Abstract
Accurate and inexpensive point-of-care (POC) tests are urgently needed to control sexually transmitted infection (STI) epidemics, so that patients can receive immediate diagnoses and treatment. Current POC assays for Chlamydia trachomatis and Neisseria gonorrhoeae perform inadequately and require better assays. Diagnostics for Trichomonas vaginalis rely on wet preparation, with some notable advances. Serological POC assays for syphilis can impact resource-poor settings, with many assays available, but only one available in the U.S. HIV POC diagnostics demonstrate the best performance, with excellent assays available. There is a rapid assay for HSV lesion detection; but no POC serological assays are available. Despite the inadequacy of POC assays for treatable bacterial infections, application of technological advances offers the promise of advancing POC diagnostics for all STIs.
Keywords: Chlamydia trachomatis, HIV, HSV-2, Neisseria gonorrhoeae, PCR, point-of-care diagnostics, resource-limited settings, serology, syphilis, Trichomonas vaginalis
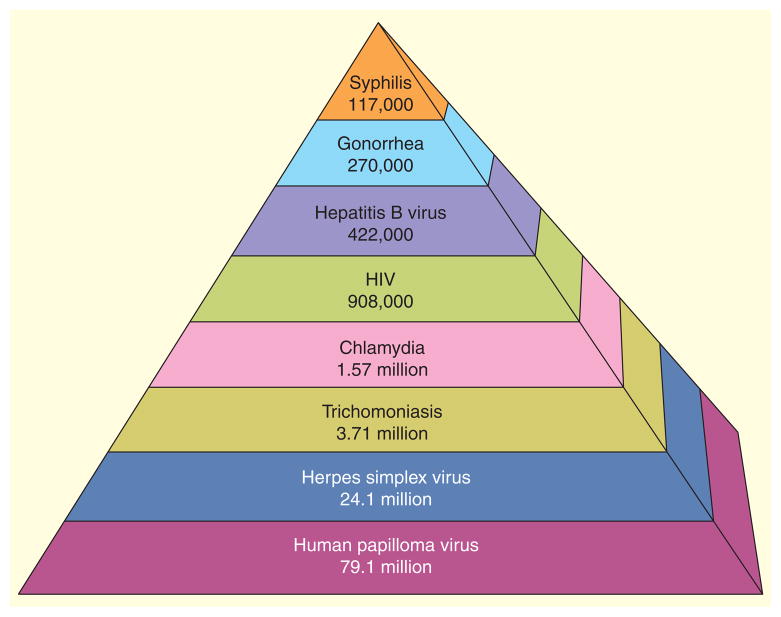
Sexually transmitted infections (STIs) represent a significant global public health burden, not only from the high morbidity and mortality but also from costs associated with treating and managing these infections. In the USA alone, total lifetime costs per year for the 19.7 million incident cases of eight major STIs that occurred among persons of all ages in 2010, [1], was estimated to be US$15.6 billion [2]. Total morbidity costs were estimated to be US$516.7 million for Chlamydia trachomatis (CT), US$162.1 milllion for Neisseria gonorrhoeae (NG), US $50.7 million for hepatitis B virus (HBV), US $12.6 billion for HIV, US$1.7 billion for human papillomavirus (HPV), US$540.7 milllion for herpes simplex virus (HSV) type 2, US $39.3 million for syphilis and US$24.0 million for Trichomonas vaginalis (TV) [2]. The prevalence of these STIs in the USA is estimated at 110,197,000 (Figure 1) [1]. Prevalences ranged from the lowest for syphilis (117,000) to the highest for HPV (79.1 million), with CT (1.57 million) in the middle [1]. Youth are disproportionately affected; >20% of infections are among those aged 15–24 years. For example, of the 1.4 million CT cases reported to the US CDC in 2011, the rates were 3416.5 and 3722.5 cases per 100,000 population for adolescents 15–19 and 20–24 years of age, respectively [3].
Figure 1.
Estimated prevalence of sexually transmitted infections in the USA (total 110,197,000).
Numerous factors contribute to the persistent STI public health burden, but of particular importance is delay in treatment resulting from lengthy diagnostic protocols. Point-of-care (POC) tests offer significantly important strategies to address STI epidemics, since diagnosis and prompt treatment can offer immediate intervention, preventing onward transmission. The former are drivers of POC diagnostics, but many barriers exist, including low accuracy, regulatory issues, information system integration, the physical and electric environment and economic concerns as examples [4]. Rapid diagnosis also offers opportunity for counseling messages about sexual risk behavior and prevention of loss to follow-up, often a significant problem with STI patients. When routine diagnostics result in treatment delays in patients with active infections, they serve as reservoirs, passing their infection onto other individual before becoming aware of their infection status. Treatment delay is further complicated by the asymptomatic nature of all STIs, particularly HIV, CT and NG, although HSV-1/2, TV and syphilis can be mostly asymptomatic as well.
Traditional diagnostic approaches
Despite limitations, diagnostic tests have markedly improved for many STIs. Traditional STI diagnostics utilize culture or serological techniques. Although techniques such as Gram stain microscopy, wet preparation and direct fluorescent antibody can be considered POC tests, these have limited utility outside of a laboratory and many are insensitive and non-specific. Other immunological techniques such as ELISA are no longer recommended by the CDC for chlamydia due to lower sensitivity. Nucleic acid amplifications tests (NAATs) are considered the current gold standard assays for detection of CT and NG with several approved commercial assays available [5–11]. They are recommended by the CDC because of high sensitivity, specificity and rapidity compared with culture techniques (hours instead of days) [12]. Although NAATs have markedly improved diagnostics available for STI detection, their cost and time limitations may indirectly contribute to the STI public health burden, due to non-use in resource-poor settings and also the time required before a result is used for treatment of infected individuals, during which, further transmission can occur. NAATs are now also available for TV, HPV and HSV-1/2, but are not yet widely used [13–15]. Although NAATs are commonly utilized in developed countries, they are often impractical for deployment in resource-limited countries because of cost and infrastructure required for proper utilization. Due to NAAT limitations, large segments of the STI-positive populations receive either delayed or no treatment, contributing to the persistently high incidence and prevalence of STIs, both in the USA and worldwide. Some bacterial STIs, such as syphilis and viral STIs such as HIV and HSV were traditionally diagnosed by serology, but can now benefit from newer POC tests used with fingerstick blood, with the most advanced tests being for HIV [16].
POC tests
In general, sensitivity and specificity are important parameters for the use and evaluation of POC tests and are particularly critical in a screening situation. These parameters, as well as overall performance vary in real-world conditions, and are influenced by the training of staff and their ability to interpret test results. New sensitive and specific POC tests may have the ability to circumvent insufficiencies of traditional diagnostics, allowing patients to receive results and treatment before leaving the clinic or doctor’s office. Recently, many technological advances allowed POC diagnostics to offer promise reducing testing times while providing sensitive and specific results. However, many existing POC assays also suffer from markedly decreased sensitivity for certain STIs [17]. There is the possibility though that some newer technologies are lower in complexity and cost than NAATs, can be deployed in resource-poor settings, thus allowing disadvantaged, at-risk populations potential for testing and treatment. Issues regarding POC uptake is whether they are sensitive, specific, inexpensive and simple enough to affect STI burden. Modeling studies have indicated that even less sensitive POCs can result in additional patients being treated [18,19]. Methodologies to measure these metrics, estimate and weigh these variable parameters are drastically needed [20]. Another important issue in the adoption of POC tests is to determine the needs of clinicians who use POC tests [21,22].
WHO has provided developers and users of POC diagnostics guidelines, known as ASSURED to aid in developing and utilizing POC diagnostics for STIs that have true utility, both in developed and developing countries. ASSURED stands for: Affordable by those at risk for infection, Sensitive, very few false negatives; Specific, very few false positives; User-friendly, very simple to perform (minimal steps required with minimal training); Rapid and Robust, to enable treatment at first visit (rapid) and does not require refrigerated storage (robust), Equipment-free, easily collected non-invasive specimens (e.g., saliva and urine) and not requiring complex equipment and Delivered to end users [23]. The focus of this review is to address shortcomings and advances of some POCs and offer future perspectives for POC diagnostics for STIs caused by CT, NG, TV, HSV-1/2, syphilis and HIV.
Chlamydia trachomatis
Overview
CT is a significant public health problem in the USA and worldwide, and in the USA is the most frequently reported pathogen (1,412,791 cases in 2011) to the CDC [3]. Untreated CT can cause serious complications, including pelvic inflammatory disease, chronic pelvic pain, infertility and ectopic pregnancy [24]. Several rapid tests for detecting CT were developed between 1998 and 2007. These include the optical immunoassay (OIA) (Inverness [formerly BioStar], Princeton, NJ, USA) [25,26], Clearview Chlamydia (Alere Health Care, Waltham, MA, USA) [27–29], QuickVue (Quidel, San Diego, CA, USA) [30,31]. All are cleared by the US FDA, but lack sensitivity compared with NAAT assays [20]. The chlamydia rapid test (CRT, Diagnostics for the Real World, Ltd., Cambridge, UK) is only available in Europe [32]. Two assays with online literature, OneStep (CLIA Waived, Inc., San Diego, CA, USA) and the magnetic immunochromatographic test (ICT; MagnaBioSciences, Green Cross Medical Sciences Corp., San Diego, CA, USA), have no published literature or FDA clearance [20].
Evaluation of POC tests currently available
The most commonly evaluated POC diagnostics for CT are ICTs and OIA, both of which are based on antigen and antibody interactions. These platforms are typically very rapid, with <30 min testing time and low technical complexity, with little infrastructure requirements, but are quite insensitive [20,33–35]. Unfortunately, many of these platforms were originally evaluated by comparison with culture as the reference standard; this comparison can inflate reported sensitivity. Generally, sensitivities associated with CT POC tests ranged from approximately 33% (compared with NAAT) to 79–95% (compared with culture) depending on sample type and comparison method, with specificities >95% for most assays. However, since CT culture is now known to have much lower sensitivity than NAATs, when POC tests are compared against NAATs, they are woefully inadequate (Table 1) [17,27,36].
Table 1.
