Abstract
Background
Attention problems are among the most prevalent neurobehavioral morbidities affecting very preterm (VPT) born children. The first study aim was to document rates of persistent attention/hyperactivity problems from ages 4 to 9 years in a regional cohort of VPT born children. The second aim was to examine the extent to which persistent problems were related to cerebral white matter abnormality and structural development on neonatal MRI.
Methods
Data were drawn from a prospective longitudinal study of 110 VPT (≤32 weeks’ gestation) and 113 full-term (FT) children born from 1998 to 2000. At term equivalent, all VPT and 10 FT children underwent cerebral structural MRI, with scans analyzed qualitatively for white matter abnormalities and quantitatively for cortical and subcortical gray matter, myelinated and unmyelinated white matter, and cerebrospinal fluid volumes. At ages 4, 6, and 9 years, each child’s parent and teacher completed the Inattention/Hyperactivity subscale of the Strengths and Difficulties Questionnaire.
Results
VPT born children had a 5-fold increased risk of persistent attention/hyperactivity problems compared to FT children (13.1% vs. 2.8%; p=.002). No association was found between neonatal white matter abnormalities and later persistent inattention/hyperactivity risk (p≥.24). In contrast, measures of cerebral structural development including volumetric estimates of total cerebral tissue and cerebrospinal fluid relative to intracranial volume were associated with an increased risk of persistent attention/hyperactivity problems in VPT born children (p=.001). The dorsal prefrontal region showed the largest volumetric reduction (↓3.2–8.2ml). These brain-behavior associations persisted and in some cases, strengthened after covariate adjustment for postmenstrual age at MRI, sex, and family socioeconomic status.
Conclusions
Just over one in 10 VPT born children are subject to early onset and persistent attention/hyperactivity problems during childhood. These problems appear to reflect, at least in part, neonatal disturbances in cerebral growth and development rather than the effects of white matter injury.
Keywords: attention, brain development, low birth weight, magnetic resonance imaging, very preterm, white matter injury
Introduction
Attention problems are one of the most common neurobehavioral morbidities associated with very preterm (VPT) birth, with around 10%–50% of children affected (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002). A meta-analysis of 7 case-control studies published from 1980 to 2001 reported that VPT born children were 2.6 times more likely to be diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD) than full-term (FT) born children at school age (Bhutta, et al., 2002). A more recent meta-analysis of 9 case-control studies published from 1998 to 2008 found that VPT born children obtained parent and teacher rated attention problems scores that were on average 0.4 to 0.6 standard deviations higher than their FT peers (Aarnoudse-Moens, et al., 2009).
Almost all studies concerned with the risks of attention problems and ADHD following VPT birth have been cross-sectional with many relying on data from a single report source or measure (Aarnoudse-Moens, et al., 2009; Bhutta, et al., 2002). As a result, little is known about the development of symptoms over time or the extent to which those with attention problems represent a stable group. Identifying VPT born children with persistent attention/hyperactivity problems and/or ADHD symptomatology is important given their likely elevated risks of academic underachievement and social difficulties (Biederman, et al., 2009; Biederman, Petty, Evans, Small, & Faraone, 2010). Thus, the first aim of this study was to examine rates of inattention/hyperactivity across multiple time-points during early/middle childhood and determine the extent of persistent problems in children born VPT.
The second major, related, focus was to examine the neonatal neurological factors that may place VPT born children at an increased risk of persistent attention/hyperactivity problems. Existing studies suggest two potential factors. These include perinatal cerebral injury and altered cerebral structural development (Nosarti, Allin, Frangou, Rifkin, & Murray, 2005; Whitaker, et al., 2011). While perinatal cerebral injuries such as germinal matrix hemorrhage-intraventricular hemorrhage with ventriculomegaly or periventricular hemorrhagic infarction have been linked with ADHD/attention problems (Whitaker, et al., 2011; Whitaker, et al., 1997), they are now relatively rare. In contrast, noncystic, diffuse white matter injuries/abnormalities remain common and have been shown to be strongly predictive of neuromotor and cognitive risk (Spittle, et al., 2011; Woodward, Anderson, Austin, Howard, & Inder, 2006; Woodward, Clark, Bora, & Inder, 2012). The extent to which these cerebral white matter abnormalities (WMAs) are also predictive of later risk of inattention/hyperactivity is not known.
