Abstract
Purpose
Ad5.SSTR/TK.RGD is an infectivity-enhanced adenovirus expressing a therapeutic thymidine kinase suicide gene and a somatostatin receptor that allows for noninvasive gene transfer imaging. The purpose of this study was to identify the MTD, toxicities, clinical efficacy and biologic effects of Ad5.SSTR/TK.RGD in patients with recurrent gynecologic cancer.
Experimental Design
Eligible patients were treated intraperitoneally (IP) for 3 days with 1×109 to 1×1012 vp/dose of Ad5.SSTR/TK.RGD followed by intravenous ganciclovir for 14 days. Toxicity and clinical efficacy were assessed utilizing CTC Adverse Events grading and RECIST criteria. Imaging utilizing In-111 pentetreotide was obtained before and after treatment. Tissue samples were obtained to evaluate for gene transfer, generation of wild-type virus, viral shedding and antibody response.
Results
Twelve patients were treated in three cohorts. The most common vector-related clinical toxicities were grade 1–2 constitutional or pain symptoms, experienced most often in patients treated at the highest dose. MTD was not identified. Five patients demonstrated stable disease; all others experienced progressive disease. One patient with stable disease experienced complete resolution of disease and normalization of CA125 on further follow-up. Imaging detected increased In-111 pentetreotide retention in patients treated at the highest dose. Ancillary studies demonstrated presence of Ad5.SSTR/TK.RGD virus and HSV1-tk expression in ascites samples collected at various time points in most patients treated within the higher dose cohorts.
Conclusions
This study demonstrates the safety, potential efficacy, and possible gene transfer imaging capacity of Ad5.SSTR/TK.RGD in patients with recurrent gynecologic cancer. Further development of this novel gene therapeutic appears to be warranted.
Keywords: Suicide gene therapy, gene transfer imaging, infectivity enhanced adenoviral vectors, ovarian cancer, endometrial cancer
Introduction
Nearly 22,000 women are diagnosed annually with ovarian cancer (1). Due to a lack of effective screening strategies and the nonspecific nature of presenting signs and symptoms, most patients with epithelial ovarian cancer will be diagnosed with advanced stage disease. Advances in surgical debulking and chemotherapy have clearly led to improvements in median survival for these patients (2–5). However, most patients diagnosed with ovarian cancer will ultimately develop recurrent disease, become resistant to further therapy, and eventually succumb to their disease. Thus, there has been a clear need for the development of new therapeutic approaches for those patients affected by ovarian cancer as well as other selected advance stage or recurrent gynecologic cancers such as primary peritoneal cancer, fallopian tube cancer, and endometrial cancer.
Over the past two decades, various gene therapeutic approaches for ovarian cancer have been investigated (6, 7). One such approach is suicide gene therapy, alternatively known as molecular chemotherapy. This strategy is based upon delivery of gene that will express an enzyme capable of converting a prodrug into an active toxic metabolite. The most commonly investigated suicide gene therapy based gene therapeutic has been the thymidine kinase (TK) gene from the herpes simplex virus (HSV) given in combination with the nucleoside analog ganciclovir (GCV). Expression of the HSV-TK enzyme allows for tumor cells to ultimately convert GCV into a triphosphate configuration, which will inhibit DNA synthesis and mediate tumor cell apoptosis. The therapeutic effect of this approach is augmented by a “bystander effect,” whereby triphosphorylated GCV is transferred to other nontransduced cells via gap junctions that, in turn, mediate additional tumor cell cytotoxicity (8).
Prior studies of ours using an adenoviral vector mediated HSV-TK suicide gene therapy approach confirmed the utility of this approach in preclinical models of ovarian cancer (9, 10). A subsequent phase I trial of ours demonstrated the safety of intraperitoneal administration of an HSV-TK expressing adenovirus in combination with intravenous GCV in a cohort of 14 patients with persistent/recurrent ovarian cancer (11). Others had demonstrated the feasibility of utilizing a HSV-TK mediated suicide gene approach using retroviral based vectors (12, 13). While toxicity with this approach was limited, no significant clinical responses were noted in our initial clinical trial or in those conducted by others. In addition, while gene transfer was noted in ascites samples obtained from patients in these trials, the copy number of HSV-TK genes may have been too low to produce a meaningful clinical effect. Moreover, the manner in which tissue is retrieved for gene transfer evaluation was invasive, cumbersome and associated with a high degree of sampling variability.
To address the limitations of adenoviral mediated cancer cell transfection and clinical activity noted in our previous trial, we have investigated various strategies to enhance adenoviral infectivity. For example, we have modified the adenoviral fiber knob of the type 5 adenovirus to incorporate an arginine-glycine-asparate (RGD-4C) motif in the HI loop of the knob (14, 15). This modification directs adenoviral transfection via cell surface integrins rather than its normal receptor, the Coxsackie Adenoviral Receptor (CAR). Proof of principle studies have demonstrated that RGD-modified adenoviruses mediate enhanced gene transfer to established and primary ovarian cancer cells (16). In addition, we have also developed various novel non-invasive strategies to assess gene transfer in vivo (17, 18). One such method relies upon incorporation of the SSTR-expressing construct, which allows for non-invasive gene transfer imaging utilizing conventional nuclear medicine imaging. The utility of this approach in preclinical models has also been previously validated (19–21). Advantages of SSTR include the availability of a human-approved imaging agent (In-111 pentetreotide) for gamma camera and SPECT imaging, as well as excellent PET tracers targeting SSTR for imaging and therapy. Imaging SSTR was more sensitive than imaging TK for detection of low doses of adenoviral-mediated gene transfer, and SSTR showed a linear relationship between adenoviral dose and retention of the imaging agent targeting the SSTR, while a linear relationship was not found between the adenoviral dose and trapping of iodinated FIAU that was specific for TK (21). Detailed comparisons between SSTR and TK were published previously (21).
We have incorporated these infectivity and non-invasive gene transfer assessment enhancements into our adenoviral mediated suicide gene therapy approach. Specifically, Ad5.SSTR/TK.RGD, a bicistronic RGD modified adenovirus encoding both a HSV-TK and a somatostatin receptor expressing construct, has been developed (22). Preclinical studies have confirmed the anti-tumor activity and gene transfer imaging capacity of this reagent in preclinical modes of ovarian cancer (23, 24). Safety studies have also identified toxicity and biodistribution of Ad5.SSTR/TK.RGD in Syrian hamsters (25). The primary purpose of this study was to determine the maximum tolerated dose and spectrum of toxicities associated with intraperitoneal administration of Ad5.SSTR/TK.RGD given in combination with intravenous GCV to patients with recurrent ovarian and other selected gynecologic cancers. The ability to image gene transfer, the potential clinical efficacy and the biologic effects of this novel therapeutic strategy were also assessed.
Materials and Methods
General Study Design
This study was a phase I dose-escalating trial evaluating intraperitoneal administration of Ad5.SSTR/TK.RGD in combination with intravenous GCV in cohorts of eligible patients. With the standard 3 + 3 design, this study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board (IRB) and Institutional Biosafety Committee. Approvals were also obtained from the NIH Recombinant DNA Advisory Committee and the US Food and Drug Administration (FDA).
Patient Eligibility
Eligible patients included women aged 19 years or older with histologically-proven recurrent epithelial ovarian, primary peritoneal, fallopian tube, or endometrial cancer, who had been previously treated with conventional surgical procedures and standard adjuvant therapies. Patients were required to have adequate organ laboratory function as defined by a white blood cell count (WBC) > 3000 μL, granulocyte count > 1,500 μL, platelets > 100,000, creatinine clearance > 80 mg/dL, creatinine < 2.0, aspartate aminotransferase or alanine aminotransferase < 2.5x the upper limit of the normal reference range, bilirubin < 2.0, and a prothrombin time/international normalized ratio or partial thromboplastin time (PT/INR/PTT) < 1.5 x the upper limit of normal reference range. Patients were also required to have an ejection fraction of >55% on echocardiogram, oxygen saturation >92% on room air, a Gynecologic Oncology Group (GOG) performance status 0–2 and a life expectancy > 3 months. Those patients found to have tumors of low malignant potential, germ cell or sex cord/stromal tumors, active cardiac or pulmonary disease, or coagulation disorders were excluded. All patients were required to sign the provided informed consent.