Sensitivity and specificity of point-or-care/near-patient tests for Chlamydia trachomatis and Neisseria gonorrhoeae.
| Organism | Test | Sample type | Sensitivity (%)† | Specificity (%)† |
|---|---|---|---|---|
| Chlamydia trachomatis | BioStar OIA chlamydia test | Cervical | 59.4–73.8 | 98.4–100 |
| Male urine | ||||
| Clearview Chlamydia test | Cervical | 49.7 | 97.9 | |
| Vaginal | 32.8 | 99.2 | ||
| QuickVue | Cervical | 25–65 | 100 | |
| Chlamydia rapid test | Vaginal | 83.5 | 98.9 | |
| Male urine | ||||
| X-pert CT/NG | Cervical | 97.4 | 99.6 | |
| Vaginal | 98.7 | 99.4 | ||
| Female urine | 97.6 | 99.8 | ||
| Male urine | 97.8 | 99.9 | ||
|
| ||||
| Neisseria gonorrhoeae | BioStar OIA GC test | Cervical | 60 | 89.9 |
| PATH GC-Check | Cervical | 70 | 97.2 | |
| Vaginal | 54.1 | 98.25 | ||
| X-pert CT/NG | Cervical | 100 | 100 | |
| Vaginal | 100 | 99.9 | ||
| Female urine | 95.6 | 99.9 | ||
| Male urine | 98.9 | 99.9 | ||
Sensitivity and specificity compared with nucleic acid amplification tests.
OIA: Optical immunoassay; PATH: Program for Appropriate Technology in Health.
Data taken from [20].
An extensive effectiveness and cost–effectiveness review for the British CRT assay was performed and compared with the Clearview Chlamydia POC assay and standard-of-care PCR testing by the Health Technology Assessment Program [35]. That review reported from several studies that the pooled sensitivity of CT for vaginal swabs was 80% and for first void urines was 77%. However for Clearview, pooled estimates from four studies indicated the sensitivity for vaginal, cervical and urethral swabs combined was 64 and was 52% for cervical samples alone. The resulting economic analysis reported that both POC tests were more costly and less effective than current practice of using NAAT assays; therefore, insufficient evidence was found to suggest that CRT could improve detection of chlamydia compared with standard practice [35].
New CT assays
Fortunately, biomedical technology has progressed in the last several years to provide some promising new POC or ‘near-patient’ assays. One, the Cepheid GenXpert assay has been FDA cleared in 2012 and combines microfluidic technology with real-time PCR. The cartridge-based assay detects DNA of CT (as well as NG) in female endocervical swabs, patient-collected vaginal swabs and for female and male urine specimens from symptomatic and asymptomatic patients [37]. It has demonstrated near perfect sensitivity (97.4–98.7%) and specificity (99.4–99.9%) in urogenital specimens [37,38]. The X-pert is a modular platform for testing samples directly from patients, which requires no hands-on manipulation from specimen loading until results are available in approximately 90 min. A mathematical model was reported for the potential of the new generation of POC tests, such as the Cepheid assay or other new POC test, to reduce the prevalence of CT and NG in the setting of high prevalence [39]. The Australian authors estimated that if the new POC test achieved sensitivity of 95% and with baseline screening coverage of 44% per year, prevalence for NG might be reduced from 7.1 to 5.7%, and CT prevalence might be reduced from 11.9 to 8.9%. If screening coverage were increased to 60 or 80%, prevalence could be as low as 0.6% for NG and 1.5% for CT. The model predicted that alone, minimizing the time between testing and treatment would have minimal impact in prevalence decline, but the use of highly sensitive POC tests have a huge potential to improve the outcome of screening since they can achieve the combined effect of reducing the delay for treatment to zero and effectively increasing the treatment rate to 100% [39].
POC tests under development
Another promising chlamydia POC assay is the Atlas Genetics platform in the UK [40]. This PCR assay has a novel electrochemical detection method for which a preclinical validation study using 306 archived clinical samples demonstrated a clinical sensitivity of 98.1% and specificity of 98.0%. The Velox™ technology contains an integrated fluidic card for sample processing and reagent handling and incorporates a novel technique for detection of proprietary ferrocene electrochemical labels and utilizes a low-cost reader instrument [40].
Another new POC CT technology in development is the microwave accelerated metal-enhanced fluorescence (MAMEF) assay [41–43]. The MAMEF assay can detect approximately 10 inclusion-forming units/ml of CT in <9 min, including DNA extraction and detection. Using a plasmid-based assay on archived clinical samples, sensitivity and specificity were 82.2% (37/45) and 92.9% (197/212), respectively [43]. In the assay, target DNA sequences bind to a fluorophore-labeled probe and additionally an anchor probe covalently bound to the assay surface. If target CT DNA sequence is present, metal enhanced fluorescence occurs through close proximity of the fluorophore to silver metallic nanoparticles. Sample lysis utilizes gold bowtie geometries, which highly focus microwaves onto clinical samples [43].
Neisseria gonorrhoeae
Overview
NG is the second most frequently reported infection to the CDC (with 321,849 cases in 2011) [3]. Similar to CT, the sequelae to untreated infections include PID, infertility, chronic pelvic pain and ectopic pregnancies. Gonorrhea is a significant public health problem worldwide, especially in light of increasing resistance to currently used antibiotics [44–46]. If a POC test could provide a result for not only NG but also for a resistance marker(s) for ciprofloxacin, for example, such a test would be invaluable for providing an immediate therapeutic option and for potentially sparing cephalosporins [47]. Unfortunately, no such POC diagnostic options currently exist.
Evaluation of POC tests currently available
For currently available NG POC tests, sensitivities generally range from 50 to 70% depending on sample type and comparison method, with specificity >95% for most sample types and comparison methods [20]. These assays include: the OIA (Inverness [formerly BioStar]), the Program for Appropriate Technology in Health GC-Check (PATH, Seattle, WA, USA) and OneStep (Cortez Diagnostics, Inc., Calabasas, CA, USA) [20].
A recent review article which compared LE dip stick (not NG specific but measures the white cell leukocyte esterase enzyme) studies (median sensitivity 71% [range 23–81%]), three ICT strip studies and microscopy for the detection of NG reported no adequate POC test for NG [48]. The three ICT studies (all requiring 15–30 min and 5–7 steps) in that review (GC Check, performed in Benin [49]; BioStar, performed in Brazil [50] and NOW Gonorrhea Test, performed in Japan) [51] demonstrated a median sensitivity of only 70% (range 60–90%) and a median specificity of 96% (range 89–97%) (Table 1) [48]. All of these reports indicate that improved POC tests for diagnosing NG are urgently needed [48].
New NG assays
Similar to the assay for CT, the Cepheid GenXpert assay for NG, promises to offer near-patient tests combining microfluidic technology with real-time PCR. The cartridge-based assay detects DNA from NG in female endocervical swabs, patient-collected vaginal swabs and for female and male urine specimens from symptomatic and asymptomatic patients [37]. Sensitivities and specificities ranged from 95.6 to 100% and 99.9 to 100%, respectively. Additionally, there have been no false-positive results with near neighbors in Neisseria sp. or other commensal respiratory bacteria [38]. Since this assay is newly commercially available, future evaluations will indicate its potential use in routine practice as a near-patient test.
POC diagnostics for syphilis
Overview
Syphilis, caused by Treponema pallidum, is re-emerging as an important STI, particularly since evidence indicates that syphilis cases are increasing in the USA as well as globally [52,53]. Following a primary ulcer, the disease, if untreated, can progress to the latent stages where serological evidence of infection is present, but with no clinical signs or symptoms of infection. Syphilis was thought to be on the verge of elimination in the USA, but cases increased 36% between 2006 and 2010, including a 134% increase in primary and secondary syphilis in African American males, 20–24 years old, which was possibly the result of risky sexual practice, inadequate access to healthcare and a greater incidence of syphilis within networks of men who had sex with men [53]. Syphilis outbreaks in Europe are also associated with men who have sex with men, with additional outbreaks occurring among commercial sex workers. Russia and China have both experienced syphilis epidemics from 2000 to 2009; China is the nation with the largest increase in syphilis, with cases doubling between 2005 and 2009 [53]. Due to the clinical severity associated with syphilis, particularly neonates, rapid and accurate detection of the infection is important, and highlights the need for POC diagnostics for syphilis.
Evaluation of POC tests currently available
Serologic tests for syphilis have been utilized successfully to detect and identify active and previous infections, largely because culture of T. pallidum is not possible, and detection by dark field microscopy can be performed only on primary syphilis genital lesions. Non-treponemal and treponemal tests rely on antigen and antibody interactions to generate results, and are primarily performed on plasma or serum. Both non-treponemal and treponemal serologic tests are utilized for diagnosis, and the latter test has been typically utilized to confirm the results of the former, since non-treponemal serologic tests lack specificity for T. pallidum.
The traditional screening algorithm for syphilis has been to first screen with a non-treponemal test. Two commonly utilized examples are the venereal disease research laboratory or rapid plasma reagin (RPR, Becton Dickinson, Sparks, MD, USA), and confirm a positive result with a treponemal test, such as T. pallidum particle agglutination (TP-PA, Fujirebio Diagnostics, Malvern, PA, USA) or T. pallidum hemagglutination [33,54]. Only the fourfold decrease in titer of the non-treponemal venereal disease research laboratory and RPR POC tests can indicate successfully treated syphilis. The treponemal-based tests remain positive for life and cannot distinguish between past treated and untreated infection. However, a reverse testing algorithm has begun to be commonly used [55]. This metric utilizes a treponemal primary screening assay followed by a non-treponemal test if the primary treponemal assay is positive. If the secondary, non-treponemal test is reactive, then syphilis is confirmed. If the non-treponemal test is non-reactive, a second treponemal test is performed to confirm the result of the primary test [56]. The reverse testing algorithm has been adopted largely because of economic and screening throughput factors, but studies have indicated that utilizing reverse screening may identify a higher percentage of positive patients [55–57].
New syphilis assays
There are an abundance of treponemal tests available for use with either screening algorithm. Rapid POC tests have been developed which can be performed on fingerstick blood, and are increasingly being used in resource-limited settings, but only one, Trinity Health Check™ (Diagnostics Direct, LLC, Stone Harbor, NJ, USA), is FDA cleared for use in the USA. This trepomemal POC test for syphilis has a reported 95.6% positive agreement and a 90.5% negative agreement with gold standard testing, with a percent overall agreement of 90.6% [58].
An excellent review in 2010 of results from 22,000 ICT strip POC syphilis tests described in 15 studies in STI clinics and antenatal clinics reported a high sensitivity (median 86%) and a high specificity (99%) (Table 2) [59]. The authors concluded that the ICT strip assays ‘have the potential to widen the breadth and depth of screening efforts’ and concluded operational research with ICT strip syphilis testing is an important step in syphilis control. A 2006 evaluation of four POC rapid tests for syphilis was reported for assays that are not yet approved for use in the USA: Determine Syphilis TP (Abbott Laboratories, Tokyo, Japan), VisiTect Syphilis (Omega Diagnostics, Alloa, UK), Syphicheck-WB (Qualpro Diagnostics, Goa, India) and SD Bioline Syphilis 3.0 (Standard Diagnostics, Kyonggi-do, Korea) and found that specificities for all rapid tests evaluated was >95%, with sensitivities ranging from 64 to 100% for the tests evaluated (Table 2) [60]. Other evaluations of POC tests for syphilis have found similar results to this evaluation, with reported sensitivities and specificities ranging from 62 to 100% and 83 to 95%, respectively, depending on the assay evaluated (Table 2) [33].
