Abstract
Both dopamine and glutamate are critically involved in cognitive processes such as working memory. Astrocytes, which express dopamine receptors, are essential elements in the termination of glutamatergic signaling: the astrocytic glutamate transporter GLT-1 is responsible for >90% of cortical glutamate uptake. The effect of dopamine depletion on glutamate transporters in the prefrontal cortex (PFC) is unknown. In an effort to determine if astrocytes are a locus of cortical dopamine-glutamate interactions, we examined the effects of chronic dopamine denervation on PFC protein and mRNA levels of glutamate transporters. PFC dopamine denervation elicited a marked increase in GLT-1 protein levels, but had no effect on levels of other glutamate transporters; high affinity glutamate transport was positively correlated with the extent of dopamine depletion. GLT-1 gene expression was not altered. Our data suggests that dopamine depletion may lead to post-translational modifications that result in increased expression and activity of GLT-1 in PFC astrocytes.
Keywords: astrocyte, dopamine, EAAT2, GLAST, prefrontal cortex, schizophrenia
The prefrontal cortex (PFC) is critically involved in a number of cognitive processes. For example, the dopamine innervation of the PFC is required for working memory (Takahashi et al., 2012). Both dysfunction of the PFC dopamine innervation, as seen in schizophrenia (Davis et al., 1991; Barch and Ceaser, 2012), and administration of dopamine receptor antagonists (Robbins and Roberts, 2007) impair working memory. Similarly, disruption of glutamatergic signaling by the NMDA receptor antagonist ketamine elicits working memory deficits (Krystal et al., 1994). In addition, NMDA antagonists increase cortical dopamine receptor expression and decrease PFC extracellular dopamine levels (Healy and Meador-Woodruff, 1996; Tsukada et al., 2005). These data are consistent with dopamine-glutamate interactions modulating PFC function and influencing cognition (Javitt et al., 2012; Moghaddam and Krystal, 2012).
Astrocytes, which envelope excitatory synapses, express the glutamate transporters GLT-1 (EAAT2) and GLAST (EAAT1) (Lehre et al., 1995). GLT-1 is a major determinant of cortical extracellular glutamate levels, accounting for >90% of glutamate clearance from the extracellular space (Tanaka et al., 1997). Autoradiographic studies first suggested that dopamine receptors are expressed by astrocytes (Hösli and Hösli, 1986). Subsequent studies confirmed the presence of D2 receptor protein and mRNA in PFC astrocytes (Bal et al., 1994; Khan et al., 2001), and stimulation of astrocytic dopamine receptors in vitro increases astrocytic calcium levels (Parpura and Haydon, 2000).
Despite considerable interest in dopaminergic regulation of glutamatergic neurons of the PFC, scant attention has been devoted to how dopamine modulates glutamate transporters. Dopamine depletion increases striatal GLT-1 (Massie et al., 2010), but no comparable studies have examined the response of GLT-1 to PFC dopamine depletion. Because of the importance of the PFC in cognitive processes in schizophrenia, and the corresponding interest in modulating glutamatergic function as a novel therapeutic approach, we determined the effects of dopamine denervation on PFC glutamate transporters.
Materials and Methods
Subjects
Adult male Sprague–Dawley rats (Harlan; Indianapolis, IN) were group-housed with food and water available ad libitum. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Disruption of the dopamine innervation of the PFC
Animals received bilateral 6-OHDA lesions of the VTA, the source of the PFC dopamine innervation. We used this approach rather than direct PFC manipulations to avoid mechanical damage to the PFC and a resultant astrocytosis. To minimize 6-OHDA uptake into noradrenergic axons coursing near the VTA, rats received desipramine (12.5 mg/kg, i.p.) 30 and 15 minutes before infusion of 1.0 μL 6-OHDA HBr (4.0 μg/μL free base in 0.02% ascorbic acid) at 250 nL/min (AP:−5.4, ML:+/− 0.6, DV: −8.4). Sham-operated animals served as controls. Control (N=6) and 6-OHDA-injected (N=13) rats were sacrificed 3 weeks post-operatively.
Immunoblotting
The medial PFC, including both the infralimbic (area 25) and prelimbic (area 32) cortices, was dissected from 1.0 mm slices. Samples were subjected to subcellular fractionation using a series of buffers containing no detergent, 1% Triton X-100, or 1%Triton/deoxycholate, yielding cytosolic-, membrane-, and postsynaptic density (PSD)-enriched fractions, respectively (see Gustin et al., 2010). Samples were heated at 65°C and electrophoretically separated on a 10% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose and stained with Ponceau-S.