Ad5.SSTR/TK.RGD
Ad5.SSTR/TK.RGD is a tropism modified recombinant adenoviral vector developed in the Gene Therapy Center at UAB (22). The virus is a replication-defective Ad5 derivative carrying deletions in the E1 and E3 genomic regions with a modified fiber gene. A dual-expression transgene cassette has been inserted into the E1 region that results in expression of SSTR under the control of the immediate-early CMV promoter and TK under the control of the SV40 promoter. The fiber gene carries an insertion of an RGD motif.
Ad5.SSTR/TK.RGD was formulated in 20mM Tris, 25mM NaCl, 2.5% (w/v) Glycerol, pH 8.0 (GST) and was provided in sterile, single use containers that were stored at −80 degrees and kept refrigerated until administration. The daily dose of Ad5.SSTR/TK.RGD was diluted in 250 mL of 0.9% Sodium Chloride, USP bag. Ad5.SSTR/TK.RGD was manufactured with support from the NCI RAID Program at the Cell and Gene Therapy Center at Baylor College of Medicine and at the Biopharmaceutical Development Program/SAIC-Frederick, Inc. at NCI-Frederick. Stability testing of the reagent continued throughout the duration of the clinical study.
Ganciclovir (GCV)
GCV (Cytovene) was obtained in vials of sterile powder from Syntex Laboratories, Inc. and reconstituted by adding 10 mL of sterile water for injection to provide a final solution at 50mg/mL. The appropriate dose was then diluted in 100 mL of normal saline.
Treatment Plan, Ad5.SSTR/TK.RGD Dose Cohorts, and GCV Dosing
Prior to treatment, patients underwent evaluation which included: history and physical examination, toxicity grading, performance status assignment, complete blood count (CBC), chemistry panel, hepatic function panel, coagulation studies, CA-125, echocardiogram, oxygen saturation, and computed tomography (CT) of the abdomen and pelvis. Those patients who completed pretreatment evaluation and met eligibility criteria were enrolled onto study and subsequently had an intraperitoneal Quinton Curl, 22.4-inch, double-cuffed, Tenckhoff catheter (Tyco Healthcare) placed by an interventional radiologist at least one week prior to use.
Patients were then assigned into one of three Ad5.SSTR/TK.RGD dose cohorts ranging from 1 × 109 vp/d to 1 × 1012 vp/d (6 × 107 pfu/d to 6 × 1010 pfu/d). (Supplemental Table 1) Similar to the strategy validated in our preclinical studies, assigned Ad5.SSTR/TK.RGD doses were administrated via the intraperitoneal catheter daily for three consecutive days. GCV was then administered intravenously from days 5–18 at a dose of 5 mg/kg at a constant rate over 1 hour, twice daily. On days 1–4, 11, 18, and 29, a history and physical examination, performance status, toxicity grading, CBC, and chemistry panel was obtained on all study patients. CA-125 and CT scan of the abdomen and pelvis were repeated on day 29.
Peritoneal aspirates, as well as urine, saliva, and serum specimens were obtained immediately preceding Ad5.SSTR/TK.RGD dose administration on days 4, 11, 18, and 29 for planned ancillary biologic studies. All samples were processed and de-identified prior to performing ancillary biological studies.
Evaluation of Clinical Toxicity
Grading of toxicity was performed on the days previously specified using the NCI Common Toxicity Criteria (CTC), version 3.0. Dose limiting toxicity was defined as any vector-related, grade 3, non-hematologic toxicity or a hematologic toxicity as defined by any admission for neutropenic fever, absolute neutrophil count < 500 for > 5 days, or platelet count < 20,000. The maximum tolerated dose was defined as the dose exceeded by the dose at which at least two patients experienced dose-limiting toxicity.
Evaluation of Clinical Efficacy
Clinical efficacy was determined by comparing pretreatment CT findings with those noted on CT on day 29 utilizing RECIST criteria, version 1.1 (26). Measurable disease was defined as at least one lesion > 1 cm that could be accurately measured in one dimension. In each patient, up to five lesions per organ or ten lesions total were identified as target lesions. Complete response required disappearance of all target lesions and normalization of CA-125. Partial response was defined as > 30% decrease in the sum total recorded dimensions of a patient’s target lesions. Progressive disease was deemed as > 20% increase in the sum total recorded dimensions of a patient’s target lesions. Any condition that did not qualify for partial response or progressive disease was deemed stable disease.
Noninvasive Imaging of Ad5.SSTR/TK.RGD Gene Transfer
Ad5.SSTR/TK.RGD gene transfer was assessed noninvasively in 9 patients (3 patients per each dose cohort). Specifically, patients were imaged by a whole-body planar technique on a Philips Forte dual-headed gamma camera, at 4 hours, after intravenous dosing with In-111 pentetreotide (mean=6.1 mCi, 222 MBq; range 204– 238.7 MBq). There were two separates doses and imaging sessions for each patient, separated in time by at least 1 week. The first session was obtained the week before Ad5.SSTR/TK.RGD therapy and the second imaging session was performed 1 day after the final dose of therapy.
Radionuclide images were graded qualitatively (and blinded to Ad5.SSTR/TK.RGD dose group) as showing no uptake beyond expected biodistribution (0), mild uptake (less than expected bowel uptake) (1+), or uptake equal to or greater than bowel (2+). For each imaging session a ratio of activity in the abdominal cavity to background (mediastinum) was calculated in two ways. First, the average counts in three adjacent regions of interest (ROI) that included the entire abdomen and pelvis was determined and divided by counts in an identical sized ROI placed over the mediastinum. Second, an identical size ROI was drawn around the region in the abdomen of “apparent” increased uptake (unrelated to normal excretion) and divided by the same mediastinal ROI.
Evaluation of Ad5.SSTR/TK.RGD Gene Transfer and Wild Type Adenovirus Generation in Ascites Samples
Total DNA from cellular material obtained from ascites samples was isolated using a QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s protocol. Real-time quantitative PCR was employed to evaluate for Ad5.SSTR/TK.RGD gene transfer and for the generation of wild type (WT) virus using primers and probe specific for the RGD-4C peptide coding sequence and the early viral E1A gene, respectively. Both E1A and RGD primers were confirmed to only amplify the virus being tested. To adjust for the amount of DNA varying between samples, the primers and probe for the human β-actin housekeeping gene were used to determine the cellular DNA content present in each sample. Primers and probe sets, which were utilized in this study, as designed by the Primer Express 1.5 software and synthesized by Sigma-Aldrich, are detailed in Supplemental Table 2. FastStart TaqMan Probe Master mix (Roche Applied Science) was used to run duplexing PCR reactions in triplicate wells with each ascites sample along with the standard dilutions containing 108, 106, 104 and 102 copies/μL of Ad5.SSTR/TK.RGD or wild type Ad5 genome, and 50, 5.0, 0.5 or 0.05 ng/μL of human DNA on a LightCycler 480 (Roche Molecular Biochemicals, Indianapolis, Indiana). Thermal cycling conditions were set to 8 minutes, at 95°C, followed by 45 cycles of 10 seconds at 95°C and 40 seconds at 60°C. Data were analyzed with the LightCycler 480 1.5.0 SP1 software and the resultant numbers of RGD- or E1A-containing viral genomes were normalized to the amount of human DNA detected in the same sample to allow for comparison between patients and at different time points.