Table 2.
Sensitivities and specificities for point-of-care diagnostics for syphilis.
| Organism | Test | Sample type | Sensitivity† (%) | Specificity† (%) |
|---|---|---|---|---|
| Treponema pallidum (syphilis) | Abbott Determine Syphilis TP | Whole blood/serum | 59.6–100 | 95.7–100 |
| Omega VisiTect Syphilis | Whole blood/serum | 72.7–98.2 | 98.1–100 | |
| Qualpro Syphicheck-WB | Whole blood/serum | 64–97.6 | 98.4–99.7 | |
| Standard SD Bioline Syphilis 3.0 | Whole blood/serum | 85.7–100 | 95.5–99.4 | |
| Trinity syphilis health check | Whole blood/serum | 98.2‡ | 97.3‡ |
Expressed as a range of sensitivities and specificities from across four countries, utilizing whole blood and serum sensitivities from clinics and laboratories.
Based on overall sensitivity and specificity reported for treponemal test comparisons in Trinity syphilis health check package insert [58].
Data taken from [60].
A recent evaluation of eight treponemal assays, including fluorescent treponemal antibody-absorption (FTA-ABS, Zeus Scientific, Raritan, NJ, USA), LIASON Treponema assay (Diasorin, Stillwater, MN, USA), SD Bioline 3.0 Rapid POC test (Standard Diagnostics, Korea), INNO-LIA (Biokit, Barcelona, Spain) and CAPTIA IgG, Trep-ID and Trep-Sure (Trinity Biotech, Jamestown, NJ, USA) were all evaluated qualitatively, as well as quantitatively, to determine their concordance with RPR and TP-PA, as well as to determine the end point detection for reactive samples by testing various serial dilutions [54]. All treponemal assays evaluated performed very well in the qualitative evaluation, at >94 and >98% concordance for reactive and non-reactive samples, respectively. However, there were substantial differences in the lowest end point titer dilution detection for each assay [54]. Overall, FTA-ABS had the lowest median end point at 1:4, while Trep-Sure had the highest at 1:512. The evaluation illustrated that when utilizing treponemal tests in the reverse testing algorithm, it is important to utilize primary and confirmation treponemal tests with the same end point of detection to limit discordance between primary and confirmation tests [54].
The POC diagnostic from this same evaluation, SD Bioline, performed well qualitatively with 100 and 98.9% concordance to TP-PA for reactive and non-reactive specimens, respectively [54]. The end point dilution detection for this assay was 1:32, which was statistically different from TP-PA (end point of 1:16) as well as other tests evaluated (p < 0.0001).
Another recent study performed in Tanzania with a cohort of pregnant women evaluated SD Bioline and compared the results with TP-PA and the syphilis enzyme immunoassay (EIA; Lab 21 Healthcare, Kentford, UK) with an accessibility component added to the study in addition to the performance evaluation [61]. SD Bioline had a sensitivity and specificity of 59.6 and 99.4%, respectively compared with TP-PA, while the EIA displayed higher sensitivity and lower specificity at 95.3 and 97.78%, respectively. For active cases of syphilis sensitivities of SD Bioline and the EIA were 82 and 100%, respectively [61]. Notably, the accessibility evaluation performed in conjunction with the performance evaluation indicated that there was a huge discrepancy between pregnant women, who visit health centers and dispensaries (where the POC diagnostic was implemented) than those who visited district hospitals [61]. In fact, the study found that using the 2.3% prevalence determined in the study, that 82% (n = 63) of women with active syphilis infections in these districts would have been treated with implementation of the POC test, whereas without it, only 16% (n = 12) would be successfully treated, which would translate into a huge improvement in the implementation of POC tests for syphilis control.
POC tests for syphilis have been successfully deployed in other areas of the world as well, including Peru and China [62,63]. In these areas, POC testing for syphilis proved feasible and valuable particularly in China, where a high incidence of primary syphilis was observed in pregnant women from rural areas of the country [62].
The variability in sensitivity of POC diagnostics for syphilis will remain an issue; however, the benefits of being able to provide early treatment to infected individuals may outweigh the variability and sometimes lower sensitivity of the assays [33], and the ease of use of these assays suggest they could be coupled to other rapid screening initiatives, particularly if utilized in conjunction with rapid HIV testing [63]. In summary, the most efficient use of POC assays for syphilis appears to be in resource-limited settings rather in the USA, where the prevalence of syphilis is generally much lower; however, there may be a place for their use in men who have sex with men in urban areas of high prevalence in the USA.
Trichomonas vaginalis
Overview
Trichomonas infections, caused by the parasite TV, are highly prevalent STIs, with estimates of 3.7 million infections annually in the USA and 180 million globally [64]. They represent the most common curable STI in young, sexually active women [65,66] and have been associated with poor reproductive outcomes such as low birth weight (LBW) and premature birth [67,68]. The National Health and Nutrition Examination Survey 2001–2004 estimated that 3.1% of women in the USA have TV [69]. The National Longitudinal Study of Adolescent Health cohort study indicated that 2.8% of women 18–26 years were positive for TV, with infections in African American women ranging from 10.5 to 13% [70]. Data from the National Health and Nutrition Examination Survey demonstrated that TV was associated with other STIs among women in the civilian US population in a sample of 3648 women, 14–49 years old [71] with a TV prevalence of 3.2, and >80% of cases being asymptomatic. Of important interest is the association of TV with HIV [72]. Evidence suggests that TV increases transmission and acquisition HIV among women and several models suggest positive effects of TV therapy related to HIV viral burden and estimating the number of transmitted HIV infections attributable to TV [73,74]. Of note, most studies of TV have involved women, but more attention may be directed toward men in the future as POC assays improve and potentially provide better methods to access and test men [75,76].
Evaluation of POC tests currently available
Currently available POC assays for TV [77] include wet preparations (microscopic examination of saline mount of vaginal secretions for motile trichomonads), OSOM TV Trichomonas Rapid Test (Sekisui, formerly Genzyme Diagnostics, Cambridge, MA, USA), the Affirm VPIII Microbial Identification Test (Becton Dickinson, Sparks, MD, USA) and the Kalon Tv latex test (Kalon Biological, Surrey, UK). The Kalon test uses latex beads coated with antibodies specific to TV proteins, but is not FDA cleared or CE (Conformité Européenne) marked in Europe [77]. The XenoStrip-Tv (Xenotope Diagnostics, Inc., San Antonio, TX, USA) is an ICT diagnostic detecting TV membrane proteins with reported sensitivities of 78.5 and 90% in two published studies, but it is no longer available [20].
OSOM TV Trichomonas Rapid Test is an ICT capillary flow (dipstick) assay that detects TV membrane proteins, with an additional internal control. It is Clinical Laboratory Improvement Amendments (CLIA) waived and requires five steps to complete, with results in 10 min. An early study compared the sensitivity and specificity of OSOM, wet mount and culture performed on vaginal swabs from 449 sexually active women [78]. The overall prevalence of TV was 23.4% by a gold standard of either positive wet mount or positive culture. For the vaginal swabs, OSOM displayed 83.3 and 98.8% sensitivity and specificity, respectively, and it performed better than wet preparation. In another comparison with wet preparation, culture and a NAAT assay, the OSOM performed very well with a sensitivity and specificity of 90 and 100%, respectively in 330 sexually active females aged 14–21 years [79]. The prevalence of trichomoniasis in this population was 18.5% (61/330); the sensitivity of wet preparation ranged from 50 to 54% and for culture was 75%. In symptomatic women, the sensitivity of OSOM was 92.5% and for the NAAT, 97.5%. Similar low sensitivities for culture and wet preparation were reported in another publication (Table 3) [80].
Table 3.
Sensitivities and specificities for point-of-care diagnostics for Trichomonas vaginalis.
An innovative algorithm in another study utilized OSOM and culture for subjects who tested wet mount negative. Wet mount saline was utilized as an InPouch TV culture inoculum, and the remaining swab after wet mount was tested with OSOM. The best strategy to detect TV using two tests was wet mount followed by OSOM, with a sensitivity of 86.4% [81].
A recent study utilized the OSOM test in India in 450 sexually active women compared with wet preparation and InPouch culture [82]. Compared with a composite reference standard of wet preparation and culture, OSOM demonstrated a sensitivity and specificity of 86.1 and 100%, respectively, with positive predictive value (PPV) and negative predictive value (NPV) of 100 and 97.1%, respectively.
Affirm VPIII Microbial Identification Test is a test that detects T. vaginalis, Candida sp. and Gardnerella vaginalis. It uses synthetic nucleic acid capture probes and color development detection probes complementary to unique genetic sequences of the target organisms [20]. It is considered a moderately complex test with at least 10 steps and requires 45 min to achieve results. Early evaluations that compared it with wet preparation and culture demonstrated a range of sensitivity of 91.8 and 89.2%, respectively, while specificity ranged from 98.1 to 99.3% [20]. However, a recent study which compared the TV results with a NAAT for TV reported a sensitivity and specificity of 46.3 and 100%, respectively (Table 3) [83].
New TV POC assays under development
A new rapid prototype TV POC assay has been designed for use in conjunction with the Atlas io POC platform [84]. The assay features novel electrochemical end point detection, with a multi-copy region of the TV genome as a target. In a preliminary performance study, 90 clinical vaginal swab samples were used to verify the performance of the prototype assay, demonstrating a sensitivity and specificity of 95.5% (42/44) and 95.7% (44/46), respectively [85].
Herpes simplex virus 1 & 2
Overview
Herpes simplex viruses type 1 and type 2 cause ulcerative lesions that are typically painful, and infection is a persistent lifelong disease. HSV-1 infections typically recur 1- to 2-times per year, whereas HSV-2 infection can recur anywhere from 4- to 10-times per year [85,86]. Although HSV-1 typically causes oral lesions, and HSV-2 causes about 80% of anogenital lesions, there is considerable crossover of types with more anogenital HSV-1 noted in younger individuals [33,87,88]. Data from the CDC estimate that there are 776,000 new HSV-2 infections a year in the USA [1], and that 50 million persons in the USA are infected with HSV-2 [87]. There is no cure for HSV-1 or HSV-2, although the disease can be managed through suppressive therapy with antiviral drugs, based on CDC recommendations for treatment of primary, episodic or recurrent genital herpes [86,87]. Current pathogenesis research indicates that a vaccine for HSV-1 and HSV-2 may be possible, and efforts are advancing toward that end [89,90]. It is important that infected individuals are aware of their status to allow counseling and behavioral intervention by providers, and importantly to be offered antiviral therapy to reduce the risk of transmission to current and/or future partners [33].