After a blocking step, the membranes were incubated in primary antibodies overnight at 4° C. The antibodies were: GLT-1 (Tocris #20631, Minneapolis, MN, 1:100,000 and Cell Signaling Technologies #3838, Beverly, MA, 1:5000), GLAST (Novus Biologicals #NB100-1869, Littleton, CO, 1:3,000), EAAC1 (Alpha Diagnostics #EAAC11-A, San Antonio, TX, 1:500), and GFAP (Millipore #MAB360, Billerica, MA, 1:1000). The membranes were incubated in peroxidase-conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) before being developed using chemiluminescence (Plus-ECL; PerkinElmer; Waltham, MA). Bands representing the proteins of interest were densitometrically scanned using ImageJ and normalized to total protein levels from the Ponceau-stained membranes (Romero-Calvo et al., 2010).
RT-PCR
RNA was extracted from the PFC (control N=12; 6-OHDA N=12) using an RNeasy Mini kit (Qiagen; Germantown, MD). Subsequently, cDNA was made from 0.5 μg total RNA using Superscript Vilo reverse transcriptase (Life Technologies; Grand Island, NY). RT-PCR was performed with SYBR Green (Bio-Rad; Hercules, CA) as the reporter using the following primers: GLT-1 (5′-ATGCCGCACACAACTCTGTCGT-3′ and 5′-TCAGCTGACTTTCCATTGGCCGC-3′); GLAST (5′-CCTCAGGCCGGTCTAGTCACCA-3′ and 5′-GGTGGTGGTTCGGAGGCGGT-3′); EAAC1 (5′-CTTCCTGCGGAATCACTGGCTG-3′ and 5′-GAGCTCACTGTGTCCTCGAACC-3′); GAPDH (5′-GGGCTCTCTGCTCCTCCCTGT-3′ and 5′-CCAGGCGTCCGATACGGCCA-3′). Cycle threshold values were subjected to statistical analyses after normalization to gapdh.
Catecholamine determinations
The PFC dorsal to the prelimbic cortex (including the pregenual cingulate [area 24b] and shoulder cortices) was dissected for determination of catecholamine concentrations by HPLC-EC (see Deutch and Cameron, 1992).
Glutamate uptake
Minced mPFC tissue from lesioned (N=11) and control (N=7) animals was suspended in Krebs-Ringer-Hepes buffer to 10 μg protein/mL and incubated in 100 nM [3H]-glutamate for 10 min at 37° C in a shaking waterbath. Non-specific uptake was assessed at 4° C. Uptake was terminated by filtration in a Brandell Cell Harvester, and the filters rinsed and placed overnight in scintillation fluid. Accumulated radioactivity was determined by scintillation spectrometry, with specific uptake calculated by subtracting non-specific from total uptake.
Stereology
The number of glial fibrillary acidic protein (GFAP)-immunoreactive (-ir) astrocytes in the PFC of sham- (N=4) and 6-OHDA-lesioned (N=4) animals was determined using sterological methods (optical dissector; 2500 μm2 sampling area; MBF Bioscience; Williston, VT). Activation of astrocytes, as reflected by branching complexity, was assessed by measuring the number of GFAP-ir processes crossing two of the stereological counting square boundaries (50 μm/side).
Statistical analysis
Statistical comparisons were made by Student’s two-tailed t tests, which were controlled for multiple comparisons by setting the experiment-wise α level at 0.05.
Results
Extent of dopamine depletion
Dopamine concentration in the PFC of control animals averaged 1.05 ± 0.09 ng/mg protein. Mean PFC dopamine concentration in 6-OHDA-treated rats was reduced by 70.4 ± 3.6% (t17= 9.02, p<0.001). Norepinephrine concentrations (control concentration, 5.14 ± 0.19 ng/mg protein) were not significantly decreased by dopamine denervation (t17=0.16, NS).