Assessment of Ad5.SSTR/TK.RGD-mediated Expression of hSSTR2 and HSV1-tk genes
Total RNA was isolated from ascites samples by use of the QIAamp RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer instructions and used as a template for quantitative reverse transcription PCR (RT-PCR) as follows. RT-PCR was carried out using the TaqMan one step PCR master mix supplemented with RT enzyme mix (Applied Biosystems) and both the hSSTR2 target and hGAPDH housekeeping gene-specific primers and probes (Supplemental Table 2) were added at a final concentration of 100 nM and delivered into the LightCycler 480System (Roche Molecular Biochemicals). Known amounts of Ad5.SSTR/TK.RGD genome (108, 106, 104 or 102 copies/μL) and human genomic DNA (50, 5.0, 0.5 or 0.05 ng/μL) were used to generate standard curves for quantification of cDNA copies synthesized on hSSTR2 or hGAPDH mRNA isolated from ascites samples, respectively. For this assay, each unknown and standard sample was subjected to duplexing RT-PCR reactions in triplicate wells under thermal cycling conditions set to 30 minutes at 48 °C, 10 minutes at 95 °C, 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. Data was analyzed with LightCycler 480 1.5.0 SP1 software and the resultant hSSTR2 copies were normalized to the amount of housekeeping gene (hGAPDH) detected in the same sample to allow for comparison between patients and at different time points.
To determine the expression levels of the HSV1-tk gene in ascites cells infected with Ad5.SSTR/TK.RGD vector both the HSV1-tk gene and hGAPDH housekeeping gene-specific primers and probes (Supplemental Table 2) were used to run duplexing RT-PCR with RNA samples as described above.
Assessment for Ad5.SSTR/TK.RGD Viral Shedding
Total DNA from the patients’ blood and saliva specimens was isolated using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer protocol. Urine specimens were initially concentrated with Millipore Amicon Ultra-4 Centrifugal Filter Units and then used to isolate viral DNA with the QIAampMinElute Virus Spin Kit (QIAGEN), according to the manufacturer’s instructions. The DNA samples isolated from blood, saliva, and urine were used to detect the Ad5.SSTR/TK.RGD genome with primers and probes (Supplemental Table 2) specific for the RGD-4C coding sequence as described above.
Evaluation of an Anti-Adenoviral Neutralizing Antibody Response
To evaluate for an anti-adenoviral neutralizing antibody response after treatment, a nonreplicative, luciferase-expressing virus, Ad5-RGD-Luc1, was neutralized by either serum or ascites before infection of SKOV3.ip1 cells, a cell line derived from the implantation of SKOV3 cells (American Type Culture Collection) in nude mice. After neutralization in respective samples, Ad5-RGD-Luc1 transduction efficacy was determined by a luciferase assay. Triplicates of SKOV3.ip1 cells were plated into 96-well plates (10,000 per well) and grown overnight prior to infection. A 1:2 dilution of serum or ascites of each time point specimen was prepared in Opti-MEM (Media Preparation Shared Facility, UAB) in a normalized volume. Nonreplicative Ad5-RGD-Luc1 at 100 plaque-forming unit per cell was mixed with each dilution for 30 minutes at room temperature before addition to appropriate wells. This infection was allowed to proceed for 48 hours. A luciferase assay was carried out using a luciferase assay system (Promega) on an Orion microplate luminometer (Berthold) reading Culturplate-96 wells (Research Parkway) according to the manufacturer’s protocols.
Statistical Analysis
A de-identified list of patients was provided with dose level of Ad5.SSTR/TK.RGD, age, race, cancer type, treatment date, and number of prior chemotherapy regimens. Clinical and laboratory adverse events were tabulated by category and grade. Formal statistical tests were limited to a 2-way ANOVA for non-invasive imaging and in regard to detecting an adenoviral neutralizing antibody response.
Results
Patient Demographics and Treatment
From August of 2009 to November of 2010, 12 patients were screened and consented to participate in this phase I trial. Table 1 summarizes study patient demographics. The median patient age was 61 (range 50–68 years). Ninety-two percent of patients were Caucasian, and 75% had recurrent ovarian cancer. The median number of prior chemotherapy regimens was 4 (range 1–11). All patients had successful placement of an intraperitoneal catheter. Patients were enrolled in escalating Ad5.SSTR/TK.RGD dose cohorts (1 × 109 vp/d to 1 × 1012 vp/d) and all completed treatment with Ad5.SSTR/TK.RGD according to protocol. In-111 pentetreotide was not commercially available during treatment of the first three patients in the highest dose cohort. After obtaining FDA and IRB approval, three additional patients were enrolled in the highest dose cohort to facilitate noninvasive assessment of gene transfer in such patients.
Table 1.
Selected patient demographics and treatment cohorts.
| Dose | Pt ID | Age | Race* | Cancer | No. of prior chemotherapy regimens |
|---|---|---|---|---|---|
| 1×109 vp/d | 101 | 66 | C | Ovarian | 8 |
| 102 | 64 | C | Endometrial | 2 | |
| 103 | 63 | C | Ovarian | 2 | |
| 5×1010 vp/d | 201 | 61 | C | Ovarian | 4 |
| 202 | 59 | C | Ovarian | 3 | |
| 203 | 68 | C | Ovarian | 6 | |
| 1×1012 vp/d | 301 | 66 | A | Endometrial | 2 |
| 302 | 61 | C | Endometrial | 1 | |
| 303 | 54 | C | Ovarian | 11 | |
| 304 | 51 | C | Ovarian | 4 | |
| 305 | 50 | C | Ovarian | 10 | |
| 306 | 61 | C | Ovarian | 8 |
C=Caucasian, A=African American.
Toxicity Associated with Intraperitoneal Administration of Ad5.SSTR/TK.RGD
All reportable clinical and laboratory adverse events by grade noted in patients enrolled in this study are summarized in Table 2. One patient had a grade 1 intraperitoneal catheter-associated infection prior to administration of Ad5.SSTR/TK.RGD that appropriately responded to antibiotics. Of the 107 clinical adverse events noted, 94 were classified as not or unlikely to be related to Ad5.SSTR/TK.RGD treatment. Thirteen were classified as attributable to Ad5.SSTR/TK.RGD treatment, 7 as “possibly” and 6 as “probably.” The clinical adverse events attributable to Ad5/SSTR/TK.RGD occurred in 6 patients and appeared to be dose dependent (1 patient in the intermediate dose cohort, and 5 patients in the highest dose cohort). These events were grade 1–2 in nature and included fatigue (1), fever (2), flu-like symptoms (3), headache (2), abdominal pain (4), or back pain (1). All were in general transient and managed medically. There were no grade 3/4 dose-limiting clinical toxicities attributable to Ad5.SSTR/TK.RGD.
Table 2.
Clinical (A) and laboratory (B) toxicity noted in study patients by category and grade.