Worldwide, HSV is highly prevalent with estimates of 536 million by WHO. A recent longitudinal analysis of STI incidence at a Brazilian healthcare center indicated the HSV incidence was 10.6% from 1999 to 2009 [91]. In Sub-Saharan Africa, of 40 countries with available data, the HSV-2 prevalence was below 20% in 32 of those, while the prevalence was >50% in the other eight countries [92,93]. Previous infection with HSV-1 contributes to a threefold increase in asymptomatic HSV-2 infection, and HSV-2 infection has been established as a principal risk factor for acquisition and transmission of HIV [33,86,94].
Evaluation of HSV tests currently available
Currently, there are no POC tests for serological detection of antibodies to HSV that are FDA cleared.
The high prevalence of HSV-1 and HSV-2, plus the contribution of HSV-2 to increased HIV acquisition and transmission emphasize the need for POC diagnostics for HSV.
Formerly, traditional diagnosis of infection was performed by scraping a herpetic lesion and performing either Tzanck smear or cytology, but this diagnostic method has been discontinued due to low sensitivity and specificity [86]. Viral culture of lesions has been utilized for many years and had good specificity, but lacks excellent sensitivity, especially in older healing lesions. PCR is a sensitive and specific technique that can also be utilized on ulcers, and is adaptable for typing [33,86]. Most PCRs have been Laboratory Developed Tests for research and clinical use, but now there is an amplification assay that is FDA cleared by Becton Dickinson [15]. Serology has been the mainstay diagnostic test to identify present or previous HSV-1 and HSV-2 infections, since often lesions are not present and type-specific serology has been widely implemented to demonstrate exposure [33,86,95–98].
Serology for HSV detection depends on antibody and antigen interactions. One potential cost savings screening algorithm utilizing serology for HSV detection is the Alberta Algorithm, which consists of a primary screen utilizing a non-specific serology kit (Behring Enzygnost IgG, Siemens Healthcare, Munich, Germany) for any anti-HSV antibody, and if this test is reactive, screen with a secondary serology test specific for anti-HSV-2 antibodies (Focus HerpeSelect 2, Focus Diagnostics, Cypress, CA, USA). If the secondary test is positive, HSV-2 is confirmed and if the secondary test is negative, the sample is an HSV-1 presumed positive [95]. Evaluation of that algorithm, using western blot as a gold standard, demonstrated sensitivity and specificity of 92 and 97%, respectively for HSV-2 detection, and 94 and 100%, respectively for HSV-1 (Table 4).
Table 4.
Sensitivities and specificities for point-of-care diagnostics for herpes simplex virus 2.
| Organism | Test | Sample type | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Herpes simplex virus 2 | HerpeSelect | Serum | 80–100† | 41–100† |
| HerpeSelect | Serum | 91‡ | 97‡ | |
| Kalon HSV-2 gG2 | Serum | 84–98.6† | 83.2–100† | |
| Rapid real-time PCR | Genital secretions, genital lesions, buffer solutions | 96.7§ | 99.6§ | |
| IsoAMP® HSV | Genital swabs | 97.1¶ | 93.4¶ |
Utilizing serological testing to detect HSV infections has been controversial among STI practitioners and willingness to pay appears to drive uptake by patients, with acceptance being high when the test was offered free, but low among high-risk women who were charged US$10 [96,97].
Although serology has been successfully utilized in multiple venues, certain barriers including cost limitations prevent utilization globally. A recent systematic review and meta-analysis of 10 articles illustrated some of these barriers [98]. The review and analysis focused on 21 studies of HerpeSelect and 12 studies that utilized Kalon gG2 ELISA (Kalon Biologicals Ltd., Guilford, UK), and found that sensitivities and specificities for these HSV-2 varied by setting when evaluated in sub-Saharan Africa, and were generally lower than reported in the USA and UK (Table 4) [98]. Specifically, that analysis found that HerpeSelect had low specificity, estimated at 69%, when utilizing the manufacturer’s cut-off, and that in general Kalon performed better than HerpeSelect when utilized in the same population. Potential reasons for this discrepancy include: potential African HSV-2 strain variants that are uncommon in North America, cross-reactivity with unidentified antibodies that are common in African populations, or nucleotide polymorphisms gG2 sequences that are specific to African populations [98]. One recommendation to improve specificity of these assays in sub-Saharan Africa is to increase the cut-off recommended by the manufacturer. Ideally, for a diagnostic to be implemented for routine use throughout the world, standardization of that assay across populations would be a key feature.
New HSV POC tests
There is one recently FDA-cleared POC assay for the detection of HSV in lesions, the IsoAMP® HSV (Biohelix Corp., Beverly, MA, USA), discussed below. Advances in biomedical technology continue to drive diagnostics for HSV forward. PCR has not been routinely utilized outside of diagnostic and core laboratories due to the 4 h time requirement and associated complexity. A recent study with the emphasis on developing and validating a 2 h result with a real-time PCR, using pre-aliquoted reagents, as well as an ABI 7500HT FAST instrument (Applied Biosystems, Foster City, CA, USA) was validated for women in labor [99]. The assay had sensitivity and specificity of 99.6 and 96.7%, respectively compared with a previously validated real-time PCR for HSV detection (Table 4). Although this PCR does not meet the definition of a POC assay, application of additional advances in technology, such as isothermal amplification or LAMP could reduce assay time, and complexity. However, until amplification technologies can be encapsulated into a low complexity, low-cost device that can be deployed in areas like sub-Saharan Africa, they remain far from being true POC diagnostics for HSV.
New HSV POC assays & assays under development
Microfluidic technology has made advancements recently, and is beginning to yield quality results, in terms of new POC diagnostics [100]. Recently, promising microfluidic technology utilizing luciferase coupled antigens for detection of HSV-2 antibodies was demonstrated [101]. The luciferase immunoprecipitation system presented in the publication relies on light emitting recombinant antigens to measure antibody titers, and demonstrated 100% sensitivity and specificity for detection of HSV-2 antibodies in 20 human plasma samples [101]. With a detection time of <10 min, this platform has great potential for automation and portability, although further evaluation will be required. Devices like luciferase immunoprecipitation system are well suited for antibody detection, and as a potential replacement for standard serology, particularly in developing countries where inexpensive, rapid and highly sensitive and specific assays are required.
For primary outbreaks of HSV, the IsoAMP HSV (Biohelix Corp.) has recently received the FDA approval for detection of HSV nucleic acid from genital and oral lesions [102]. The technology utilizes isothermal helicase-dependent amplification (HAD), which uses Bst DNA polymerase, and by obviating the nucleic acid extraction process, can offer results in 1.5 h. Analytical sensitivity has been measured at 5.5 and 34.1 copies/reaction for HSV-1 and HSV-2, respectively [103]. In clinical performance, IsoAMP demonstrated sensitivity for HSV-1 and -2 of 98.6% and a specificity of 98.8% for both oral and genital lesions (no typing) (Table 4) [102]. Compared with ELVIS® typing system, the technology offered a sensitivity of 97.1% for genital lesions and 93.8% for oral lesions and specificities of 93.4 and 87.4% in genital and oral lesions, respectively [102]. From five study sites in the USA, and after discrepant analysis, overall agreement of IsoAMP with ELVIS was 98.8%, with a 37.0% overall prevalence [104]. These results are comparable with PCR and offered high reproducibility of results among the laboratories where it was evaluated. This technology represents the future of diagnostics for primary HSV infections, as well as for other STIs, due to the fast time to result, easy operation and high sensitivity and specificity [102].
Human immunodeficiency virus
Overview
There are an estimated 41,400 new HIV infections in the USA each year [1]. Unfortunately, of 1.1 million infected individuals in the USA, 20% are unaware of their HIV infection [105]. These persons and their partners remain at high risk of unknowing transmission and acquisition of HIV. HIV incidence remains highest in African Americans, accounting for 44% of all new infections, and the rate of 68.9/100,000 population was 7.9-times higher than the rate in Caucasians (8.7/100,000) [106]. Because of the need to test those who are at most risk as well as the urgency for rapid results, POC tests are most appealing to use in situations where patients interact with the healthcare system, as well as to reach outside of routine clinical situations, such as emergency departments, schools, mobile vans, healthcare fairs and even self-testing [107–111]. Rapid POC tests are designed to detect anti-HIV antibodies within approximately 20 min so that results are available within the medical encounter. Most assays use whole blood from fingersticks, plasma, serum or oral fluid [112,113]. POC tests are especially valuable by allowing patients assessment for immediate care or when results are required for immediate treatment decisions, as for pregnant women in labor [113].
Evaluation of POC tests currently available
Currently available rapid POC tests in the USA include: OraQuick (OraSure Technologies, Bethlehem, PA), Reveal (Medmira, Inc., Halifax, Nova Scotia), Multispot HIV-1/HIV-2 Rapid Test (Bio-Rad Laboratories, Redmond, WA), Uni-Gold Recombigen HIV test (Trinity Biotech PLC, Dublin, Ireland), Clearview HIV-1/2 STAT-PAK and Clearview Complete (self-contained closed system) HIV-1/2 (Alere Health Care, Waltham, MA), Chembio Dual Path Platform (DPP) HIV-1/2 Assay (Chembio Diagnostic Systems, Inc., Medford, NY) and INSTI (Biolytical Laboratories Inc., Richmond, BC, Canada; Chicago, IL) [20,114,115]. Many of the advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by test generation and platform type, including POC rapid tests have been recently reviewed in an excellent manner by the CDC [116]. There are also WHO-approved assays that are not FDA cleared for use in the USA, such as the Determine HIV-1/2 (Inverness Medical Japan Co. Ltd., Tokyo, Japan) that are widely used outside the USA [117,118]. For example, the new Determine assay, HIV-1/2 AG/AB Combo test, now includes p24 antigen detection and has been evaluated in Africa with excellent performance characteristics (sensitivity 99.4% and specificity 99.2%) for the antibody portion, although the antigen portion had a sensitivity of 0.000 and a specificity of 0.983. [119].
OraQuick Rapid HIV-1/2 Antibody Test is FDA-approved CLIA waived and can be used with oral fluid and blood, but for plasma, it is rated as moderately complex [20]. It relies on antibody and antigen interactions and is commonly used for oral fluid samples. The result can be read between 20 and 40 min after the sample is inserted into the test vial; 2 lines (control and test lines) indicate a positive result and 1 line (control only) indicates a negative result. [120]. In one study, comparing 6811 specimens with EIA testing, OraQuick detected 91% of the antibody positive results with a specificity, positive predictive value (PPV) and NPV values of 99.6, 98.1 and 99.4%, respectively (Table 5) [121].
Table 5.