Alterations of GLT-1 expression
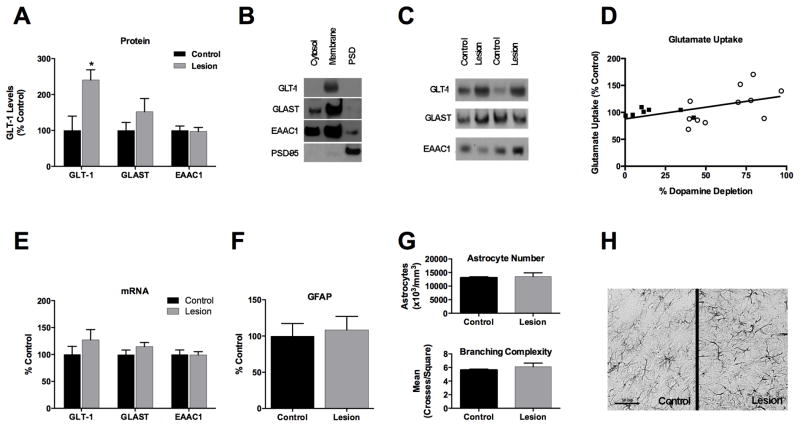
Antibodies to GLT-1 detected a clear band at ~62 kDa with a much lighter “smear” visible between 150 and 250 kDa. The majority of GLT-1 was present in the membrane-enriched fraction (Fig. 1). Dopamine depletion markedly increased PFC GLT-1 levels in the membrane fraction compared to controls (t16=2.88, p=0.011; Fig. 1). The increase in GLT-1 levels after dopamine denervation was replicated in a second experiment using a different GLT-1 antibody (data not shown). No changes were observed in either GLAST or EAAC1 protein levels (Fig. 1).
Figure 1.
A). Dopamine loss in the PFC increases GLT-1 protein levels in the membrane- enriched fraction; there is no significant change in GLAST or EAAC1. B). Glutamate transporters are most abundant in the membrane-enriched fraction. C). Representative immunoblot showing the glutamate transporters in control and dopamine-depleted (lesion) animals. D). The magnitude of the decrease in PFC dopamine is positively correlated to the degree to which glutamate uptake is increased. E). There is no change in mRNA levels of GLT-1 or other glutamate transporters. F). No change in level of the astrocytic protein GFAP is seen in immnoblot studies, and G) stereological evaluation indicates no increase in the number of GFAP-positive astrocytes (top) or astrocytic processes (bottom) in dopamine- denervated animals. H). Representative immunohistochemical staining for GFAP; scale bar = 50μm.
*p = .011
Glutamate transporter mRNA levels
Real-time PCR revealed no significant change in PFC mRNA levels of GLT-1, GLAST, or EAAC1 in response to DA depletion (Fig. 1).
Glutamate uptake
There was no significant difference in high-affinity glutamate uptake (HAGU) between control and dopamine-denervated rats. However, there was a significant correlation between the extent of dopamine loss in the PFC and HAGU (r = .494, p = .039; Fig. 1).
Astrocyte number and morphology
Immunoblot analysis did not detect a change in PFC levels of the astrocytic marker GFAP (Fig. 1). Similarly, stereological studies of GFAP-ir astrocytes revealed no increase in the number of astrocytes or astrocyte processes in response to dopamine denervation (Fig. 1).
Discussion
Dopamine depletion of the PFC increased levels of the astrocytic glutamate transporter GLT-1 and increased HAGU but had no significant effect on levels of the two other neocortical glutamate transporters, GLAST and EAAC1. The increase in cortical GLT-1 protein levels was not attributable to astrocytosis or increased GLT-1 gene expression.
Dopamine in the PFC
PFC dopamine loss in lesioned animals averaged ~70%, spanning 40–91%. The incomplete dopamine denervation is consistent with previous studies, including those that injected 6-OHDA directly into the PFC (Bubser, 1994). The partial dopamine denervation presumably reflects very low or absent expression of the dopamine transporter in a subset of VTA dopamine neurons, including some of those projecting to the PFC (Sesack et al., 1998). Given the range of dopamine depletions, future studies will be required to determine the threshold level of dopamine depletion that must be achieved to increase GLT-1, as suggested by the significant correlation between extent of PFC dopamine loss and HAGU.
Increased GLT-1 protein expression
All three cortical glutamate transporters were most abundant in the membrane-enriched fraction, consistent with immunohistochemical data indicating that GLT-1 is primarily localized to the plasma membrane and EAAC1 to the plasma membrane and to a lesser degree the cytosol (Conti et al., 1998). The increase in membrane-associated GLT-1 was not accompanied by a detectable change in transporter levels in the other two fractions.