Another factor that may also contribute to attention/hyperactivity problems in VPT born children is altered cerebral structural development characterized by delayed maturation or impaired growth as a result of perinatal cerebral injury or the clinical complications of VPT birth (Inder, et al., 1999; Thompson, et al., 2007). Many neuroanatomical areas affected by VPT birth overlap with brain regions implicated in the pathogenesis of attention problems and ADHD in the general population. For example, ADHD has been linked with structural alterations of the corpus callosum, caudate nucleus, amygdala, hippocampus, and lateral ventricles in the general population (Seidman, Valera, & Makris, 2005). Similarly, poor postnatal growth and thinning of the corpus callosum has been documented in younger and older children born VPT (Inder, Wells, Mogridge, Spencer, & Volpe, 2003; Skranes, et al., 2007). Significant reductions of hippocampal and caudate nuclei volumes have also been reported at both term equivalent and adolescence (Abernethy, Palaniappan, & Cooke, 2002; Thompson, et al., 2008).
Global and regional neuroanatomical alterations at term equivalent in VPT born children have been associated with a range of adverse neurodevelopmental outcomes, particularly oculomotor function, working memory, cognitive and psychomotor development (Peterson, et al., 2003; Shah, et al., 2006; Thompson, et al., 2008; Woodward, Edgin, Thompson, & Inder, 2005). However, the relationship between neonatal cerebral growth and maturation and later risk of attention problems remains unclear. Nonetheless, studies have shown associations between ADHD symptoms and altered cerebral morphometry, and in particular the hippocampus, corpus callosum, and caudate nucleus, in VPT born adolescents (Abernethy, et al., 2002; Indredavik, et al., 2005; Nosarti, et al., 2005). Neuroanatomical alterations in regions involved in the frontostriatal circuitry have also been found, with these potentially contributing to later inattention/hyperactivity risk (Thompson, et al., 2007; Woodward, et al., 2005).
Accordingly, the specific study aims were to:
Document rates of inattention/hyperactivity at ages 4, 6, and 9 years, and to assess the extent of persistent problems across assessment time-points in VPT and FT born children.
Examine within the VPT born group, associations between a) qualitatively defined cerebral WMAs, b) volumetric estimates of global and regional cerebral tissues, as identified on magnetic resonance imaging (MRI) at term equivalent and later persistent inattention/hyperactivity.
METHODS
Sample
The sample included two groups of children. A descriptive profile of the neonatal clinical and social background characteristics of the sample is provided in Table 1. The first group consisted of a regional cohort of 110 VPT born infants (≤32 weeks’ gestation) admitted consecutively to the level III Neonatal Intensive Care Unit of Christchurch Women’s Hospital (New Zealand) from 1998 to 2000 (92% recruitment). Infants with congenital anomalies, fetal alcohol syndrome, and/or non-English speaking parents were excluded. There were no significant differences in the perinatal characteristics of recruited and non-recruited infants. Excluding deaths (n=3), 92% (n=99) of VPT born infants were assessed at ages 4, 6, and 9 years.
Table 1.
Neonatal Clinical and Social Characteristics of the Sample
| Measure | Full-Term (N=110) | Very Preterm (N=107) | p |
|---|---|---|---|
| Infant clinical characteristic | |||
| Gestational age at birth, M ± SD, weeks | 39.5 ± 1.2 | 27.8 ± 2.4 | <.001 |
| Birth weight, M ± SD, grams | 3,579.5 ± 409.3 | 1,061.6 ± 314.2 | <.001 |
| Male sex, % | 53.6 | 50.5 | .64 |
| Twin birth, % | 3.6 | 33.6 | <.001 |
| Small for gestational age, % | 0.9 | 10.3 | .003 |
| Antenatal corticosteroid use, % | – | 84.1 | |
| Postnatal dexamethasone use, % | – | 5.6 | |
| Oxygen therapy at 36 weeks, % | – | 34.6 | |
| Necrotizing enterocolitis, % | – | 6.5 | |
| Confirmed sepsis, % | – | 28.0 | |
| Patent ductus arteriosus, % | – | 43.9 | |
| Intraventricular hemorrhage grade III/IV, % | – | 5.6 | |
| Cystic periventricular leukomalacia, % | – | 5.6 | |
| White matter abnormalities on MRI | |||
| None, % | – | 24.5 | |
| Mild, % | – | 57.5 | |
| Moderate–severe, % | – | 17.9 | |
| Social background characteristic | |||
| Maternal age, M ± SD, years | 31.0 ± 4.4 | 30.7 ± 5.4 | .62 |
| Maternal smoking during pregnancy, % | 16.2 | 36.8 | .001 |
| Mother not a high school graduate, % | 19.1 | 39.3 | .001 |
| Single parenthood, % | 11.8 | 18.7 | .16 |
| Minority ethnicity, % | 11.8 | 14.0 | .63 |
| Family socioeconomic status | |||
| Professional/managerial, % | 35.5 | 26.2 | |
| Technical/skilled, % | 54.5 | 43.0 | |
| Semiskilled/unskilled/unemployed, % | 10.0 | 30.8 | .001 |
The second group included 113 FT born infants (38–41 weeks’ gestation). These infants were identified from hospital records (N=7,200 live births) by selecting for each VPT infant, the second previous or the second next same sex term born infant in the birth-register (62% recruitment). Infants with congenital anomalies, fetal alcohol syndrome, and/or non-English speaking parents were excluded. Recruited and non-recruited infants did not differ on measures of infant gestational age, birth weight, sex, family type, ethnicity, or socioeconomic status. Comparison with regional census data showed that families of FT born infants were highly representative of the population from which they were recruited (Statistics New Zealand, 2001). Overall, 94% (n=106) of FT born infants were assessed at ages 4, 6, and 9 years. Study protocols were approved by the Regional Health and Disability Ethics Committee and written informed consent obtained from all parents/guardians.