Sensitivities and specificities for point-of-care diagnostics for HIV.
| Organism | Test | Sample type | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| HIV† | OraQuick advance rapid HIV-1/2 antibody test | Oral fluid, whole blood/serum | 99.6 | 100 |
| Reveal G3 rapid HIV-1 antibody test | Serum/plasma | 99.8 | 99.9 | |
| Multispot HIV-1/HIV-2 rapid test | Serum/plasma | 100 | 99.9 | |
| Uni-Gold Recombigen HIV test | Whole blood/serum/plasma | 100 | 99.7 | |
| Clearview HIV-1/2 STAT-PAK or Clearview Complete HIV-1/2 | Whole blood/serum/plasma | 99.7 | 99.9 | |
| Chembio DPP HIV-1/2 assay | Oral fluid, whole blood/serum/plasma | 99.8 | 99.9 | |
| INSTI HIV-1 antibody test | Whole blood/plasma | 99.8 | 99.5 |
Reveal is a single-use, qualitative immunoassay to detect anti-HIV-1 antibodies in serum or plasma and is categorized as moderately complex, with reported sensitivity and specificity of 99.8 and 99.9%, respectively (Table 5) [113,122].
The Multispot HIV-1/HIV-2 Rapid Test is a single-use, qualitative immunoassay to detect and differentiate circulating anti-HIV-1 and anti-HIV-2 antibodies in fresh or frozen serum and plasma [123]. It is rated as moderately complex but not CLIA waived with reported sensitivity and specificity of 100 and 99.9%, respectively, and is recommended as a confirmatory and differentiation test between HIV-1 and HIV-2 utilized with the new HIV testing algorithm beginning with fourth-generation antigen/antibody assays (Table 5) [118,124].
Uni-Gold Recombigen HIV is a single-use, rapid test for the detection of anti-HIV-1 antibodies in plasma and serum, where it is considered moderately complex and whole blood from venipuncture, where it is CLIA waived. Uni-Gold Recombigen HIV test is intended for use in POC settings for confirmation of HIV-1 infection. This test is suitable for use in appropriate multi-test algorithms designed for the statistical validation of rapid HIV test results and has been reported sensitivity and specificity of 100 and 99.7%, respectively (Table 5) [125,113].
The Clearview HIV-1/2 STAT-PAK assay is a single-use, rapid ICT qualitative test used to detect anti-HIV-1 and anti-HIV-2 antibodies in fingerstick whole blood, venous whole blood, serum and plasma specimens, with reported 99.7% sensitivity and 99.9% specificity in 15–20 min (Table 5) [126]. It is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results. The Clearview Complete is a closed system and self-contained for minimal exposure to healthcare workers.
New HIV POC tests
The Chembio DPP HIV-1/2 Assay is a single-use ICT for the detection of anti-HIV-1 and anti-HIV-2 antibodies in oral fluid, fingerstick whole blood, venous whole blood, serum or plasma samples, with reported sensitivity for fingerstick whole blood at 99.8% (926/964, 95% CI: 99.2–99.9%). Specificity has documented at 99.9% (Table 5). The intended use is as a POC test to aid in the diagnosis HIV-1and HIV-2 infection, and it is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results [127].
The INSTI HIV-1 Antibody Test is a single-use, rapid, in vitro qualitative immunoassay for anti-HIV-1 antibody detection in human venipuncture whole blood, fingerstick blood or plasma specimens. The newly FDA-cleared test is intended for use by trained personnel in POC and laboratory situations to aid in detection of HIV-1 infections. If multiple rapid HIV tests are available, this test is suitable for use in appropriate multi-test algorithms [128]. Sensitivity analysis was performed on HIV-1-positive specimens (n = 1076) with matching fingerstick whole blood, ethylenediaminetetraacetic acid (EDTA) whole blood and EDTA plasma. Additionally, unscreened, matching fingerstick whole blood, EDTA whole blood and EDTA plasma samples (n = 782) with 22 positives via an FDA-licensed test were also evaluated. Overall sensitivity and for fingerstick whole blood from confirmed and know HIV-1-positive samples was 99.8% (1095/1097, 95% CI: 99.3–99.9%), while specificity, minus invalid results, was 99.5% (1375/1382, 95% CI: 99.3–99.9%) (Table 5) [128].
Acceptability of standard and rapid tests for HIV testing is high among patients and the feasibility of such testing appears to be adequate [129,130]. In an urban sexually transmitted disease clinic, 80% of eligible patients accepted HIV testing and 87% of those accepted POC HIV tests [131]. Patients will even accept to perform their own HIV test in Emergency Departments with some being recruited via kiosks [107,108,110].
Many evaluations of rapid POC testing strategies have been performed with excellent results, some advocating either parallel (two separate POC tests) or serial algorithms (where initial positive tests are confirmed by a second more specific test), and are enabling moving testing from the laboratory to the patient [16,132–134]. One comparison of three rapid test kits (Determine, STAT-PAK and Uni-Gold) demonstrated that parallel testing had a sensitivity and specificity of 99.7 and 99.8%, respectively, whereas if STAT-PAK was utilized as the initial serial test, overall sensitivity and specificity were 99.6 and 99.7%, respectively. However, if Determine was used as the first serial test, the overall sensitivity dropped to 97.3% and the specificity increased to 99.9%, indicating the Determine was a slightly less sensitive, although more specific screening test [133]. Serial testing is likely to be cost saving though, and this should be measured against variations in sensitivity and specificity in various testing algorithms to determine if impactful cost savings mitigate a reduced number of positive detections.
Rapid HIV testing at home is receiving attention especially since the approval of the OraQuick home testing kit by the FDA [135,136]. This approval is expected to empower individuals to identify their HIV status and overcome barriers to HIV testing. However, research regarding self-testing is currently scarce and relevant research is needed, especially cost–effectiveness studies and systems for monitoring self-testing, as well as studies regarding self-reporting for individuals who test positive [109,111].
Although not a POC test for an STI, there is increasing use of rapid CD4 POC tests in resource-limited sites to augment rapid HIV POC tests, and the evaluation of the Pima™ assay (Alere Health Care, Waltham, MA, USA) appears to show reasonable overall agreement with laboratory-based flow cytometry assays [137–140]. Field use of such CD4 assays has the potential to monitor improved adherence to HIV therapy. Recent nanotechnology and microfluidic advances will make even simple, low-cost, and robust POC HIV viral load assays possible in the near future [141,142]. As research into new POC diagnostic platforms, such as microfluidic paper-based analytic devices advances, we may expect to see healthcare in resource-limited areas without trained medical clinicians become realized [100,143].
Expert commentary & five-year view
There is no debate that accurate and inexpensive POC tests are urgently needed to control the costly epidemics of STIs in the world today, so that patients can receive timely diagnoses and needed treatment for these infections [144]. The exciting advances in microfluidic technology represent the future of improved diagnostics for POC diagnostics for STIs. Excellent POC serological assays have been developed for the detection of anti-HIV antibodies and p24 antigen, as well as syphilis, but development of true POC tests for the detection of the curable bacterial STIs, such as chlamydia and gonorrhea are lagging and thus far have performed inadequately. A substantial investment in terms of capital, time and research effort is required to further develop POC diagnostics for these infections. The threat of emerging antimicrobial resistance for gonorrhea represents a substantial hurdle, both as a public health problem and highlights the urgent need for new POC assays and rapid tests to detect antibiotic sensitivity or resistance to currently available antibiotics in order to curtail development resistance and aid in antibiotic-sparing measures.
It is important to recognize the necessity of evaluating the performance of new POC tests for STIs in real-world settings. Although sensitivity and specificity are often used to evaluate the performance of a test in trial situations, it is the PPV and NPV that may be more important with regards to evaluating assays, since the prevalence affects these values. Thus, it will be important to recognize the predictive values of a test that will depend on the population prevalence of the infection, which if is very low, can substantially affect test performance in the particular setting.
Despite of the current lack of adequate POC assays for treatable bacterial infections, with the application of new technological advances, for example, low-cost, low-complexity microfluidic paper-based platforms and the ability to couple new technologies to healthcare infrastructures, promising advancements should be expected in the next few years [100,143].
The POC assays for trichomonas are promising, but are not yet as sensitive as NAATs; further refinement is required, but should be forthcoming in the near future as technologies such as isothermal amplification and microfluidic advances add the promise of bringing NAAT sensitivity and specificity to POC assay for TV. Better FDA-cleared POC assays are required for serological assays for syphilis and HSV and hopes are high that new assays under current development will meet the need and demand for such assays.
The future for the development of new inexpensive and accurate POCs will depend on the commitment of public health officials and industry to successfully partner to bring such assays, such as those based on chip technology and biosensors, [145–149], through regulatory requirements and into actual use in the USA and in resource-limited settings. The POC test pipeline should be filled with new POC assays in the next 5 years.
Key issues.
Although current point-of-care (POC) tests for Chlamydia trachomatis and Neisseria gonorrhoeae have demonstrated less than ideal performance, there are near-patient assays (GenXpert) that have displayed excellent performance characteristics, and new microfluidic-based technologies that offer the promise of excellent POC tests for Chlamydia trachomatis and Neisseria gonorrhoeae in the near future.
Traditional POC tests for Trichomonas vaginalis still rely largely on wet preparation for detection, although advances have been made with POC tests, notably the OSOM test. The Kalon Tv latex test is another promising technology, although it is not US FDA approved or CE marked, and requires further evaluation before implementation.
The reverse testing algorithm for syphilis has provided a strategy to identify increased cases of new and previous syphilis infections, although with variable sensitivity. POC tests for syphilis are best utilized in resource-poor settings, although they may have some utility in developed countries among men who have sex with men, or where incidence and prevalence are high.
There is a FDA-cleared rapid test (IsoAMP) for detection of herpes simplex viruses (HSVs) from lesions, but POC serological assays to measure antibody are not available. Utilizing enzyme immunoassay serological testing to detect HSV antibody is effective in developed countries, although willingness to pay, as well as acceptance by sexually transmitted infection practitioners, are factors affecting acceptability. In developing countries, sensitivity and specificity remain variable, although alterations in testing algorithms and manufacturer cut-offs may improve sensitivity, specificity and consistency in results. For the detection of primary HSV infections, there are some promising new technologies in development (e.g., luciferase immunoprecipitation system).
Technological advances have led to numerous POC tests for the detection of anti-HIV antibodies, the majority of which show excellent performance in serial or multi-test algorithms. Many are available only outside the USA. The possibility of performing home HIV testing has been advanced with FDA approval for the OraQuick home testing kit, although cost–effectiveness and self-monitoring studies are required to understand the impact home testing will make on overcoming barriers to HIV testing. Numerous technological advances offer the promise of incorporating CD4 as well as p24 antigen testing into POC diagnostics.
