the University of Tennesse, and received a Physicians Medical Grant from the University of Tennessee ($10,000) and Young Investigator Grant from the American Medical Society for Sports Medicine ($5,000).
References
- 1. Averill KH, Fosmoe RJ, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects: IV. Wolff-Parkinson-White syndrome. Am J Cardiol. 1960;6:108-129 [DOI] [PubMed] [Google Scholar]
- 2. Benson DW, Jr, Dunnigan A, Benditt DG. Follow-up evaluation of infant paroxysmal atrial tachycardia: transesophageal study. Circulation. 1987;75:542-549 [DOI] [PubMed] [Google Scholar]
- 3. Bershader RS BC, Cecchin F. Exercise testing for risk assessment in pediatric Wolff-Parkinson-White syndrome. Heart Rhythm. 2007;4:S138-S139 [Google Scholar]
- 4. Bricker JT, Porter CJ, Garson A, Jr, et al. Exercise testing in children with Wolff-Parkinson-White syndrome. Am J Cardiol. 1985;55:1001-1004 [DOI] [PubMed] [Google Scholar]
- 5. Calvo N, Brugada J, Sitges M, Mont L. Atrial fibrillation and atrial flutter in athletes. Br J Sports Med. 2012;46(suppl 1):i37-i43 [DOI] [PubMed] [Google Scholar]
- 6. Celiker A, Kafali G, Karagoz T, Ceviz N, Ozer S. The results of electrophysiological study and radiofrequency catheter ablation in pediatric patients with tachyarrhythmia. Turk J Pediatr. 2003;45:209-216 [PubMed] [Google Scholar]
- 7. Cohen MI, Triedman JK, Cannon BC. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congentinal Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), and American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society(CHRS). Heart Rhythm. 2012;9:1006-1024 [DOI] [PubMed] [Google Scholar]
- 8. Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516-524 [DOI] [PubMed] [Google Scholar]
- 9. Crick JC, Davies DW, Holt P, Curry PC, Sowton E. Effect of exercise on ventricular response to atrial fibrillation in Wolff-Parkinson-White syndrome. Br Heart J. 1985;54:80-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czosek RJ, Anderson JB, Marino BS, Mellion K, Knilans TK. Noninvasive risk stratification techniques in pediatric patients with ventricular preexcitation. Pacing Clin Electrophysiol. 2011;34:555-562 [DOI] [PubMed] [Google Scholar]
- 11. Davidoff R, Schamroth CL, Myburgh DP. The Wolff-Parkinson-White pattern in health aircrew. Aviat Space Environ Med. 1981;52:554-558 [PubMed] [Google Scholar]
- 12. Deal BJ, Keane JF, Gillette PC, Garson A., Jr Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol. 1985;5:130-135 [DOI] [PubMed] [Google Scholar]
- 13. Dick M, 2nd, O’Connor BK, Serwer GA, LeRoy S, Armstrong B. Use of radiofrequency current to ablate accessory connections in children. Circulation. 1991;84:2318-2324 [DOI] [PubMed] [Google Scholar]
- 14. Dimarco JP, Garan H, Ruskin JN. Complications in patients undergoing cardiac electrophysiologic procedures. Ann Intern Med. 1982;97:490-493 [DOI] [PubMed] [Google Scholar]
- 15. Dreifus LS, Haiat R, Watanabe Y, Arriaga J, Reitman N. Ventricular fibrillation: a possible mechanism of sudden death in patients and Wolff-Parkinson-White syndrome. Circulation. 1971;43:520-527 [DOI] [PubMed] [Google Scholar]
- 16. Fukatani M, Tanigawa M, Mori M, et al. Prediction of a fatal atrial fibrillation in patients with asymptomatic Wolff-Parkinson-White pattern. Jpn Circ J. 1990;54:1331-1339 [DOI] [PubMed] [Google Scholar]
- 17. Guize L, Soria R, Chaouat JC, Chretien JM, Houe D, Le Heuzey JY. Prevalence and course of Wolff-Parkinson-White syndrome in a population of 138,048 subjects [in French]. Ann Med Interne (Paris). 1985;136:474-478 [PubMed] [Google Scholar]
- 18. Hejtmancik MR, Herrmann GR. The electrocardiographic syndrome of short P-R interval and broad QRS complexes; a clinical study of 80 cases. Am Heart J. 1957;54:708-721 [DOI] [PubMed] [Google Scholar]
- 19. Hindman MC, Last JH, Rosen KM. Wolff-Parkinson-White syndrome observed by portable monitoring. Ann Intern Med. 1973;79:654-663 [DOI] [PubMed] [Google Scholar]
- 20. Hiss RG, Lamb LE. Electrocardiographic findings in 122,043 individuals. Circulation. 1962;25:947-961 [DOI] [PubMed] [Google Scholar]
- 21. Horowitz LN, Kay HR, Kutalek SP, et al. Risks and complications of clinical cardiac electrophysiologic studies: a prospective analysis of 1,000 consecutive patients. J Am Coll Cardiol. 1987;9:1261-1268 [DOI] [PubMed] [Google Scholar]
- 22. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301:1080-1085 [DOI] [PubMed] [Google Scholar]
- 23. Klein GJ, Prystowski EN, Yee R, Sharma AD, Laupacis A. Asymptomatic Wolff-Parkinson-White. Should we intervene? Circulation. 1989;80:1902-1905 [DOI] [PubMed] [Google Scholar]
- 24. Kolditz DP, Wijffels MC, Blom NA, et al. Persistence of functional atrioventricular accessory pathways in postseptated embryonic avian hearts: implications for morphogenesis and functional maturation of the cardiac conduction system. Circulation. 2007;115:17-26 [DOI] [PubMed] [Google Scholar]