Glutamate uptake
Prefrontal cortical dopamine deafferentation did not significantly increase HAGU relative to control animals. When one considers the variable degree to which dopamine was decreased in the PFC of lesioned rats, and the significant correlation between the degree of PFC dopamine loss and the increase in HAGU, it is not surprising that there was not an overall difference between dopamine-denervated and control animals. However, there was a substantial difference between the magnitude of the increase in GLT-1 protein and the degree of increase in glutamate uptake. The stoichiometry of GLT-1 protein to transport activity in vivo is unclear, and the difference in degrees to which protein and transport are increased may reflect a trafficking issue. Future work will need to determine the precise mechanisms underlying the changes in glutamate transport in the dopamine-denervated PFC.
GLT-1, GLAST or EAAC1 gene expression
Real-time PCR did not detect significant changes in relative abundance of GLT-1, GLAST, or EAAC1 mRNA levels in dopamine-depleted subjects. The large increase in GLT-1 protein levels that occurs after cortical dopamine denervation, without a concurrent increase in mRNA levels, suggests that the increase in GLT-1 protein is not due to induction of the transporter.
Schmitt et al. (2003) reported that chronic treatment with the D2 receptor antagonist haloperidol decreases GLT-1 mRNA in the PFC. However, we did not detect a change in GLT-1 gene expression after PFC dopamine depletion. The difference between our results and those of Schmitt and colleagues may be attributable to the fact that the haloperidol dose used by Schmitt et al. results in complete occupancy of D2 receptors in vivo (see Perez-Costas et al., 2008), whereas our lesions resulted in partial dopamine denervation.
Absence of astrocytosis or increased astrocyte activation
A possible explanation for the increase in GLT-1 protein levels is that dopamine denervation increases PFC astrocyte number. We did not observe a change in levels of the astrocytic protein GFAP in the dopamine-denervated PFC, nor did stereological analysis reveal a change in the number of GFAP-ir astrocytes. Astrocytes are dynamic cells that upon activation extend processes. We therefore examined the number of astrocytic processes, which was not changed in the dopamine-denervated PFC. These data suggest that the increase in GLT-1 is not due to astrocytic activation.
Potential post-translational modification
The immunoblots showed a clear ~62 kDa (GLT-1) band, consistent with previous reports (Schmitt et al., 2003). There was also a much fainter smear of high molecular weight proteins between 150–250 kDa, suggesting the presence of post-translationally modified forms of GLT-1. Ubiquitination of GLT-1 is required for its internalization and subsequent degradation (Boehmer et al., 2006). Densitometry of the bands between 150–250 kDa revealed decreased levels of these proteins in the dopamine-depleted PFC (t11=2.20; p<0.05). This raises the possibility that accumulation of GLT-1 in astrocyte membranes may result from decreased ubiquitination and a resultant decrease in internalization and degradation of the transporter.
Our efforts to detect polyubiquitinated GLT-1 in the PFC using multiple approaches (immunoprecipitation of GLT-1 and ubiquitin, as well as pull down assays using ubiquitin-binding small molecules) were unsuccessful (data not shown), presumably because of the high expression of deubiquitinating enzymes and the transient nature of polyubiquitinated proteins. Future studies will be required to determine if post-translational modifications account for the accumulation of membrane-associated GLT-1 after dopamine depletion.
Functional implications
Changes in both glutamate and dopamine have been suggested to contribute to the cognitive deficits in schizophrenia (Davis et al., 1991; Laruelle et al., 2003; Seamans and Robbins, 2009). The expression of dopamine receptors on astrocytes offers a potential means whereby dopamine can modulate PFC glutamate through the astrocyte.
Neither acute nor chronic treatment with the dopamine receptor antagonist haloperidol changes PFC extracellular glutamate levels (Daly and Moghaddam, 1993; Yamamoto and Cooperman, 1994). However, Yamamoto and colleagues showed that K+-evoked depolarization of the PFC in animals treated chronically with haloperidol elicits a trend toward an increase in PFC glutamate levels, suggesting that an increase in neuronal glutamate release is dampened by a compensatory increase in GLT-1.
Post-mortem studies of GLT-1 in the PFC of schizophrenia subjects have yielded inconsistent results. A preliminary study of unmedicated patients, but not those treated with antipsychotic drugs, reported increased PFC GLT-1 protein and mRNA levels (Matute et al., 2005). Bauer et al. (2008) found no change in GLT-1 protein in a cohort including both medicated and unmedicated patients, while a third study of haloperidol-treated patients reported a decrease in GLT-1 mRNA (Ohnuma et al., 1998). The variability seen in postmortem studies may be due to drug treatment or a variety of agonal or post-mortem factors.