Footnotes
Financial & competing interests disclosure
This study was funded by NIH U-54 EB 007958 and NIAID NIH U-01 AM AI 068613. C Gaydos has received research funding and speaker fees from the following: Abbott Molecular, Becton Dickinson, Cepheid and Hologic Gen-Probe. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1•.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates 2008. Sex Transmit Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. Important new estimates of incidence and prevalence. [DOI] [PubMed] [Google Scholar]
- 2.Owusu-Edusei JK, Chesson HW, Gift TL, et al. The estimated direct medical costs of selected sexually transmitted infections in the United States 2008. Sex Transmit Dis. 2013;40:197–201. doi: 10.1097/OLQ.0b013e318285c6d2. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2011. Atlanta: U.S. Department of Health and Human Services; 2012. pp. 1–156. CDC 2012. [Google Scholar]
- 4.Pothier K, Kirtland M, Gupta S. Has Point-of care come of age? Point Care. 2010;9:147–50. [Google Scholar]
- 5.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA. Combo 2 assay for the multiplex detection of Chlamydia trachomatis and Neisseria gonorrheae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41:304–9. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaydos CA. Nucleic acid amplification tests for gonorrhea and chlamydia: practice and applications. In: Zenilman JM, Moellering RC Jr, editors. Infect Dis Clin N Am. Elsevier Saunders; Philadelphia, PA, USA: 2005. [DOI] [PubMed] [Google Scholar]
- 7.Gaydos CA, Cartwight CP, Colaninno P, et al. Performance of the Abbott RealTime CT/NG for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2010;48:3236–43. doi: 10.1128/JCM.01019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SN, Van Der Pol B, Lillis R, et al. Clinical evaluation of the BD. ProbeTecTM Chlamydia trachomatis (CT) Qx amplified DNA assay on the BD ViperTM system with XTRTM technology. Sex Transm Dis. 2011;38:603–9. doi: 10.1097/OLQ.0b013e31820a94d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Der Pol B, Taylor S, Lebar W, et al. Clinical evaluation of the BD ProbeTec™ Neisseria gonorrhoeae Qx amplified DNA assay on the BD viper™ system with XTR™ technology. Sex Transmit Dis. 2012;39:147–53. doi: 10.1097/OLQ.0b013e3182372fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Pol B, Liesenfeld O, Williams JA, et al. Performance of the cobas CT/NG test compared to the aptima AC2 and viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2012;50:2244–9. doi: 10.1128/JCM.06481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Pol B, Taylor SN, Liesenfeld O, et al. Vaginal swabs are the optimal specimen for detection of genital Chlamydia trachomatis or Neisseria gonorrhoeae using the cobas 4800 CT/NG test. Sex Transmit Dis. 2013;40:247–50. doi: 10.1097/OLQ.0b013e3182717833. [DOI] [PubMed] [Google Scholar]
- 12•.Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR. 2014 In press Important new CDC laboratory guidelines for sexually transmitted infections. [PMC free article] [PubMed] [Google Scholar]
- 13.Schwebke JR, Hobbs M, Taylor S, et al. Molecular testing for Trichomonas vaginalis in women: results of a pivotal US clinical trial. J Clin Microbiol. 2011;49:4106–11. doi: 10.1128/JCM.01291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Der Pol B, Warren T, Taylor SN, et al. Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test. J Clin Microbiol. 2012;50:3466–71. doi: 10.1128/JCM.01685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shott JP, Galiwango RMm, Reynolds SJ. A Quality management approach to implementing point-of-care technologies for HIV diagnosis and monitoring in sub-Saharan Africa. J Trop Med. 2012;2012:1–8. doi: 10.1155/2012/651927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dommelen L, van Tiel FH, Ouburg S, et al. Alarmingly poor performance in Chlamydia trachomatis point-of-care testing. Sex Transm Infect. 2010;86:355–9. doi: 10.1136/sti.2010.042598. [DOI] [PubMed] [Google Scholar]
- 18.Gift TL, Pate M, Hook IEW, Kassler WJ. The rapid test paradox: when fewer cases detected lead to more cases treated, a decision analysis of tests for Chlamydia trachomatis. Sex Trans Dis. 1999;26:232–40. doi: 10.1097/00007435-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 19•.Huang W, Gaydos CA, Barnes M, et al. Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting. Sex Transmit Infect. 2013;89:108–14. doi: 10.1136/sextrans-2011-050355. Model showing point-of-care (POC) diagnostic assays have the potential to be cost-effective and prevent more sequelae due to chlamydia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huppert JS, Hesse E, Gaydos CA. What’s the point? How point-of-care sexually transmitted infection tests can impact infected patients. Point Care. 2010;9:36–46. doi: 10.1097/POC.0b013e3181d2d8cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh YH, Hogan MT, Barnes M, et al. Perception of an ideal point-of-care test for sexually transmitted infections – a qualitative study of eight focus group discussions. PLoS One. 2010;5:e14144. doi: 10.1371/journal.pone.0014144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Hsieh YH, Gaydos CA, Hogan MT, et al. What qualities are most important to making a point of care test desirable for clinicians and others offering sexually transmitted infection testing? PLoS One. 2011;6:e19263. doi: 10.1371/journal.pone.0019263. Information about the essential qualities that clinicians desire for new POC tests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeling RW, Holmes KK, Mabey D, et al. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect. 2006;82:v1–6. doi: 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaydos CA. Chlamydia trachomatis. In: Marlene G, Rebecca T, Katherine R, editors. Women and Health. Academic Press; Elsevier; NY, USA: 2013. [Google Scholar]
- 25.Bandea CI, Koumans EH, Sawyer MK, et al. Evaluation of the rapid BioStar optical immunoassay foe detection of Chlamydia trachomatis in adolescent women. J Clin Microbiol. 2009;47:215–16. doi: 10.1128/JCM.01338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pate MS, Dixon PB, Hardy K, et al. Evaluation of the BioStar chlamydia OIA assay with specimens from women attending a sexually transmitted disease clinic. J Clin Microbiol. 1998;36:2183–6. doi: 10.1128/jcm.36.8.2183-2186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Y-P, Peeling RW, Chen X-S, et al. Clinic-based evaluation of Clearview chamydia MF for detection of Chlamydia trachomatis in vaginal and cervical specimens from women at risk in China. Sex Transm Infect. 2006;82(Suppl):v33–7. doi: 10.1136/sti.2006.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratton NJ, Hirsch L, Harris F, et al. Evaluation of the rapid Clearview Chlamydia test for direct detection of chlamydiae from cervical specimens. J Clin Microbiol. 1991;29:1551–3. doi: 10.1128/jcm.29.7.1551-1553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skulnick M, Small GW, Simor AE, et al. Comparison of the Clearview Chlamydia test, chlamydiazyme, and cell culture for detection of Chlamydia trachomatis in women with a low prevalence of infection. J Clin Microbiol. 1991;29:2086–8. doi: 10.1128/jcm.29.9.2086-2088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steingrimsson O, Pawlak C, Van Der Pol B, et al. Multicenter comparative evaluation of two rapid immunoassay methods for the detection of Chlamydia trachomatis antigen in endocervical specimens. Clin Microbiol Infect. 1997;3:663–7. doi: 10.1111/j.1469-0691.1997.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 31.Rani R, Corbitt G, Killough R, Curless E. Is there any role for rapid tests for Chlamydia trachomatis? Int J STD AIDS. 2002;13:22–4. doi: 10.1258/0956462021924569. [DOI] [PubMed] [Google Scholar]
- 32.Mahilum-Tapay L, Laitila V, Wawrzyiak JJ, et al. New point of care chlamydia rapid test – bridging the gap between diagnosis and treatment: performance evaluation study. BMJ. 2007;335:1190–4. doi: 10.1136/bmj.39402.463854.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer L, Wendel JG. Rapid diagnostic methods in sexually transmitted infections. Infect Dis Clin North Am. 2008;22:601–17. doi: 10.1016/j.idc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 34.van der Helm JJ, Sabajo LOA, Gruneberg AW, et al. Point-of-care tests for detection of urogenital chlamydia in women shows low sensitivity. A performance evaluation study in two clinics in Suriname. PLoS One. 2012;7:e32122. doi: 10.1371/journal.pone.0032122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Hislop J, Quayyum Z, Fleet G, et al. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men. Health Technol Assess. 2010;14:1–125. doi: 10.3310/hta14290. Very detailed analysis of the effectiveness of POC tests showing that the one POC reviewed did not show cost–effectiveness. [DOI] [PubMed] [Google Scholar]
- 36.Jain A, Ison CA. Chlamydia point-of-care testing: where are we now? Sex Transmit Infect. 2013;89:88–9. doi: 10.1136/sextrans-2012-050834. [DOI] [PubMed] [Google Scholar]
- 37•.Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the cepheid CT/NG X-pert rapid PCR test for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51:1666–72. doi: 10.1128/JCM.03461-12. Newly US FDA-cleared important real-time PCR test that is ‘near patient’ and can be potentially used to treat patients before they leave the clinic or emergnecy department of doctors’ office. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabrizi SN, Unemo M, Golparian D, et al. Analytical evaluation of the GenXpert CT/NG, the first genetic point-of-care assay for simultaneous detection of Neisseria gonorrhoeae and Chlamydia trachomatis. J Clin Microbiol. 2013;51:1945–7. doi: 10.1128/JCM.00806-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui BB, Wilson DP, Ward JS, et al. The potential impact of new generation molecular point-of-care tests on gonorrhoea and chlamydia in a setting of high endemic prevalence. Sex Health. 2013;10:348–56. doi: 10.1071/SH13026. [DOI] [PubMed] [Google Scholar]
- 40•.Pearce DM, Shenton DP, Holden J, Gaydos CA. Evaluation of a novel electrochemical detection method for Chlamydia trachomatis: application for point-of-care diagnostics. IEEE Trans BioMed Eng. 2011;58:755–8. doi: 10.1109/TBME.2010.2095851. Promising new POC test for chlamydia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pribik R, Dragan AI, Zhang Y, et al. Metal-enhanced fluorescence (MEF): physical characterization of silver-island films and exploring sample geometrics. Chem Phys Lett. 2009;478:70–4. doi: 10.1016/j.cplett.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang Y, Agreda P, Kelly S, et al. Development of a microwave-accelerated metal -enhanced fluorescence 40 second, 100 cfu/mL point of care assay for the detection of Chlamydia trachomatis. IEEE Trans BioMed Eng. 2011;58:781–4. doi: 10.1109/TBME.2010.2066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Melendez JH, Huppert JS, Jett-Goheen M, et al. Blind evaluation of the microwave-accelerated metal-enhanced fluorescence ultrarapid and sensitive Chlamydia trachomatis test by use of clinical samples. J Clin Microbiol. 2013;51:2913–20. doi: 10.1128/JCM.00980-13. Promising new POC test for chlamydia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonoccocal infection. N Eng J Med. 2012;366:485–7. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein E, Kirkcaldy RD, Reshef D, et al. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg Infect Dis. 2012;18:1290–7. doi: 10.3201/eid1808.111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin I, Sawatzky P, Allan V, et al. Emergence and characterization of Neisseria gonorrhoeae isolates with decreases susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010. Sex Transmit Dis. 2012;39:316–23. doi: 10.1097/OLQ.0b013e3182401b69. [DOI] [PubMed] [Google Scholar]
- 47.Sadiq ST, Dave J, Butcher PD. Point-of-care antibiotic susceptibility testing for gonorrhea: improving therapeutic options and sparing the use of cephalosporins. Sex Transmit Infect. 2010;86:445–6. doi: 10.1136/sti.2010.044230. [DOI] [PubMed] [Google Scholar]
- 48•.Smith LAW, Hillman R, Ward J, et al. Point-of-care tests for the diagnosis of Neisseria gonorrhoeae infection: a systematic review of operational and performance characteristics. Sex Transm Infect. 2012;89:320–6. doi: 10.1136/sextrans-2012-050656. Review demonstrating the need for better POC tests for gonorrhea are required. [DOI] [PubMed] [Google Scholar]
- 49.Alary M, Gbenafa-Agossa C, Aina G, et al. Evaluation of a rapid point-of-care test for the detection of gonococcal infection among female sex workers in Benin. Sex Transm Infect. 2006;82(Suppl 5):v29–32. doi: 10.1136/sti.2006.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benzaken AS, Galban EG, Antunes W, et al. Diagnosis of gonococcal infection in high risk women using a rapid test. Sex Transm Infect. 2006;82(Suppl 5):v26–8. doi: 10.1136/sti.2006.022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szuki K, Matsumoto T, Murakami H, et al. Evaluation of a rapid antigen detection test for Neisseria gonorrhoeae in urine sediment for diagnosis of gonococcal urethritis in males. J Infect Chemother. 2004;10:208–11. doi: 10.1007/s10156-004-0322-6. [DOI] [PubMed] [Google Scholar]
- 52.Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MR, Watts DH, editors. Sexually transmitted disease. McGraw Hill; NY, USA: 2008. [Google Scholar]
- 53.Stamm LV, Mudrak B. Old foes, new challenges: syphilis, cholera and TB. Future Microbiol. 2013;8:177–9. doi: 10.2217/fmb.12.148. [DOI] [PubMed] [Google Scholar]
- 54.Castro A, Jost H, Cox D, et al. A comparison of the analytical level of agreement of nine treponemal assays for syphilis and possible implications for screening algorithms. BMJ Open. 2013;3:e003347. doi: 10.1136/bmjopen-2013-003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binnicker MJ. Which algorithm should be used to screen for Syphilis? Curr Opin Infect Dis. 2012;25:79–85. doi: 10.1097/QCO.0b013e32834e9a3c. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal tests for initial screening: four laboratories. New York City, 2005–2006. MMWR Morb Mortal Wkly Rep. 2008;57:872–5. [PubMed] [Google Scholar]
- 57•.Centers for Disease Control and Prevention. Discordant results from reverse sequence Syphilis screening: five laboratories, United States 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:133–7. Important recommendations for using reverse syphilis algorithm. [PubMed] [Google Scholar]
- 58.Syphilis health check. Available from: www.diagnosticsdirect2u.com/images/PDF/Syphilis-Product-Sheet-May-2013.pdf.