| A. | |||||
|---|---|---|---|---|---|
| Body System | Toxicity Grades (NCI CTC V.3) | Total Occurrences | |||
| 1 | 2 | 3 | 4 | ||
| Constitutional | 8 | 2 | 2 | 0 | 12 |
| Dermatology/Skin | 1 | 0 | 0 | 0 | 1 |
| Gastrointestinal | 11 | 4 | 6 | 0 | 21 |
| Infection | 5 | 0 | 0 | 1 | 6 |
| Lymphatics | 4 | 1 | 0 | 0 | 5 |
| Musculoskeletal | 1 | 0 | 0 | 0 | 1 |
| Neurology | 3 | 0 | 0 | 0 | 3 |
| Pain | 25 | 20 | 1 | 0 | 46 |
| Respiratory | 2 | 2 | 0 | 2 | 6 |
| Renal/Genitourinary | 0 | 0 | 2 | 0 | 2 |
| Syndromes | 3 | 0 | 0 | 0 | 3 |
| Vascular | 0 | 0 | 0 | 1 | 1 |
| Total | 63 | 29 | 11 | 4 | 107 |
| B. | |||||
|---|---|---|---|---|---|
| Lab Tests | Toxicity Grades (NCI CTC V.3) | Total Occurrences | |||
| 1 | 2 | 3 | 4 | ||
| Anemia | 18 | 10 | 4 | 0 | 32 |
| Hyperglycemia | 0 | 1 | 0 | 0 | 1 |
| Hyperkalemia | 1 | 0 | 1 | 0 | 2 |
| Elevated Creatinine | 3 | 2 | 1 | 0 | 6 |
| Neutropenia | 9 | 3 | 0 | 0 | 12 |
| Hypocalcemia | 0 | 1 | 0 | 0 | 1 |
| Thrombocytopenia | 7 | 0 | 0 | 0 | 7 |
| Elevated PTT | 2 | 0 | 1 | 0 | 3 |
| Elevated INR | 0 | 0 | 1 | 0 | 1 |
| Total | 40 | 17 | 8 | 0 | 65 |
Four patients, one in the second dose cohort and 3 in the third dose cohort, experienced a total of eleven grade 3 and four grade 4 clinical toxicities and eight grade 3 laboratory toxicities. The patient in the second dose cohort experienced symptoms of a small bowel obstruction after placement of her intraperitoneal catheter and prior to treatment with Ad5.SSTR/TK.RGD; her symptoms resolved and she was treated according to protocol without further significant adverse effects. A second patient developed disease related symptoms of fatigue, small bowel obstruction, anemia, and electrolyte abnormalities (elevated potassium) several weeks after treatment per protocol. She was also diagnosed with a disease related pulmonary embolism, was treated with anticoagulants and had appropriate rise in coagulation laboratories. She was ultimately referred for hospice care. A third patient developed a disease related pleural effusion approximately one month after completing protocol treatment that was complicated by aspiration pneumonia and sepsis; she required intubation and ultimately expired due to progressive respiratory failure. A fourth patient experience disease related symptoms of a small bowel obstruction, dehydration, anemia, and acute renal insufficiency which caused her treatment with GCV to be interrupted after four days. She did not receive further therapy and was placed on hospice care due to her progressive disease and bowel obstruction symptoms. None of the patients who developed bowel obstructive symptoms after Ad5.SSTR/TK.RGD treatment were noted to have symptoms or signs of a treatment related inflammatory response that may have contributed to their obstructive symptoms. None of these grade 3/4 clinical toxicities were deemed attributable specifically to the Ad5.SSTR/TK.RGD treatment.
The most common laboratory abnormality noted in treated patients was grade 1/2 anemia; 10 of 12 patients had some degree of anemia noted. There were no other grade 3/4 laboratory abnormalities reported other than the eight grade 3 laboratory abnormalities previously described. All laboratory abnormalities were deemed disease related and none were attributed specifically to Ad5.SSTR/TK.RGD treatment.
Clinical Efficacy
All patients were assessed approximately one month after intraperitoneal Ad5.SSTR/TK.RGD and intravenous GCV utilizing RECIST version 1.0 criteria by comparing post treatment abdominal pelvic CT examinations to that obtained pretreatment and pre- and post-treatment CA-125 responses (Table 3). Five patients (42%) demonstrated RECIST-defined stable disease while seven patients (58%) experienced RECIST-defined progressive disease. Three patients (25%) had a decrease in CA-125 levels and 9 (75%) had an increase in CA-125.
Table 3.
Clinical efficacy of Ad5.SSTR/TK.RGD treatment as noted by RECIST and CA-125 response.
| Dose | Patient ID | Cancer | Best Response by RECIST* | CA-125 | Subsequent treatment | Current Status+ | Survival (months) | |
|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | |||||||
| 1×109 vp/d | 101 | Ovarian | SD | 3006.6 | 3918.0 | Yes | DOD | 20 |
| 102 | Endometrial | SD | 13.8 | 144.0 | Yes | AWD | 29 | |
| 103 | Ovarian | PD | 84.9 | 123.1 | Yes | AWD | 28 | |
| 5×1010 vp/d | 201 | Ovarian | PD | 319.1 | 409.2 | Yes | DOD | 7 |
| 202 | Ovarian | PD | 9.6 | 14.3 | Yes | DOD | 15 | |
| 203 | Ovarian | PD | 337.9 | 354.0 | Yes | DOD | 8 | |
| 1×1012 vp/d | 301 | Endometrial | PD | 25.7 | 78.1 | No | DOD | 1 |
| 302 | Endometrial | SD | 845.7 | 53.8 | No | Alive, NED | 25 | |
| 303 | Ovarian | PD | 274.8 | 277.3 | No | DOD | 4 | |
| 304 | Ovarian | SD | 299.0 | 255.9 | No | Died of other causes | 1 | |
| 305 | Ovarian | PD | 533.0 | 947.5 | Yes | AWD | 16 | |
| 306 | Ovarian | SD | 400.1 | 385.1 | No | DOD | 1 | |
SD=Stable Disease, PD=Progressive Disease.
DOD=Dead of disease, AWD=Alive with disease, NED=No evidence of disease
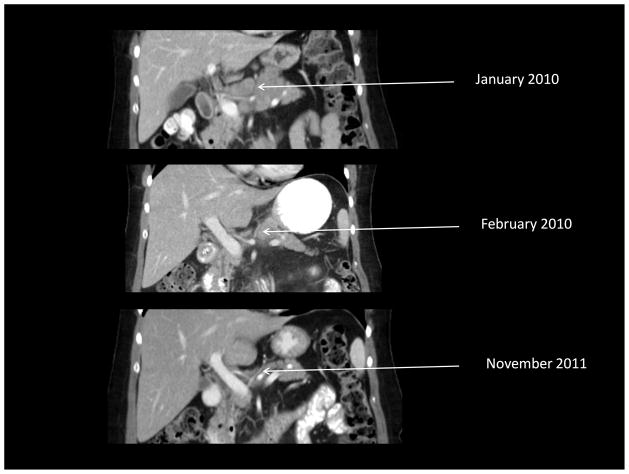
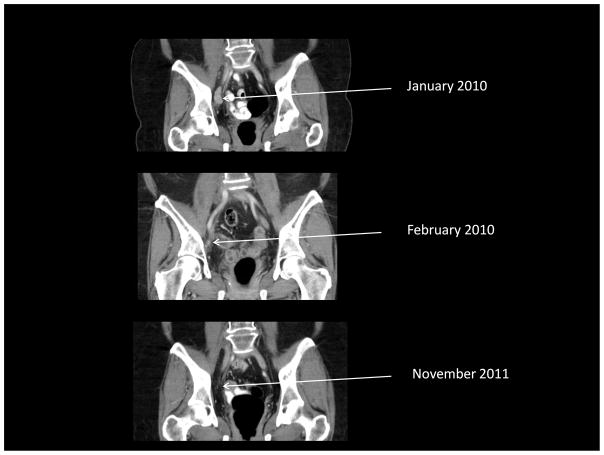
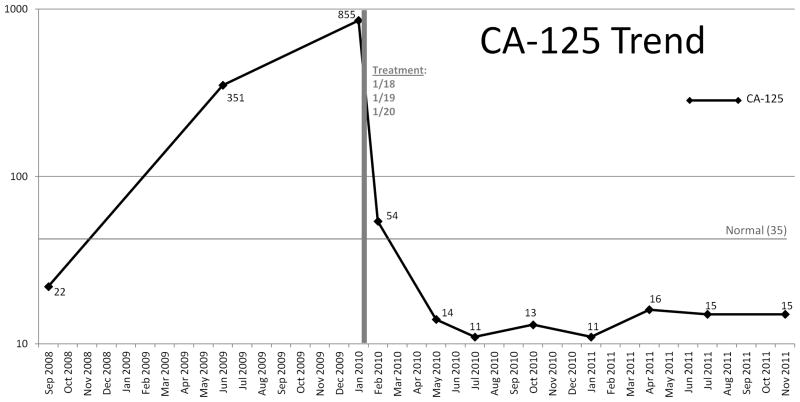
One patient (#302) with RECIST-defined stable disease at day 29 did not receive additional treatment and was eventually noted to have near complete resolution of disease by 5 months after her treatment (Figure 1). She also had a >50% reduction in her CA125 at day 29 and subsequently normalized her CA-125 levels which would have characterized her as having a response to therapy according to internationally accepted criteria (27). She continues to be followed without evidence of disease progression approximately 25 months after treatment.