Measures
MRI Acquisition and Analysis (Term Equivalent Age)
At term equivalent, all VPT born infants underwent a cerebral structural MRI scan without sedation. In addition, a subsample of 10 FT infants without cerebral injury was also scanned on the week of their due date. Prior to imaging, infants were fed, wrapped, swaddled, and placed in a vacuum-fixation bean bag (Vac-Fix; S & S X-ray Products, Brooklyn, NY) to minimize motion artifacts. Images were acquired using a 1.5-Tesla General Electric Signa System (GE-Medical Systems). Imaging protocols included 1) a three-dimensional T1 spoiled gradient recalled sequence (1.5mm coronal slices, flip angle 45°, repetition time 35ms, echo time 5ms, field of view 18cm, matrix 256×256) and 2) a T2 double-echo (interleaved acquisition) spin echo sequence (3mm axial slices, repetition time 3000ms, echo times 36ms and 162ms, field of view 18cm, matrix 256×256).
Images were analyzed in two ways. First, the nature and severity of cerebral WMAs were assessed using a qualitative grading scheme (Inder, et al., 2003; Woodward, et al., 2006). Each MRI scan was independently rated by a pediatric neuroradiologist and neonatal neurologist. Inter-rater agreement was 95%, with discrepant cases assigned a consensus rating. WMAs were graded on five 3-point scales assessing: 1) the nature and extent of white matter signal abnormality; 2) periventricular white matter volume loss; 3) cystic abnormality; 4) ventricular dilatation; and 5) thinning of the corpus callosum. A composite WMAs score was computed and children classified as showing no (scores 5–6), mild (scores 7–9), or moderate–severe (scores 10–15) cerebral WMAs.
Second, images were quantitatively analyzed for volumetric estimates of different cerebral tissue subtype. A sequence of image processing algorithms was used to reduce imaging system noise and align T1 and T2 images (Huppi, et al., 1998). Each image was then segmented into cortical and subcortical gray matter, myelinated and unmyelinated white matter, and cerebrospinal fluid, using a spatially varying model through alignment with an anatomical template of a 40-week-old infant (Warfield, Kaus, Jolesz, & Kikinis, 2000). The total cerebral tissue volume was computed as the sum of all gray and white matter. The intracranial cavity volume was computed as the sum of all gray matter, white matter, and cerebrospinal fluid within the skull. For regional volumes, each image was segmented using the Talairach parcellation scheme (Peterson, et al., 2000) into dorsal prefrontal, orbitofrontal, premotor, subgenual, sensorimotor, midtemporal, parieto-occipital, and inferior occipital with cerebellum, using a combination of one axial and three coronal planes. The axial plane was passed through the anterior commissure and posterior commissure line. The first coronal plane was positioned at the most anterior part of the genu of the corpus callosum, the second coronal plane at the anterior border of the anterior commissure, and the third coronal plane through the posterior commissure. Volumetric image processing was feasible for 82% (n=90) of VPT born infants, with remaining scans affected by motion artifacts and MRI signal intensity errors that limited registration and tissue segmentation. There were no significant differences in neonatal clinical and social background characteristics of infants included and excluded from this analysis. For the FT born group, volumetric processing was feasible for 8 out of 10 infants.
Results for volumetric analysis have been reported as absolute volume (ml) as well as relative proportion within the intracranial cavity (i.e., [absolute volume/intracranial cavity volume] × 100). As the proportion of cerebral tissues differs greatly in absolute volume, results have also been reported as relative difference in order to estimate the magnitude of volumetric reduction for different tissue subtype. Relative differences were calculated by dividing the absolute mean difference for each tissue subtype by the absolute mean volume of the comparison group, after adjusting for the intracranial cavity volume.