- 59•.Tucker JD, Bu J, Brown LB, et al. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect Dis. 2010;10:381–6. doi: 10.1016/S1473-3099(10)70092-X. Excellent review. [DOI] [PubMed] [Google Scholar]
- 60.Mabey D, Peeling RW, Ballard R, et al. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect. 2006;82(Suppl V):v13–16. doi: 10.1136/sti.2006.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smit PW, Mabey D, Changalucha J, et al. The trade-off between accuracy and accessibility of Syphilis screening assays. PLoS One. 2013;8:e75327. doi: 10.1371/journal.pone.0075327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang LG, Tucker JD, Liu FY, et al. Syphilis screening among 27, 150 pregnant women in South Chinese rural areas using point-of-care tests. PLoS One. 2013;8:e72149. doi: 10.1371/journal.pone.0072149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia PJ, Caramo CP, Chiappe M, et al. Rapid Syphilis tests as catalysts for health systems strengthening: a case study from Peru. PLoS One. 2013;9:e66905. doi: 10.1371/journal.pone.0066905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections –2008 Trichomonas. WHO; Geneva, Switzerland: 2012. [Google Scholar]
- 65.Van Der Pol B, Williams JA, Orr DP, et al. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis. 2005;192:2039–44. doi: 10.1086/498217. [DOI] [PubMed] [Google Scholar]
- 66.Weinstock H, Berman S, Cates W. Sexually transmitted disease among American youth: incidence and prevalence estimates. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 67.Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transmit Dis. 1997;24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 68•.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Review. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. Important review of trichomoniasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Inf Dis. 2007;45:1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 70.Miller WC, Swygard H, Hobbs MM, et al. The prevalence of trichomoniasis in young adults in the United States. Sex Tansmit Dis. 2005;32:593–8. doi: 10.1097/01.olq.0000179874.76360.ad. [DOI] [PubMed] [Google Scholar]
- 71.Allsworth JE, Ratner JA, Peipert JF. Trichomonas and other sexually transmitted infections: results from the 2001–2004. National Health and Nutrition Examination Surveys. Sex Tansmit Dis. 2009;36:738–44. doi: 10.1097/OLQ.0b013e3181b38a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transmit. 2013;89:426–33. doi: 10.1136/sextrans-2012-051005. Important review demonstrating the association of trichomonas with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African Women. Sex Transmit Dis. 2012;39:638–42. doi: 10.1097/OLQ.0b013e31825725ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinlivan EB, Patel SN, Grodensky CA, et al. Modeling the impact of Trichomonas vaginalis infection on HIV transmission in HIV-infected individuals in medical care. Sex Transm Dis. 2012;39:671–7. doi: 10.1097/OLQ.0b013e3182593839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaydos CA, Barnes MR, Quinn N, Jett-Gohneen M. Trichomonas vaginalis infection in men who submit self-collected penile swabs after internet recruitment. Sex Transm Infect. 2013;89:505–8. doi: 10.1136/sextrans-2012-050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reagen MM, Xu H, Shih SL, et al. A randomized trial of home versus clinic-based sexually transmitted disease screening among men. Sex Transm Dis. 2012;39:842–7. doi: 10.1097/OLQ.0b013e3182649165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect. 2013;89:434–8. doi: 10.1136/sextrans-2013-051057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huppert JS, Batteiger BE, Braslins P, et al. Use of an immunochromatographic assay for rapid detection of Trichomonas vaginalis in vaginal specimens. J Clin Microbiol. 2005;43:684–7. doi: 10.1128/JCM.43.2.684-687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huppert JS, Mortensen JE, Reed JL, et al. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin Infect Dis. 2007;45:194–8. doi: 10.1086/518851. [DOI] [PubMed] [Google Scholar]
- 80.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol. 2009;200:188.e1–188.e7. doi: 10.1016/j.ajog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Pattullo L, Griffeth S, Ding L, et al. Stepwise diagnosis of Trichomonas vaginalis infection in adolescent women. J Clin Microbiol. 2009;47:59–63. doi: 10.1128/JCM.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madhivanan P, Li T, Trammell S, et al. Performance of the OSOM. Trichomonas rapid test for the diagnosis of Trichomonas vaginalis infection among women in Mysore, India. Sex Health. 2013;10:320–4. doi: 10.1071/SH13015. [DOI] [PubMed] [Google Scholar]
- 83•.Cartwright CP, Lembke BD, Ramachandran K, et al. Comparison of nucleic acid amplification assays with BD Affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol. 2013;51:3694–9. doi: 10.1128/JCM.01537-13. Manuscript demonstrating the lack of sensitivity of the Affirm assay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Pearce DM, Styles DN, Hardick J, Gaydos CA. A new rapid molecular point-of-care assay for Trichomonas vaginalis: preliminary performance data. Sex Transm Infect. 2013;89:495–7. doi: 10.1136/sextrans-2012-051000. Report of a promising new trichomonas POC test under development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MR, Watts DH, editors. Sexually transmitted diseases. McGraw Hill Medical; NY, USA: 2008. [Google Scholar]
- 86.Markle W, Conti T, Kad M. Sexually transmitted diseases. Prim Care Clin Office Pract. 2013;40:557–87. doi: 10.1016/j.pop.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines 2010. CDC. 2010;59:1–110. Available from: www.cdc.gov/std/treatment/2010 • Traditional treatment guidelines and recommendations for sexually transmitted infections. [Google Scholar]
- 88.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 89.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2013;121:4600–9. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rompalo A. Preventing sexually transmitted infections: back to basics. J Clin Invest. 2012;12:4580–3. doi: 10.1172/JCI61592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fagundes LJ, Vieira EE, Jr, Moyses ACMC, et al. Sexually transmitted diseases in a specialized STD healthcare center: epidemiology and demographic profile from January 1999 to December 2009. An Bras Dermatol. 2013;88:523–9. doi: 10.1590/abd1806-4841.20132149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kenyon C, Colebunders R, Buve A, Hens N. Partner-concurrency associated with herpes simplex virus 2 infection in young South Africans. Inter J STD AIDS. 2013;24:804–12. doi: 10.1177/0956462413482810. [DOI] [PubMed] [Google Scholar]
- 93.Kenyon C, Colebunders R, Hens N. Determinants of generalized herpes simplex virus-2 epidemics: the role of sexual partner concurrency. Inter J STD AIDS. 2013;24:375–82. doi: 10.1177/0956462412472816. [DOI] [PubMed] [Google Scholar]
- 94.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 95.Zahariadis G, Severini A. Evaluation of a novel serology algorithm to detect herpes simplex virus 1 or 2 antibodies. Sex Transmit Dis. 2010;37:696–9. doi: 10.1097/OLQ.0b013e3181e2cdab. [DOI] [PubMed] [Google Scholar]
- 96.Roth AM, Van Der Pol B, Fortenberry JD, et al. Herpes simplex virus type 2 serological testing at a community court: predictors of test acceptance and seropositivity among female defendants. Inter J STD AIDS. 2013;24:169–74. doi: 10.1177/0956462412472442. [DOI] [PubMed] [Google Scholar]
- 97.Roth AM, Dodge BM, Van Der Pol B, Reece M, Zimet GD. Low acceptance of HSV-2 testing among high-risk women. Inter J STD AIDS. 2013;22:329–31. doi: 10.1258/ijsa.2010.010399. [DOI] [PubMed] [Google Scholar]
- 98•.Biraro S, Mayaud P, Morrow A, et al. Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from sub-Saharan Africa: a systematic review and meta-analysis. Sex Transmit Dis. 2011;38:140–7. doi: 10.1097/OLQ.0b013e3181f0bafb. Well-written comparison of HSV-2 antibody assays. [DOI] [PubMed] [Google Scholar]
- 99.Gardella C, Huang ML, Wald A, et al. Rapid polymerase chain reaction assay to detect herpes simplex virus in the genital tract of women in labor. Obstet Gynecol. 2010;115:1209–16. doi: 10.1097/AOG.0b013e3181e01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chin CD, Laksanasopin T, Cheung YK, et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17:1015–19. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 101.Zubair A, Burbelo PD, Vincent LG, et al. Microfluidic LIPS for serum antibody detection: demonstration of a rapid test for HSV-2 infection. Biomed Microdevices. 2011;13:1053–62. doi: 10.1007/s10544-011-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lemieux B, Kong H, Tang YW. Near instrument-free, simple molecular device for rapid detection of herpes simplex viruses. Expert Rev Mol Diagn. 2012;12:437–43. doi: 10.1586/erm.12.34. [DOI] [PubMed] [Google Scholar]
- 103.Kim HJ, Tong Y, Tang W, et al. A rapid and simple isothermal nucleic acid amplification test for detection of herpes simplex virus types 1 and 2. J Clin Virol. 2011;50:26–30. doi: 10.1016/j.jcv.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104•.Miller NS, Yen-Lieberman B, Poulter MD, et al. Comparative clinical evaluation of the IsoAMP® HSV Assay with ELVIS® HSV culture/ID/typing test system for the detection of herpes simplex virus in genital and oral lesions. J Clin Virol. 2012;54:355–8. doi: 10.1016/j.jcv.2012.04.004. New POC test for HSV-2 antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall H, Song R, Hodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Centers for Disease Control and Prevention. HIV Surveillance report: estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17:1–26. [Google Scholar]