Figure 1.
Demonstration of delayed clinical response (complete resolution of disease on CT and normalization of CA-125) in one patient (#302) who had stable disease by RECIST criteria one month after Ad5.SSTR/TK.RGD treatment. A. Upper abdominal CT. B. Pelvic CT. C. Graph of CA-125 values.
Three other patients were alive with disease 16 to 29 months after treatment per protocol and were being treated with other therapies. As previously stated, one patient had died of other causes (respiratory failure) soon after treatment per protocol. Seven patients had died of disease 1 to 20 months following treatment per protocol; four of these patients had been treated with other therapies after participating in this trial.
Noninvasive Imaging of Gene Transfer and Assessment of Somatostatin Expression
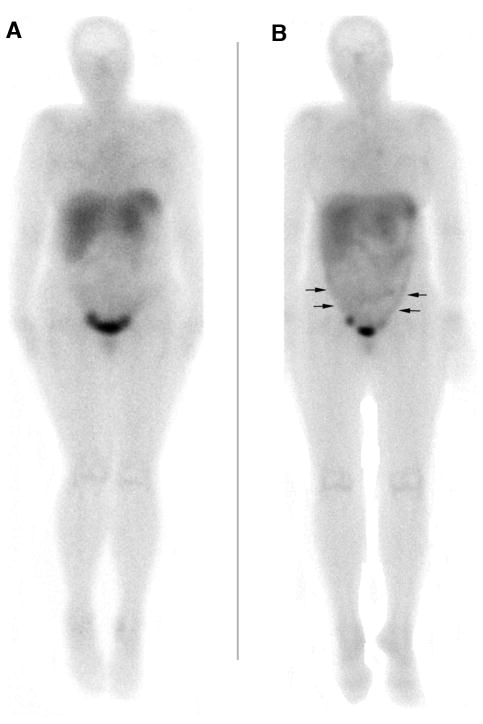
Patients receiving the lowest and middle Ad5.SSTR/TK.RGD doses were graded qualitatively as the same, with no significant difference in In-111 pentetreotide images before and after therapy. In contrast, all 3 evaluable patients in the highest Ad5.SSTR/TK.RGD dose group showed a qualitative increase in In-111 pentetreotide retention when comparing images before and after therapy (e.g. patient #1, 1+ to 2+; patient #2, 0 to 1+; patient #3, 1+ to 2+). By ROI analyses the average ratio (entire abdomen/pelvis) for all 9 evaluable patients before Ad5.SSTR/TK.RGD therapy was 1.07 (SE=0.04), and there was a borderline increase (p=0.051 by two-way repeated measures ANOVA) in the ratio to 1.21 (SE=0.06) after therapy. The sample was too small to determine if there was a dose-response effect. However, for the 3 evaluable patients in the highest Ad5.SSTR/TK.RGD dose group, the ratio for the regions of “apparent” uptake was 1.07 (SE=0.1) before therapy, and significantly increased (p=0.036) to 1.42 (SE=0.1) for the same regions after therapy. Images from a representative patient before and after Ad5.SSTR/TK.RGD therapy are demonstrated in Figure 2.
Figure 2.
Anterior whole body, planar In-111 pentreotide gamma camera images of a representative patient before (A) and after (B) Ad treatment (highest Ad dose cohort) show diffuse peritoneal activity following therapy (arrows) as well as normal distribution in liver, spleen, bowel, and urine. The image in (B) was at 1 day after the last Ad5.SSTR/TK.RGD dose (of 3 doses given over 3 days). Therefore, the single imaging time point post Ad5.SSTR/TK.RGD therapy was at 24, 48, and 72 hours after the three respective Ad5.SSTR/TK.RGD doses. This time point is coincided with the highest levels of TK expression according to our ancillary studies evaluating TK expression in peritoneal aspirates.
Ancillary Biologic Studies
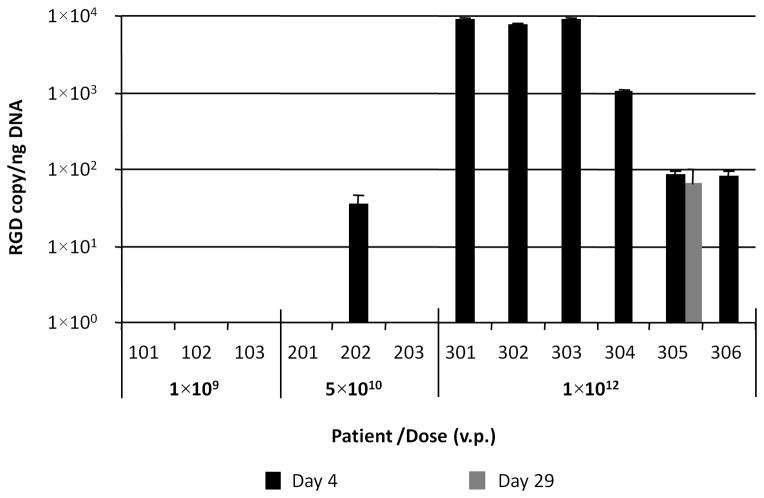
Various tissue samples were obtained prior to and at several time points after Ad5.SSTR/TK.RGD vector administration to assess for gene transfer and expression, viral shedding, generation of wild type virus, and generation of an anti-adenovirus neutralizing antibody response utilizing methodologies previously described. There clearly appeared to be a correlation between detectable vector and Ad5.SSTR/TK.RGD dose. Figure 3A illustrates the detection of Ad5.SSTR/TK.RGD vector in ascites samples by quantitative PCR with an RGD-specific set of primers and probe (Supplemental Table 2). These data demonstrate that detectable levels of Ad5.SSTR/TK.RGD vector were found on day 4 following administration of virus in the peritoneal lavage samples obtained from one patient in the intermediate dose cohort (5×1010 vp/dose) and from all patients in the highest dose cohort (1×1012 vp/dose).
Figure 3.
Figure 3A. Quantification of Ad5.SSTR/TK.RGD vector in ascites samples. The Ad5.SSTR/TK.RGD genome copies were quantified in each patient’s ascites sample (in triplicate) with primers and probe specific for RGD-4C-coding sequence (RGD) and then, normalized to amount of cellular DNA detected in the same sample with primers and probe for human μ-actin (housekeeping gene) using duplexing quantitative PCR settings. No RGD DNA was detected pretreatment (data not depicted in figure).
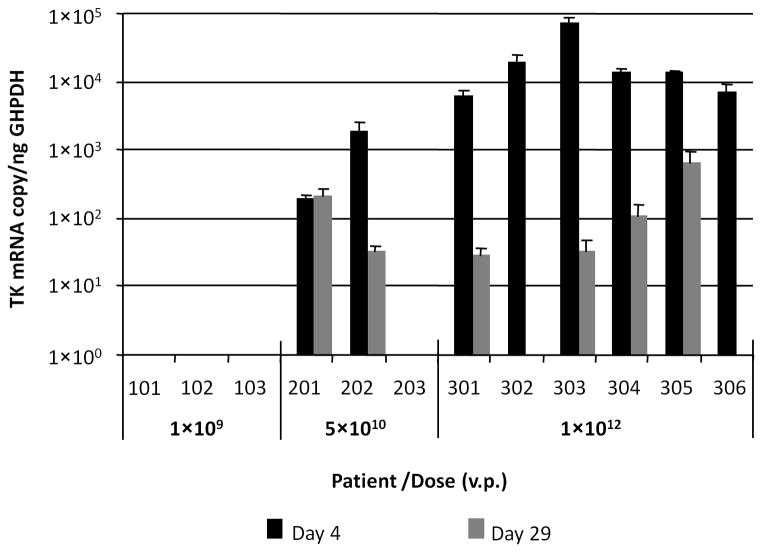
Figure 3B. Assessment of vector-mediated expression of HSV1-tk gene in ascites samples. HSV1-tk gene-specifc mRNA was quantified using total mRNA isolated from ascites samples as template (in triplicate) and then, normalized to the amount of human GAPDH (housekeeping gene) mRNA, which was detected in the same sample using duplexing RT-PCR settings. No TK mRNA was detected pretreatment (data not depicted in figure).