Behavioral Inattention/Hyperactivity Screening (Ages 4, 6, 9 years)
At ages 4 and 6 (corrected) and 9 years (uncorrected), all study children were screened for behavioral attention/hyperactivity problems based on parent and teacher ratings using the Inattention/Hyperactivity subscale of the Strengths and Difficulties Questionnaire, SDQ (Goodman, 1997). This scale assesses child behavior over the last 6 months using 5-items rated on a 3-point Likert scale. The SDQ has been widely used in epidemiological research and clinical practice as a behavioral screening measure, and has been consistently shown to have good concurrent and predictive validity (Goodman & Scott, 1999; Mathai, Anderson, & Bourne, 2004). Specifically, the Inattention/Hyperactivity scale has moderate sensitivity (68%–74%) and good specificity (92%–93%) for a DSM-IV ADHD diagnosis across ages 5 to 15 years (Goodman, 2001).
For this study, at each age assessment, children were classified as showing clinically significant attention/hyperactivity problems if their parent or teacher ratings exceeded the 90th percentile (defined on the basis of the FT born group). This approach of determining the clinical cut-point is widely used internationally in epidemiological research, and has also been endorsed by follow-up studies of children born very preterm (Delobel-Ayoub, et al., 2009; Larroque, et al., 2011). To be included in the persistent attention/hyperactivity problems group, children were required to meet the clinical cut-point for problems at all three age assessments. Children with missing parent and teacher data at any age (VPT: n=8; FT: n=4) were excluded from this analysis. However, to minimize data loss, children with only their parent (VPT: n=4; FT: n=1) or teacher (VPT: n=14; FT: n=8) data missing at a single time-point were classified as showing no problem, unless clear difficulties were reported by the same informant at both the preceding and subsequent assessment. These identified cases were also cross-validated against a DSM-IV ADHD diagnosis assigned by a child psychiatrist blinded to children’s perinatal history and group status and based on a structured psychiatric interview at age 9 years. All children who met criteria for early-onset persistent attention/hyperactivity problems also received a diagnosis of ADHD. Children not meeting the criteria for persistent attention/hyperactivity problems but showed symptoms at least at one age assessment were included in the transient problems group. Two children with persistent problems and two other children with transient problems were on prescribed ADHD medication at age 9 years.
RESULTS
Rates of Inattention/Hyperactivity across Ages 4 to 9 Years
Table 2 describes the extent to which VPT and FT born children showed clinically significant attention/hyperactivity problems based on either parent or teacher report at ages 4, 6, and 9 years. Also shown are the proportions of children in each group characterized by transient and persistent attention/hyperactivity problems. At each cross-sectional assessment, VPT born children had relative risks of inattention/hyperactivity that were 1.6 to 2.2 times higher than their FT born peers (OR=1.8–3.0). Almost half of VPT and nearly a quarter of FT born children met the criteria for transient attention/hyperactivity problems (OR=2.6). Persistent problems were much less common across both groups, but especially among FT born children (13.1% vs. 2.8%; OR=7.7). These between-groups differences persisted even after statistical control for sex and family socioeconomic status.
Table 2.
Attention/Hyperactivity Problems among Full-Term and Very Preterm Born Children
| Time-Point | Attention/Hyperactivity Problem, % (n)
|
p | Odds Ratio (95% Confidence Interval) | Adjusteda Odds Ratio (95% Confidence Interval) | |
|---|---|---|---|---|---|
| Full-Term (N=110) | Very Preterm (N=107) | ||||
| Age 4 years | 18.7 (20) | 29.1 (30) | .08 | 1.8 (0.9–3.4) | 1.8 (0.9–3.4) |
| Age 6 years | 22.2 (24) | 43.3 (45) | .001 | 2.7 (1.5–4.8) | 2.7 (1.4–5.0) |
| Age 9 years | 17.3 (19) | 38.8 (40) | <.001 | 3.0 (1.6–5.7) | 2.7 (1.4–5.1) |
| Transient, Age 4 to 9 years | 30.2 (32) | 46.5 (46) | .002 | 2.6 (1.4–4.6) | 2.5 (1.4–4.6) |
| Persistent, Age 4 to 9 years | 2.8 (3) | 13.1 (13) | .002 | 7.7 (2.1–28.6) | 7.5 (1.9–29.0) |
Adjusted for sex and family socioeconomic status.
Neonatal Cerebral WMAs and Persistent Inattention/Hyperactivity
Table 3 shows the relationship between cerebral WMAs identified on MRI at term equivalent and children’s later risk of persistent attention/hyperactivity problems. This analysis was confined to VPT born children given the absence of WMAs in the subsample of FT children with an MRI scan. As shown, no significant associations were found between the severity of overall cerebral WMAs on term MRI, or any of the individual scales, and VPT children’s later risk of persistent attention/hyperactivity problems (p≥.24). This pattern of findings persisted after adjustment for postmenstrual age at MRI, sex, and family socioeconomic status.
Table 3.