- 107.Gaydos CA, Hsieh YH, Harvey L, et al. Will Patients “Opt-In” to perform their own rapid HIV test in the emergency department? Ann Emerg Med. 2011;(Suppl 58):S74–8. doi: 10.1016/j.annemergmed.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nour S, Hsieh Y-H, Jett-Goheen M, et al. Patients can accurately perform their own rapid HIV point-of-care test in the emergency department. Point Care J. 2012;11:176–9. doi: 10.1097/POC.0b013e3182666eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mavedzenge SN, Baggaley R, Corbett E. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126–38. doi: 10.1093/cid/cit156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Gaydos CA, Solis M, Hsieh YH, et al. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Inter J STD AIDS. 2013;24:714–21. doi: 10.1177/0956462413487321. Demonstration that patients are willing to perform their own HIV POC test in an emergency department setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walensky RP, Paltiel AD. Rapid HIV Testing at home: does it solve a problem or create one? Ann Intern Med. 2006;145:459–62. doi: 10.7326/0003-4819-145-6-200609190-00010. [DOI] [PubMed] [Google Scholar]
- 112.Arora DR, Maheshwari M, Arora B. Rapid point-of-care testing for detection of HIV and clinical monitoring. ISRN AIDS. 2013;2013:287269. doi: 10.1155/2013/287269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Branson BM. State of the art for diagnosis of HIV infection. Clin Infect Dis. 2007;2007(Suppl):S221–5. doi: 10.1086/522541. [DOI] [PubMed] [Google Scholar]
- 114.Gaydos CA. Rapid tests for sexually transmitted diseases. Current Infect Dis Reports. 2006;8:115–24. doi: 10.1007/s11908-006-0007-7. [DOI] [PubMed] [Google Scholar]
- 115.Pandori MW, Branson BM. 2010 HIV Diagnostics conference. Expert Rev Anti Infect Ther. 2010;8:631–3. doi: 10.1586/eri.10.48. [DOI] [PubMed] [Google Scholar]
- 116•.Centers for Disease Control and Prevention. Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform CDC. 2013 10-25-0013 Excellent comparison of advantages and disadvantages of HIV immunoassays. [Google Scholar]
- 117.Hanafiah KM, Garcia M, Anderson D. Point-of-care testing and the control of infectious diseases. Biomark Med. 2013;7:333–47. doi: 10.2217/bmm.13.57. [DOI] [PubMed] [Google Scholar]
- 118.Branson BM, Stekler JD. Detection of acute HIV infection: we can’t close the window. J Infect Dis. 2012;205:521–4. doi: 10.1093/infdis/jir793. [DOI] [PubMed] [Google Scholar]
- 119.Rosenberg NE, Kamanga G, Phiri S, et al. Detection of acute HIV infection: a field evaluation of the Determine HIV-1/2 Ag/Ab Combo test. Detection of acute HIV infection: a field evaluation of the Determine HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012;205:528–34. doi: 10.1093/infdis/jir789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. [[Last accessed 25 October 2013]];OraQuick Advance Rapid HIV-1/2 Antibody Test [package insert] 2004 Available from: www.oraquick.com/
- 121.Stekler JD, Swenson PD, Coombs RW, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. 2009;49:444–53. doi: 10.1086/600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reveall G3. [Last accessed 25 October 2013];Rapid HIV-1 antibody test [package insert] 2006 Available from: www.medmira.com/images/uploads/RevealG3_NCCLS.pdf.
- 123. [Last accessed 25 October 2013];Multispot HIV-1/HIV-2 Rapid Test [package insert] 2004 Available from: www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm091384.pdf.
- 124.Centers for Disease Control and Prevention. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm-United States, 2011–2013. MMWR. 2013;62:489–94. [PMC free article] [PubMed] [Google Scholar]
- 125. [Last accessed 25 October 2013];Uni-Gold Recombigen HIV [package insert] 2004 Available from: www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm093428.pdf.
- 126. [[Last accessed 25 October 2013]];Clearview HIV 1/2 STAT-PAK [package insert] 2011 Available from: www.alere.com/us/en/product-details/clearview-hiv-1-2-STAT-PAK.html.
- 127. [[Last accessed 25 October 2013]];Summary of safety and effectiveness Chembio DPP HIV 1/2 assay [package insert] 2013 Available from: www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM333941.pdf.
- 128•. [[Last accessed 25 October 2013]];INSTI HIV-1 Antibody test kit [package insert] 2013 Available from: www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM235249.pdf. Important new CDC recommendations for a HIV diagnostic testing algorithm.
- 129.Swenson RR, Hadley WS, Houck CD, et al. Who accepts a rapid HIV antibody test? The role of race/ethnicity and HIV risk behavior among community adolescents. J Adol Health. 2011;48:527–9. doi: 10.1016/j.jadohealth.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 130.Haukoos JS, Hopkins E, Conroy AA, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–92. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 131.Kendrick SR, Kroc KA, Withum D, et al. Outcomes of offering rapid point-of-care HIV testing in a sexually transmitted disease clinic. J Acquir Immune Defic Syndr. 2005;38:142–6. doi: 10.1097/00126334-200502010-00004. [DOI] [PubMed] [Google Scholar]
- 132.Stack R, Mandalia S, McOwan A. Do HIV POCT testing algorithms help in clinical practice? Sex Transm Infect. 2013;89:99. doi: 10.1136/sextrans-2012-050796. [DOI] [PubMed] [Google Scholar]
- 133.Galiwango RM, Musoke R, Lubyayi L, et al. Evaluation of current rapid HIV test algorithms in Rakai. Uganda J Vir Methods. 2013;192:25–7. doi: 10.1016/j.jviromet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wright RJ, Stringer JSA. Rapid Testing Strategies for HIV-1 Serodiagnosis in High-prevalence African settings. Amer J Prev Med. 2004;27:42–8. doi: 10.1016/j.amepre.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 135.Advisory Committee Meeting GM3N2. Approaches to over-the-counter home-use HIV test kits. [[Last accessed 28 October 2013]];FDA Blood Products. 2005 Available from: http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4190B1_01_issue%20summary.htmon.
- 136.US FDA. [[Last accessed 28 October 2013]];Testing for HIV. 2011 Available from: http://wwwfdagov/BiologicsBloodVaccines/SafetyAvailability/HIVHomeTestKits/ucm126460htm.
- 137.Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: the impact of point-of-care tests on antiretroviral therapy. Clin Epidemiol. 2013;5:387–96. doi: 10.2147/CLEP.S37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Myer L, Daskilewicz K, McIntyre J, Bekker LG. Comparison of point-of-care versus laboratory-based CD4 cell enumeration in HIV-positive pregnant women. J Inter AIDS Soc. 2013 doi: 10.7448/IAS.16.1.18649. Available from: http://dx.doi.org/10.7448/IAS.16.1.18649. [DOI] [PMC free article] [PubMed]
- 139.Morawski BM, Meya DB, Boulware DR. Accuracy of Pima point-of-care CD4 analyzer in routine use in public health clinics in Uganda. J Acquir Immune Defic Syndr. 2013;63:e113. doi: 10.1097/QAI.0b013e3182928f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thaker M, Mahajan B, Shaikh N, et al. Utility of the point of care CD4 analyzer, PIMA, to enumerate CD4 counts in the field settings in India. AIDS Research Therapy. 2012;9 doi: 10.1186/1742-6405-9-26. Available from: http://www.aidsrestherapy.com/content/9-1/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rohram BA, Leautaud V, Molyneux E, Richards-Kortum RR. A lateral flow assay for quantitative detection of amplified HIV-1 RNA. PLoS One. 2012;7:e45611. doi: 10.1371/journal.pone.0045611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang SQ, Xu F, Demirci U. Advances in developing HIV-1 viral load assays for resource-limited settings. Biotechnol Adv. 2010;28:770–81. doi: 10.1016/j.biotechadv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martinez AW, Phillips ST, Whitesides GM. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010;83:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 144.Aledort JE, Ronald A, Rafael ME, et al. Reducing the burden of sexually transmitted infections in resource-limited settings: the role of improved diagnostics. Nature Pub Group; 2006. pp. 59–72. Available from: www.nature.com/diagnostics. [DOI] [PubMed] [Google Scholar]
- 145.Schneider BH, Dickinson EL, Vach MD, et al. Optical chip immunoassay for hCG in human whole blood. Biosens Bioelectron. 2000;15:597–604. doi: 10.1016/s0956-5663(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 146.Schneider BH, Dickinson EL, Vach MD, et al. Highly sensitive optical chip immunoassays in humans serum. Biosens Bioelectron. 2000;15:13–22. doi: 10.1016/s0956-5663(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 147.Bisoffi M, Severns V, Larson R. CTSA-enhanced innovative device development. Clin Transl Sci. 2012;5:311–13. doi: 10.1111/j.1752-8062.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bisoffi M, Hjelle B, Brown DC, et al. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens Bioelectron. 2008;23:1397–403. doi: 10.1016/j.bios.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 149.Bisoffi M, Severns V, Branch DW, et al. Rapid detection of human immunodeficiency virus type 1 and 2 using an improved piezoelectric biosensor. J Clin Microbiol. 2013;51:1685–91. doi: 10.1128/JCM.03041-12. [DOI] [PMC free article] [PubMed] [Google Scholar]