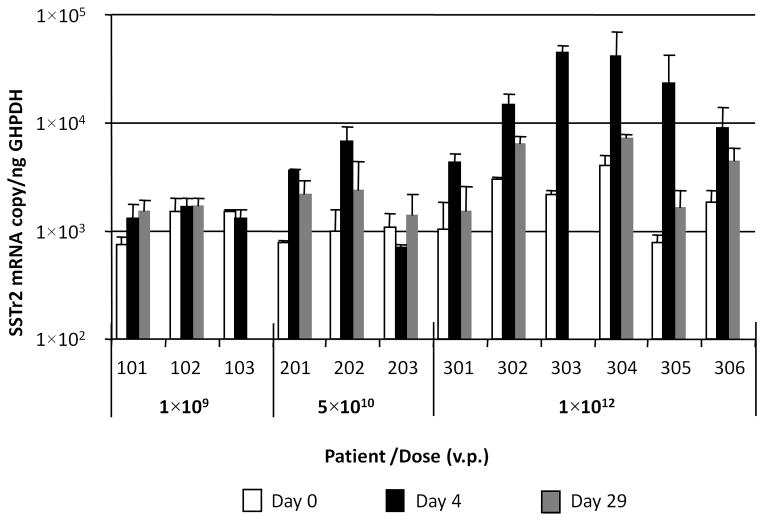
Figure 3C. Assessment of vector-mediated expression of SSTr2 gene in ascites samples. SSTr2 gene-specific mRNA was quantified using total mRNA isolated from each ascites sample as template (in triplicate) for reverse transcription reaction followed by quantitative PCR. The determined copy number of SSTr2 mRNA was normalized to the amount of human GAPDH (housekeeping gene) mRNA, which was detected in the same sample using duplexing RT-PCR settings.
To evaluate the levels of Ad5.SSTR/TK.RGD-mediated transgene expression in ascites cells we analyzed total messages using the TaqMan One Step PCR assay, which includes reverse transcription (RT) cDNA synthesis. The data presented in Figure 3B also demonstrated a correlation between HSV1-tk gene expression and dose. Detectable levels of HSV1-tk expression were noted at day 4 following administration of Ad5.SSTR/TK.RGD in two patients from the intermediate dose cohort and from all patients in the highest dose cohort. This RT-PCR assay showed higher sensitivity allowing detection of expression of heterologous HSV1-tk gene up to day 29 after Ad5.SSTR/TK.RGD administration in most patients from dose cohorts 2 and 3. The detection of HSV1-tk expression at day 29 in six of 8 patients in the higher two dose cohorts leads one to consider whether these patients could have been treated with additional GCV in hopes of further enhancing an anti-tumor effect.
Three of the five patients with stable disease had detectable vector and HSV1-tk. In two of these patients, including the patient who had a significant clinical response, HSV1-tk gene expression was not detected at day 29, suggesting the possibility that all transfected cells had been eradicated after GCV exposure. However, two of five patients with stable disease were treated in the lowest dose cohort and had no detectable Ad5.SSTR/TK.RGD vector or HSV1-tk expression at any time points. Thus, it is difficult to make a firm correlation between the detection of vector or gene expression and clinical outcome from the results of this study.
Somewhat similar data were obtained when RNA samples were analyzed for hSSTr2-specific mRNA (Figure 3C), except for the fact that the endogenous level of hSSTr2 gene expression resulted in high background values that interfered with detection fidelity. Nevertheless, a significant increase of hSSTr2 gene expression on day 4 above endogenous background levels observed on day 0 was noted in the same patients from dose cohorts 2 and 3 that were positive for HSV1-tk. None of the above methods could detect Ad5.SSTR/TK.RGD genome presence or vector-mediated transgene expression in patients from dose cohort 1 administered at the lowest viral dose.
Viral shedding studies did not reveal any detectable levels of Ad5.SSTR/TK.RGD virus in patient’s serum, saliva or urine specimens. (Data not shown) No emergence of wild type adenovirus was detected in any ascites samples. (Data not shown)
Neutralizing anti-adenoviral antibody response was determined by blocking adenoviral gene transfer in the presence of serum or ascites samples collected from patients before and after Ad5.SSTR/TK.RGD administration. High levels of preexisting immunity against adenovirus were noted on day 0 in most treated patients. (Supplemental Figure 1) Limited immune responses (ascites only) to adenovirus were observed in three patients (103, 201, and 202). A significant increase in adenoviral neutralizing antibody response was noted in most ascites samples collected from patients on day 4 and 29 as compared to day 0. (p <0.05) Despite the high levels of neutralizing antibody, all patients from Group 3 showed the presence of Ad5.SSTR/TK.RGD vector resulting in relatively high levels of HSV1-tk and SSTr2 gene expression in ascites cells on day 4.
Discussion
Prior studies of ours and those of others have utilized a variety of viral vector systems to mediate suicide gene therapy for ovarian cancer. Though demonstrated to be feasible and reasonably well tolerated, these trials demonstrated limited clinical effects in treated patients. Indeed, this has been the experience in the context of other clinical trials where suicide gene therapy strategies utilizing unmodified vector systems have been investigated for a variety of cancer sites (28–30). In the largest of these clinical trials, no significant benefit in median progression free survival was noted in 248 patients with glioblastoma multiforme treated immediately after surgical resection and radiotherapy with a HSV-TK expressing retrovirus in combination with GCV compared to those that received standard surgical resection and radiation (31).
In this study, we report the safety and potential efficacy of utilizing an infectivity-enhanced adenovirus to mediate a HSV-TK based suicide gene therapeutic approach in patients with recurrent ovarian and other selected gynecologic cancers. Specifically, we were able to administer Ad5.SSTR/TK.RGD intraperitoneally daily for three days at dosages up to 1 × 1012 vp/d. Our ancillary studies demonstrated a consistent dose related increase in detectable Ad5.SSTR/TK.RGD vector and HSV1-tk expression in ascites samples; the highest concentrations being noted within the highest dose cohort. Of note, no detectable Ad5.SSTR/TK.RGD shedding was noted. As observed in our other adenoviral mediated gene therapy trials, a robust anti-viral antibody response was noted (11, 32, 33).
The most common clinical toxicities attributable to Ad5.SSTR/TK.RGD were grade 1–2 in nature and generally consisted of manageable constitutional or pain symptoms. There clearly was a relationship between adverse events and dosage of Ad5SSTR/TK.RGD as most attributable toxicities were noted in the highest dose cohort. Other adverse events were in most instances attributable to the underlying cancer in treated patients and, in general, were medically managed. Of note, two patients experienced bowel obstructive symptoms after Ad5.SSTR/TK.RGD treatment. Though such symptoms have been noted as a result of inflammatory responses in patients treated intraperitoneally with a p53 expressing adenovirus (Ad-p53), we have not noted such in the current study or prior studies of ours evaluating other adenoviral mediated gene therapies (34). The fact that Ad-p53 was administered at higher dosages (up to 2.5 × 1013 vp/d daily over 5 days) than that utilized in the current study or prior studies of ours may have contributed in part to the increase in reagent-mediated obstructive symptoms noted in the Ad-p53 clinical trial. No vector specific laboratory abnormalities were noted. There were no grade 3/4 dose-limiting clinical or laboratory toxicities directly attributable to Ad5.SSTR/TK.RGD; thus, the maximum tolerated dose was not identified. Manufacturing constraints limited our ability to deliver higher dosages of this reagent.