Neonatal Cerebral White Matter Abnormalities and Risk of Persistent Attention/Hyperactivity Problems among Very Preterm Born Children
| Neonatal Cerebral White Matter Abnormality | Attention/Hyperactivity Problem, Age 4 to 9 Years, %
|
p | |
|---|---|---|---|
| None/Transient (N=85) | Persistent (N=13) | ||
| White matter abnormality | |||
| None | 21.2 | 15.4 | |
| Mild | 62.4 | 53.8 | |
| Moderate–severe | 16.5 | 30.8 | .34 |
| White matter signal abnormality | |||
| Normal | 45.9 | 30.8 | |
| Focal (≤2 regions) | 40.0 | 46.2 | |
| Extensive (≥2 regions) | 14.1 | 23.1 | .30 |
| Periventricular white matter volume loss | |||
| Normal | 52.9 | 38.5 | |
| Mild–moderate | 41.2 | 46.2 | |
| Diffuse | 5.9 | 15.4 | .24 |
| Cystic abnormality | |||
| Normal | 86.2 | 84.6 | |
| Focal (single, <2mm) | 9.4 | 7.7 | |
| Extensive (multiple, ≥2mm) | 2.4 | 7.7 | .50 |
| Ventricular dilatation | |||
| Normal | 38.8 | 30.8 | |
| Mild–moderate | 45.9 | 53.8 | |
| Marked | 15.3 | 15.4 | .83 |
| Thinning of the corpus callosum | |||
| Normal | 27.1 | 15.4 | |
| Focal | 58.8 | 76.9 | |
| Global | 14.1 | 7.7 | .81 |
Note. Reported associations persisted after adjustment for postmenstrual age at MRI, sex, and family socioeconomic status.
Neonatal Global Cerebral Tissue Volumes and Persistent Inattention/Hyperactivity
Table 4 describes the volumetric MRI findings at term equivalent for VPT born children with persistent attention/hyperactivity problems, VPT children with transient or no problems, and the small subsample of FT born children who had MRI data. None of these FT children met criteria for persistent or transient problems. Results show for each groups, both the absolute volume of the four tissue subtypes and cerebrospinal fluid, as well as the proportion relative to the intracranial cavity. Differences across the three study groups were examined using general linear models, with planned comparisons between VPT born children with persistent problems and the other two groups. Figure 1 supplements these results by illustrating the magnitude of volumetric reduction for different tissues.
Table 4.
Neonatal Global Cerebral Tissue Volumes and Risk of Persistent Attention/Hyperactivity Problems
| Neonatal Global Cerebral Tissue Volume, M ± SD | Attention/Hyperactivity Problem, Age 4 to 9 Years
|
p
|
||||
|---|---|---|---|---|---|---|
| None (1) | None/Transient (2) | Persistent (3) | Overall | (1) vs. (3) | (2) vs. (3) | |
|
| ||||||
| Full-Term (N=6) | Very Preterm (N=67) | Very Preterm (N=13) | ||||
| Total cerebral tissue | ||||||
| Absolute volume, ml | 441.4 ± 25.2 | 410.1 ± 49.9 | 404.2 ± 39.6 | .12 | .12 | .68 |
| Relative percentage within intracranial cavity | 94.6 ± 2.6 | 91.0 ± 4.0 | 87.8 ± 4.9 | .001 | .001 | .01 |
| Cortical gray matter | ||||||
| Absolute volume, ml | 193.4 ± 27.1 | 177.9 ± 38.3 | 176.5 ± 33.2 | .36 | .36 | .90 |
| Relative percentage within intracranial cavity | 41.5 ± 5.9 | 39.3 ± 6.6 | 38.4 ± 6.4 | .33 | .33 | .64 |
| Subcortical gray matter | ||||||
| Absolute volume, ml | 12.3 ± 3.0 | 12.6 ± 4.2 | 12.3 ± 3.5 | 1.0 | 1.0 | .82 |
| Relative percentage within intracranial cavity | 2.6 ± 0.6 | 2.8 ± 0.9 | 2.7 ± 0.7 | .94 | .94 | .64 |
| Myelinated white matter | ||||||
| Absolute volume, ml | 17.8 ± 8.2 | 13.4 ± 5.7 | 12.9 ± 5.4 | .09 | .09 | .74 |
| Relative percentage within intracranial cavity | 3.8 ± 1.6 | 3.0 ± 1.2 | 2.8 ± 1.2 | .11 | .11 | .67 |
| Unmyelinated white matter | ||||||
| Absolute volume, ml | 218.0 ± 26.2 | 206.2 ± 31.8 | 202.5 ± 31.5 | .32 | .32 | .70 |
| Relative percentage within intracranial cavity | 46.7 ± 5.1 | 45.9 ± 6.0 | 43.9 ± 5.6 | .34 | .34 | .28 |
| Cerebrospinal fluid | ||||||
| Absolute volume, ml | 25.1 ± 12.7 | 41.0 ± 19.4 | 57.7 ± 26.5 | .002 | .002 | .008 |
| Relative percentage within intracranial cavity | 5.4 ± 2.6 | 9.0 ± 4.0 | 12.2 ± 4.9 | .001 | .001 | .01 |
Note. Reported associations persisted after adjustment for postmenstrual age at MRI, sex, and family socioeconomic status.