- 25. Kugler JD, Danford DA, Houston K, Felix G. Radiofrequency catheter ablation for paroxysmal supraventricular tachycardia in children and adolescents without structural heart disease. Pediatric EP Society, Radiofrequency Catheter Ablation Registry. Am J Cardiol.1997;80:1438-1443 [DOI] [PubMed] [Google Scholar]
- 26. Lee PC, Hwang B, Chen YJ, Tai CT, Chen SA, Chiang CE. Electrophysiologic characteristics and radiofrequency catheter ablation in children with Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 2006;29:490-495 [DOI] [PubMed] [Google Scholar]
- 27. Manning GW. An electrocardiographic study of 17,000 fit, young Royal Canadian Air Force aircrew applicants. Am J Cardiol. 1960;6:70-75 [DOI] [PubMed] [Google Scholar]
- 28. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085-1092 [DOI] [PubMed] [Google Scholar]
- 29. Montenero AS, Drago F, Crea F, et al. Transcatheter radiofrequency ablation in supraventricular tachycardia in children: immediate results and mid-term follow-up [in Italian]. G Ital Cardiol. 1996;26:31-40 [PubMed] [Google Scholar]
- 30. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12:144-150 [DOI] [PubMed] [Google Scholar]
- 31. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41:239-244 [DOI] [PubMed] [Google Scholar]
- 32. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125:661-668 [DOI] [PubMed] [Google Scholar]
- 33. Pelliccia A, Zipes DP, Maron BJ. Bethesda Conference #36 and the European Society of Cardiology consensus recommendations revisited: a comparison of US and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52:1990-1996 [DOI] [PubMed] [Google Scholar]
- 34. Perry JC, Garson A., Jr Supraventricular tachycardia due to Wolff-Parkinson-White syndrome in children: early disappearance and late recurrence. J Am Coll Cardiol. 1990;16:1215-1220 [DOI] [PubMed] [Google Scholar]
- 35. Pick A, Katz LN. Disturbances of impulse formation and conduction in the pre-excitation (WPW) syndrome; their bearing on its mechanism. Am J Med. 1955;19:759-772 [DOI] [PubMed] [Google Scholar]
- 36. Pick A, Langendorf R. Recent advances in the differential diagnosis of A-V junctional arrhythmias. Am Heart J. 1968;76:553-575 [Google Scholar]
- 37. Pietersen AH, Andersen ED, Sandoe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70:38A-43A [DOI] [PubMed] [Google Scholar]
- 38. Russell MW, Dick MD. Incidence of catastrophic events associated with the Wolff-Parkinson-White syndrome in young patients: diagnostic and therapeutic dilemma. Circulation. 1993;1993:484(a) [Google Scholar]
- 39. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53:275-280 [DOI] [PubMed] [Google Scholar]
- 40. Sarubbi B. The Wolff-Parkinson-White electrocardiogram pattern in athletes: how and when to evaluate the risk for dangerous arrhythmias. The opinion of the paediatric cardiology. J Cardiovasc Med. 2005;7:271-278 [DOI] [PubMed] [Google Scholar]
- 41. Schaffer MS, Gow RM, Moak JP, Saul JP. Mortality following radiofrequency catheter ablation (from the Pediatric Radiofrequency Ablation Registry): participating members of the Pediatric Electrophysiology Society. Am J Cardiol. 2000;86:639-643 [DOI] [PubMed] [Google Scholar]
- 42. Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III. Intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:976-981 [DOI] [PubMed] [Google Scholar]
- 43. Swiderski J, Lees MH, Nadas AS. The Wolff-Parkinson-White syndrome in infancy and childhood. Br Heart J. 1962;24:561-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanel RE, Walsh EP, Triedman JK, Epstein MR, Bergau DM, Saul JP. Five-year experience with radiofrequency catheter ablation: implications for management of arrhythmias in pediatric and young adult patients. J Pediatr. 1997;131: 878-887 [DOI] [PubMed] [Google Scholar]
- 45. Timmermans C, Smeets JL, Rodriguez LM, Vrouchos G, van den Dool A, Wellens HJ. Aborted sudden death in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1995;76:492-494 [DOI] [PubMed] [Google Scholar]
- 46. Van Hare GF, Javitz H, Carmelli D, et al. Prospective assessment after pediatric cardiac ablation: demographics, medical profiles, and initial outcomes. J Cardiovasc Electrophysiol. 2004;15:759-770 [DOI] [PubMed] [Google Scholar]
- 47. Van Hare GF, Lesh MD, Scheinman M, Langberg JJ. Percutaneous radiofrequency catheter ablation for supraventricular arrhythmias in children. J Am Coll Cardiol. 1991;17:1613-1620 [DOI] [PubMed] [Google Scholar]
- 48. Vidaillet HJ, Jr, Pressley JC, Henke E, Harrell FE, Jr, German LD. Familial occurrence of accessory atrioventricular pathways (preexcitation syndrome). N Engl J Med. 1987;317:65-69 [DOI] [PubMed] [Google Scholar]
- 49. Wiedermann CJ, Becker AE, Hopperwieser T, Muhlberger V, Knapp E. Sudden death in young competitive athlete with Wolff-Parkinson-White syndrome. Eur Heart J. 1987;8:651-655 [DOI] [PubMed] [Google Scholar]
- 50. Wolff LPJ, White PD. Bundle-branch block with short PR interval in healthy young people to paroxysmal tachycardia. Am Heart J. 1930;6:685-704 [DOI] [PMC free article] [PubMed] [Google Scholar]