Though no partial or complete responses were noted in this trial, 5 patients (42%) had RECIST-defined stable disease one month after treatment and 3 (25%) had a decrease in CA-125 levels. Of note, the delayed and durable complete response noted in one patient with recurrent endometrial cancer who had stable disease one month following treatment was clearly impressive. Though she had the fewest number of prior therapies, it is likely that she would have had at best only a partial or transient response if treated with additional chemotherapy. Although such patients are often characterized as chemotherapy sensitive or resistant once they have recurrence of their cancer, it is unknown whether such characterization would apply in the context of gene therapeutics. Regardless, she remains clinically free of disease with a normalized CA-125 approximately 25 months after treatment. This delayed response is clearly promising yet the true efficacy of this approach will require further evaluation in a phase II clinical trial.
The delayed response noted in this patient raises the question as to what should be the optimal methods and timeline to assess response to gene based therapies such as that used in this study. Recent reports suggests that gene therapeutics of the nature utilized in this trial and other approaches such as oncolytics may facilitate an anti-tumor immunologic response that would result in an enhanced therapeutic response (35–37). In addition, it is becoming increasingly evident that ovarian cancer patients who appear to have evidence of an intratumoral immune response have better outcomes (38). Though not evaluated in the current trial, it would be very reasonable to evaluate anti-tumor immunologic endpoints such as select tumor infiltrating lymphocytes or cytokines in future clinical trials. In addition, it may also be prudent to utilize recently revised RECIST criteria that have been developed to address evaluation of therapies such as this that elicit a significant immune response that may initially cause tumor swelling and be falsely interpreted as tumor progression (26).
The results of this trial mimic that noted in a prior phase I study of ours which evaluated a RGD modified infectivity enhanced adenoviral virotherapeutic (33). Specifically, 21 patients with recurrent ovarian and other select gynecologic cancers were treated intraperitoneally with escalating dosages of Ad5-Δ24-RGD, an RGD modified conditionally replicative adenovirus. In this trial, patients were treated with viral dosages of up to 1 × 1012 vp/d daily for three days with limited grade 1/2 constitutional or pain type symptoms. Though no partial or complete responses were noted, 15 patients (71%) had stable disease and 7 (33%) were noted to have a decrease in CA-125 levels.
This study also evaluated a novel in vivo gene transfer imaging strategy. Ad5.SSTR/TK.RGD contains a human type 2 somatostatin receptor expressing construct that allows for gene transfer imaging utilizing In-111 pentetreotide with whole body gamma camera imaging. Imaging clearly showed statistically increased retention of the In-111 pentetreotide in patients following the highest adenoviral dose, as compared to imaging before Ad5.SSTR/TK.RGD therapy. The abdominal regions of “apparent” uptake (not including normal excretion, liver, or kidneys) had ROI ratios that averaged 1.07 (SE=0.1) before treatment, and significantly increased (p=0.036) to 1.42 (SE=0.1) for the same regions after treatment. The ROI ratios refer to the ROI for abdominal region of “apparent” uptake, divided by the same-size ROI for the mediastinum (background). This result was impressive because 3-dimensional imaging studies were not conducted. Future studies with SPECT or PET (utilizing a SSTR-avid PET probe) in combination with CT will allow improved imaging of gene transfer with this approach.
Others have utilized various serum marker and imaging approaches to monitor gene transfer noninvasively in ovarian cancer patients treated with various virotherapy strategies. Galanis et al reported the results of a phase I trial of an Edmonston vaccine strain of measles virus engineered to express the marker peptide carcinoembryonic antigen (CEA) administered intraperitoneally to patients with recurrent ovarian cancer every 4 weeks for up to 6 cycles (39). Modest increases of CEA levels in the serum (12–16 ng/mL) were observed in all three patients treated at the highest dose level (109 TCID50). Rajecki et al demonstrated the ability to perform sodium iodide symporter SPECT imaging in a patient treated with oncolytic adenovirus Ad5/3-Δ24-hNIS (40).
New strategies are being developed to enhance suicide gene therapy for cancer. These approaches include double suicide gene therapy (41), combined suicide gene/immunotherapy (42), combined suicide gene/virotherapy (43) to name but a few. Further refinements in enhancing vector infectivity and in gene transfer imaging could certainly be incorporated into these approaches. Development of Ad5/3 vectors employing dual imaging strategies is currently underway in our group.
In conclusion, this study demonstrated the feasibility, safety and potential clinical efficacy of utilizing infectivity-enhanced adenoviruses intraperitoneally to mediate suicide gene therapy for ovarian and other selected gynecologic cancers. In addition, our study established the feasibility of using SSTR as a non-invasive approach to image gene transfer. Further investigation of these approaches to enhance suicide gene therapy in these cancers appears to be warranted.
Supplementary Material
Statement of Translational Relevance.
The authors have long endeavored to develop gene therapeutic approaches for ovarian and other gynecologic cancers. The current study presents the results of a phase I clinical trial evaluating a novel infectivity-enhanced bicistronic adenoviral vector, Ad5.SSTR/TK.RGD. This vector has an RGD-modified fiber knob that enhances viral infectivity of cancer cells. Ad5.SSTR/TK.RGD encodes the suicide gene therapeutic herpes simplex virus thymidine kinase that, when given in combination with ganciclovir, induces cancer cell apoptosis. The human type 2 somatostatin receptor (SSTR) is also encoded by Ad5.SSTR/TK.RGD, allowing for assessment of gene transfer using conventional nuclear imaging. The findings of the current study provide the first human evidence of the safety and the potential clinical efficacy and gene transfer imaging capacity of Ad5.SSTR/TK.RGD in patients with gynecologic cancer.
Acknowledgments
Financial Support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. Grants that assisted in the funding this project include: NCI R01CA90547 “Infectivity-Enhanced Adenoviral Vectors for Ovarian Cancer,” NCI R01CA121187 “Monitoring of Advanced Virotherapy for Ovarian Cancer,” the P50-CA83591 Specialized Program of Research Excellence (SPORE) in Ovarian Cancer, and the CCC Core grant (Human Imaging Shared Facility, 2P30CA013148). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was also supported, in part, by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute, with support from the UAB Comprehensive Cancer Center Clinical Trials Unit PCIR and the UAB Center of Clinical and Translational Science.
The authors would like to acknowledge the assistance of Rachel Richardson and Thelma Webb in the Division of Gynecologic Oncology at the University of Alabama at Birmingham, for their meticulous data collection and monitoring; and Elena Kashentseva in the Division of Cancer Biology at Washington University in St. Louis, for her assistance with ancillary studies.