Figure 1.
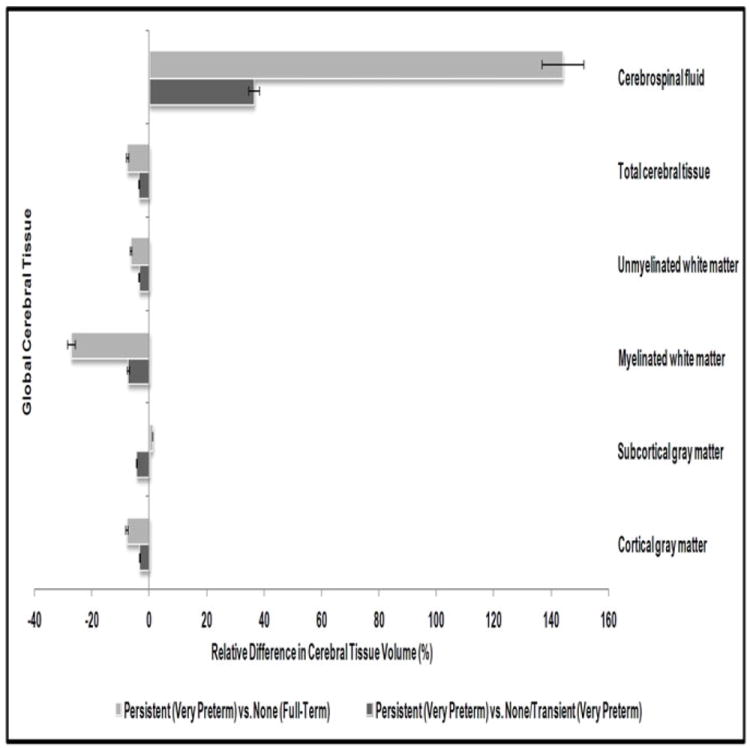
Relative difference in global cerebral tissue volume for very preterm born children with persistent attention/hyperactivity problems.
Associations between the absolute volume of total cerebral tissue and the risk of persistent attention/hyperactivity problems failed to reach statistical significance (p=.12). However, a clear linear association was evident between the relative proportion of total tissue within the intracranial cavity and risk of persistent problems (p=.001). These between-groups differences were further confirmed by pairwise comparisons (p≤.01). Consistent with the total cerebral tissue loss, there were corresponding increases in the absolute volume and relative proportion of cerebrospinal fluid (p≤.002). Specifically, as shown in Figure 1, VPT born children with persistent problems had 4% less total cerebral tissue and 36% more cerebrospinal fluid than VPT children with transient/no problems, and 8% less total tissue and 144% more cerebrospinal fluid than FT children without problems, after adjusting for the intracranial cavity volume.
Examination of the nature of neonatal cerebral tissue volume loss showed that reduced myelinated white matter volume, although not statistically significant, showed a tendency to be associated with an increased risk of later persistent attention/hyperactivity problems (p≤.11). This was particularly apparent in Figure 1 which shows that VPT born children with persistent attention/hyperactivity problems had 1.0ml (7%) and 4.7ml (27%) less myelinated white matter than VPT and FT children without these problems, respectively, after adjustment for the intracranial volume. No significant associations were found between neonatal cerebral volumes (absolute and relative proportion) of cortical gray matter (p≥.33), subcortical gray matter (p≥.94), unmyelinated white matter (p≥.32), and later persistent inattention/hyperactivity risk. These findings persisted after controlling for postmenstrual age at MRI, sex, and family socioeconomic status. Moreover, associations between proportion of total cerebral tissue within intracranial cavity, cerebrospinal fluid, and later risk of persistent problems among VPT born children became stronger after covariate adjustment (p=.004).