Our data suggest that dopamine may modulate extracellular glutamate levels in the PFC by increasing astrocytic GLT-1 levels. Increasing evidence suggests that astrocytes are not limited to a structural or support role in the CNS, but are key partners with neurons in cellular signaling. This has sparked considerable interest in the role of astrocytes in neuropsychiatric disorders, including schizophrenia. Our data point to the astrocyte as a key site for PFC dopamine-glutamate interactions.
Acknowledgments
We are indebted to Drs. Roger Colbran, Brian Wadzinski, and Karoly Mirnics for helpful comments and suggestions. This work was supported by MH077298 (AYD) and MH095044 (RDB) from the National Institute of Mental Health, the Swiss National Science Foundation (LDS), and the Neurochemistry Core of NICHD Grant P30HD15052 to the Vanderbilt Kennedy Center for Research on Human Development.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- GFAP
glial fibrillary acidic protein
- -ir
immunoreactive
- PFC
prefrontal cortex
- PSD
postsynaptic density
- VTA
ventral tegmental area
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMH, NICHD, or the NIH.
The authors declare no conflict of interest.
References
- Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, Feuerstein C. Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Mol Brain Res. 1994;23:204–212. doi: 10.1016/0169-328x(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104:108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- Bubser M. 6-Hydroxydopamine lesions of the medial prefrontal cortex of rats do not affect dopamine metabolism in the basal ganglia at short and long postsurgical intervals. Neurochem Res. 1994;19:421–425. doi: 10.1007/BF00967319. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Daly DA, Moghaddam B. Actions of clozapine and haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett. 1993;152:61–64. doi: 10.1016/0304-3940(93)90483-2. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Gustin RM, Bichell TJ, Bubser M, Daily J, Filonova I, Mrelashvili D, Deutch AY, Colbran RJ, Weeber EJ, Haas KF. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol Dis. 2010;39:283–291. doi: 10.1016/j.nbd.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DJ, Meador-Woodruff JH. Differential regulation, by MK-801, of dopamine receptor gene expression in rat nigrostriatal and mesocorticolimbic systems. Brain Res. 1996;708:38–44. doi: 10.1016/0006-8993(95)01241-9. [DOI] [PubMed] [Google Scholar]
- Hösli E, Hösli L. Binding sites for [3H]dopamine and dopamine-antagonists on cultured astrocytes of rat striatum and spinal cord: an autoradiographic study. Neurosci Lett. 1986;65:177–182. doi: 10.1016/0304-3940(86)90300-9. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci USA. 2001;98:1964–1969. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann NY Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Goursaud S, Schallier A, Vermoesen K, Meshul CK, Hermans E, Michotte Y. Time-dependent changes in GLT-1 functioning in striatum of hemi-Parkinson rats. Neurochem Int. 2010;57:572–578. doi: 10.1016/j.neuint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. Capturing the Angel in “Angel Dust”: Twenty Years of Translational Neuroscience Studies of NMDA Receptor Antagonists in Animals and Humans. Schizophr Bull. 2012;38:942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Mol Brain Res. 1998;56:207–217. doi: 10.1016/s0169-328x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115:745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential Regulation of Fronto-Executive Function by the Monoamines and Acetylcholine. Cereb Cortex. 2007;17:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocón B, Martínez-Moya P. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Analytical Biochemistry. 2010:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Zink M, Petroianu G, May B, Braus DF, Henn FA. Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. Neurosci Lett. 2003;347:81–84. doi: 10.1016/s0304-3940(03)00653-0. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Robbins TW. The Dopamine Receptors. Humana Press; Totowa, NJ: 2009. Dopamine Modulation of the Prefrontal Cortex and Cognitive Function; pp. 373–398. [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamada M, Suhara T. Functional significance of central D1 receptors in cognition: beyond working memory. J Cereb Blood Flow Metab. 2012;32:1248–1258. doi: 10.1038/jcbfm.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino EF. Chronic NMDA Antagonism Impairs Working Memory, Decreases Extracellular Dopamine, and Increases D1 Receptor Binding in Prefrontal Cortex of Conscious Monkeys. Neuropsychopharmacology. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Cooperman MA. Differential effects of chronic antipsychotic drug treatment on extracellular glutamate and dopamine concentrations. J Neurosci. 1994;14:4159–4166. doi: 10.1523/JNEUROSCI.14-07-04159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]