Footnotes
Clinical Trial Registry: clinicaltrials.gov Identifier: NCT00964756
Disclosure: The authors declare that there are no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Berek JS. Surgery for ovarian cancer: how to improve survival. Lancet. 2006;367:1558–60. doi: 10.1016/S0140-6736(06)68671-6. [DOI] [PubMed] [Google Scholar]
- 6.Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;8:441–7. doi: 10.1007/s11912-006-0073-x. [DOI] [PubMed] [Google Scholar]
- 7.Kanerva A, Raki M, Hemminki A. Gene therapy of gynaecological diseases. Expert Opin Biol Ther. 2007;7:1347–61. doi: 10.1517/14712598.7.9.1347. [DOI] [PubMed] [Google Scholar]
- 8.Freeman SM, Abboud CN, Whartenby KA, Pachman CH, Koeplin DS, Moolten FL, et al. The “bystander effect”: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–83. [PubMed] [Google Scholar]
- 9.Rosenfeld M, Feng M, Michael S, Siegal G, Alvarez R, Curiel D. Adenoviral mediated delivery of the herpes simplex virus thymidine kinase gene selectively sensitizes human ovarian carcinoma cells to ganciclovir. Clin Cancer Res. 1995;1:1571–80. [PubMed] [Google Scholar]
- 10.Rosenfeld M, Wang M, Siegal G, Alvarez R, Mikheeva G, Krasnykh V, et al. Adenoviral mediated delivery of herpes simplex thymidine kinase results in tumor reduction and prolonged survival in a SCID mouse model of human ovarian carcinoma. J Mol Med. 1996;74:455–62. doi: 10.1007/BF00217521. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez R, Gomez-Navarro J, Wang M, Barnes MN, Strong TV, Arani RB, et al. Adenoviral mediated suicide gene therapy for ovarian cancer. Mol Ther. 2000;2:524–30. doi: 10.1006/mthe.2000.0194. [DOI] [PubMed] [Google Scholar]
- 12.Robinson W, Adams J, Marrogi A, Freeman S. Vaccine therapy for ovarian cancer using herpes simplex virus thymidine kinase (HSV-TK) suicide gene transfer technique: A phase I study. Gene Ther Mol Biol. 1998;2:31–40. [Google Scholar]
- 13.Link C, Eldeman M, Tennant L, Levy J, Moorman D, Seregina T. LTKOSN.1 murine vector producer cell (VPC) for the in vivo delivery of the herpes simplex thymidine kinase (HSV-TK) gene for ovarian cancer. Am Soc Gene Ther. 1999 Abstract #915. [Google Scholar]
- 14.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. Virol. 1999;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krasnykh V, Dmitriev I, Navarro JG, Belousova N, Kashentseva E, Xiang J, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000;60:6784–7. [PubMed] [Google Scholar]
- 16.Vanderkwaak T, Wang M, Navarro J, Rancourt C, Dmitriev I, Krasnykh V, et al. An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol Oncol. 1999;74:227–34. doi: 10.1006/gyno.1999.5432. [DOI] [PubMed] [Google Scholar]
- 17.Shah K. Current advances in molecular imaging of gene and cell therapy for cancer. Cancer Biol Ther. 2005;4:518–23. doi: 10.4161/cbt.4.5.1706. [DOI] [PubMed] [Google Scholar]
- 18.Raty JK, Liimatainen T, Unelma Kaikkonen M, Grohn O, Airenne KJ, Yia-Herttuala S. Non-invasive imaging in gene therapy. Mol Ther. 2007;15:1579–86. doi: 10.1038/sj.mt.6300233. [DOI] [PubMed] [Google Scholar]
- 19.Zinn K, Buchsbaum D, Chaudhuri T, Mountz J, Grizzle W, Rogers B. Noninvasive monitoring of gene transfer using a reporter receptor imaged with a high affinity peptide radiolabeled with 99mTc or 188Re. J Nucl Med. 2000;41:887–95. [PubMed] [Google Scholar]
- 20.Zinn KR, Chaudhuri TR. The type 2 human somatostatin receptor as a platform for reporter gene imaging. Eur J Nucl Med Mol Imaging. 2002;29:388–99. doi: 10.1007/s00259-002-0764-y. [DOI] [PubMed] [Google Scholar]
- 21.Zinn KR, Chaudhuri TR, Krasnykh VN, Buchsbaum DJ, Belousova N, Grizzle WE, et al. Gamma camera dual imaging with a somatostatin receptor and thymidine kinase after gene transfer with a bicistronic adenovirus in mice. Radiology. 2002;223:417–25. doi: 10.1148/radiol.2232010501. [DOI] [PubMed] [Google Scholar]
- 22.Belousova N, Harris R, Zinn K, Rhodes-Selser MA, Kotov A, Kotova O, et al. Circumventing recombination events encountered with production of a clinical-grade adenoviral vector with a double-expression cassette. Mol Pharmacol. 2006;70:1488–93. doi: 10.1124/mol.106.025619. [DOI] [PubMed] [Google Scholar]
- 23.Hemminki A, Belousova N, Zinn K, Liu B, Wang M, Chaudri T, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–31. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- 24.Hemminki A, Zinn K, Liu B, Chaudhuri T, Desmond R, Rogers B, et al. In vivo molecular chemotherapy and non-invasive imaging with an infectivity enhanced adenovirus. J Nat Can Inst. 2002;94:741–49. doi: 10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- 25.Matthews K, Noker PE, Tian B, Grimes SD, Fulton R, Schweikart K, et al. Identifying the safety profile of Ad5.SSTR/TK. RGD, a novel infectivity-enhanced bicistronic adenovirus, in anticipation of a phase I clinical trial in patients with recurrent ovarian cancer. Clin Cancer Res. 2009;15:4131–7. doi: 10.1158/1078-0432.CCR-08-3354. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Gynecological Cancer Intergroup. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1. 1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–23. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 28.Prados MD, McDermott M, Chang SM, Wilson CB, Fick J, Culver KW, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–78. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 29.Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F, et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834–40. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- 30.Xu F, Li S, Lis XL, Guo Y, Zou BY, Xu R, et al. Phase I and biodistribution study of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene and ganciclovir administration in patients with head and neck cancer and other malignant tumors. Cancer Gene Ther. 2009;16:723–30. doi: 10.1038/cgt.2009.19. [DOI] [PubMed] [Google Scholar]
- 31.Rainov NG. A Phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez RD, Barnes MN, Gomez-Navarro J, Wang M, Strong TV, Arafat W, et al. A cancer gene therapy approach utilizing an anti-erbB-2 single chain antibody (sFv) encoding adenovirus (AD21): A phase I trial. Clin Cancer Res. 2000;6:3081–7. [PubMed] [Google Scholar]
- 33.Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W, et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16:5277–87. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buller RE, Runnebaum IB, Karlan BY, Horowitz JA, Shahin M, Buekers T, et al. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002;9:553–66. doi: 10.1038/sj.cgt.7700472. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Kim Y, Jo MY, Kim HS, Jin Y, Kim SU, et al. Combined treatment of tumor-tropic human neural stem cells containing the CD suicide gene effectively targets brain tumors provoking a mild immune response. Oncol Rep. 2011;25:63–8. [PubMed] [Google Scholar]
- 36.Onion D, Patel P, Pineda RG, James N, Mautner V. Antivector and tumor responses following adenovirus-directed enzyme prodrug therapy for the treatment of prostate cancer. Hum Gene Ther. 2009;20:1249–58. doi: 10.1089/hum.2009.078. [DOI] [PubMed] [Google Scholar]
- 37.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–8. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–8. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajecki M, Kangasmaki A, Laasonen L, Escutenaire S, Hakkarainen T, Haukka J, et al. Sodium iodide symporter SPECT imaging of a patient treated with oncolytic adenovirus Ad 5/3-Δ24-hNIS. Mol Ther. 2011;19:629–31. doi: 10.1038/mt.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu B, Zhang Y, Zhan Y, Zha X, Wu Y, Zhang X, et al. Co-expression of herpes simplex virus thymidine kinase and Escherichia coli nitroreductase by an hTERT-driven adenovirus vector in breast cancer cells results in additive anti-tumor effects. Oncol Rep. 2011;26:255–64. doi: 10.3892/or.2011.1285. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Hou J, Liu Z, Yu H, Sun W, Xiong J, et al. Gene therapy with tumor-specific promoter mediated suicide gene plus IL-12 gene enhanced tumor inhibition and prolonged host survival in a murine model of Lewis lung carcinoma. J Transl Med. 2011;9:39. doi: 10.1186/1479-5876-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leveille S, Samuel S, Goulet ML, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435–43. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.