Neonatal Regional Cerebral Tissue Volumes and Persistent Inattention/Hyperactivity
To examine possible region-specific variations in cerebral tissue loss, Table 5 shows the associations between regional tissue volumes at term equivalent and children’s later risk of persistent inattention/hyperactivity. This analysis was also confined to VPT born children and the subsample of FT born children with an MRI scan. Results showed no significant association between absolute volume of neonatal regional total cerebral tissue across all the eight anatomical subregions and children’s later risk of persistent problems. However, clear reductions in regional cerebral tissue volume were evident in terms of relative proportion of total tissue within each subregion (p≤.02). Specifically, compared to FT and VPT children with no or transient attention/hyperactivity problems, VPT born children with persistent problems had the largest volumetric reductions in the proportion of total tissue within the dorsal prefrontal (p=.001), orbitofrontal (p=.02), premotor (p=.002), sensorimotor (p=.001), and parieto-occipital (p=.003) subregions. As shown in Figure 2, of all subregions, total tissue in the dorsal prefrontal region showed the greatest volumetric reduction. VPT born children with persistent inattention/hyperactivity had 3.2ml (7%) and 8.2ml (16%) reductions in total tissue in the dorsal prefrontal region relative to their VPT and FT born peers without persistent problems, respectively, after adjusting for the intracranial cavity volume. Although these results remained unchanged after adjustment for postmenstrual age at MRI, sex, and family socioeconomic status, stronger associations were evident between proportion of regional total cerebral tissue volume and risk of persistent problems within the VPT group (dorsal prefrontal: p=.02; orbitofrontal: p=.07; premotor: p=.005; parieto-occipital: p=.05). Furthermore, all reported associations (Tables 3-5) persisted following control for child IQ and severity of cerebral palsy at age 4 years.
Table 5.
Neonatal Regional Total Cerebral Tissue Volumes and Risk of Persistent Attention/Hyperactivity Problems
| Neonatal Regional Total Cerebral Tissue Volume, M ± SD | Attention/Hyperactivity Problem, Age 4 to 9 Years
|
p
|
||||
|---|---|---|---|---|---|---|
| None (1) | None/Transient (2) | Persistent (3) | Overall | (1) vs. (3) | (2) vs. (3) | |
|
| ||||||
| Full-Term (N=6) | Very Preterm (N=67) | Very Preterm (N=13) | ||||
| Dorsal prefrontal region | ||||||
| Absolute volume, ml | 51.4 ± 15.5 | 44.7 ± 9.6 | 44.8 ± 9.1 | .19 | .19 | .96 |
| Relative percentage within intracranial cavity | 92.1 ± 5.5 | 83.7 ± 8.3 | 78.8 ± 8.3 | .001 | .001 | .05 |
| Orbitofrontal region | ||||||
| Absolute volume, ml | 11.5 ± 5.3 | 9.1 ± 3.8 | 8.7 ± 3.9 | .16 | .16 | .76 |
| Relative percentage within intracranial cavity | 93.5 ± 3.0 | 87.5 ± 8.4 | 83.8 ± 10.8 | .02 | .02 | .16 |
| Premotor region | ||||||
| Absolute volume, ml | 56.6 ± 9.2 | 50.6 ± 8.3 | 50.0 ± 6.3 | .10 | .10 | .79 |
| Relative percentage within intracranial cavity | 93.0 ± 2.6 | 88.8 ± 5.1 | 85.0 ± 5.7 | .002 | .002 | .02 |
| Subgenual region | ||||||
| Absolute volume, ml | 22.5 ± 6.3 | 22.6 ± 4.8 | 21.3 ± 4.3 | .63 | .63 | .38 |
| Relative percentage within intracranial cavity | 84.5 ± 6.6 | 84.8 ± 5.3 | 80.7 ± 5.2 | .15 | .15 | .01 |
| Sensorimotor region | ||||||
| Absolute volume, ml | 69.5 ± 7.2 | 55.0 ± 12.1 | 57.7 ± 18.0 | .07 | .07 | .50 |
| Relative percentage within intracranial cavity | 96.9 ± 1.7 | 92.7 ± 4.1 | 89.6 ± 5.2 | .001 | .001 | .01 |
| Midtemporal region | ||||||
| Absolute volume, ml | 32.5 ± 7.3 | 31.2 ± 5.6 | 30.4 ± 5.4 | .46 | .46 | .68 |
| Relative percentage within intracranial cavity | 91.5 ± 3.8 | 92.7 ± 2.9 | 91.3 ± 3.4 | .93 | .93 | .15 |
| Parieto-occipital region | ||||||
| Absolute volume, ml | 126.0 ± 8.9 | 115.9 ± 16.6 | 115.5 ± 14.0 | .18 | .18 | .94 |
| Relative percentage within intracranial cavity | 96.7 ± 2.7 | 91.9 ± 5.0 | 89.1 ± 5.8 | .003 | .003 | .06 |
| Inferior occipital region with cerebellum | ||||||
| Absolute volume, ml | 70.6 ± 13.5 | 80.3 ± 14.6 | 75.0 ± 11.6 | .53 | .53 | .22 |
| Relative percentage within intracranial cavity | 97.4 ± 1.5 | 96.5 ± 2.2 | 95.3 ± 5.7 | .16 | .16 | .18 |
Note. Reported associations persisted after adjustment for postmenstrual age at MRI, sex, and family socioeconomic status.
Figure 2.
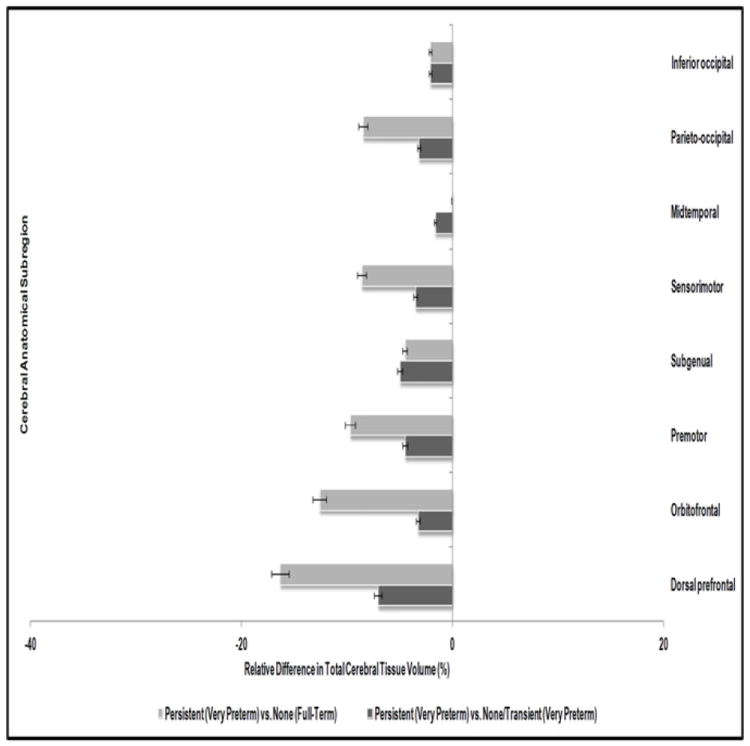
Relative difference in regional total cerebral tissue volume for very preterm born children with persistent attention/hyperactivity problems.
Neonatal Predictors of Persistent Inattention/Hyperactivity
The extent to which other neonatal clinical and social factors (as listed in Table 1) may also account for increased risk of persistent attention/hyperactivity problems among VPT born children was examined. The neonatal clinical complications significantly associated with later inattention/hyperactivity risk were grade III/IV intraventricular hemorrhage identified on cranial ultrasound (OR=8.3; p=.03) and confirmed sepsis (OR=5.3; p=.008). Being born to a younger mother also appeared to place VPT born children at increased risk of persistent inattention/hyperactivity (p=.04).
Finally, the extent to which neonatal clinical, social, and neurological factors, including global total cerebral tissue volume loss in VPT children, made a unique and independent contribution to later risk of attention/hyperactivity problems was assessed using logistic regression. After taking into account all the variables listed in Table 1, the proportion of total cerebral tissue within intracranial cavity at term equivalent age remained an independent predictor of later persistent inattention/hyperactivity (β=- 0.14; p=.07) along with confirmed sepsis (β=1.69; p=.01). No interactions were evident between these two risk factors. Jointly these two neonatal variables explained 24.1% (Nagelkerke R2) of variance and correctly classified 85% of cases.
DISCUSSION
Consistent with previous research (Delobel-Ayoub, et al., 2009; Larroque, et al., 2011), current findings show that attention/hyperactivity problems associated with VPT birth emerge early and can be readily identified with standardized screening measures. When assessed at a single time-point, parents and teachers report fairly high rates of inattention/hyperactivity in both VPT (29%–43%) and FT (17%–22%) born children, with the odds of problems being 2–3 times greater for VPT children. In contrast, examination of more serious, early-onset and persistent attention/hyperactivity problems showed that these difficulties were relatively rare, especially among FT children compared to VPT children (3% vs. 13%). In line with these results, findings from the follow-up of a large population-based cohort of low birth weight children reported a prevalence of 12% for a lifetime ADHD diagnosis (i.e., symptoms present from age 5 years) when assessed at age 16 years using the DISC-IV structured interview completed with parents (Whitaker et al., 2011). Together, these findings suggest that just over one in 10 children born VPT are at risk of persistent attention/hyperactivity problems during childhood. This rate is considerably lower than rates estimated on the basis of cross-sectional data, suggesting that for a significant proportion of both VPT and FT children, attention/hyperactivity problems are likely to be transient as opposed to chronic.
Study findings also provide useful insights into the neonatal neurological correlates that may, at least in part, help to explain why children born VPT are at an elevated risk of persistent inattention/hyperactivity. In contrast to previous studies (Spittle, et al., 2011; Woodward, et al., 2006; Woodward, et al., 2012) linking neonatal cerebral WMAs with later adverse motor and cognitive outcomes, no such association was found for attention/hyperactivity problems. Rather, global cerebral tissue volume loss during the neonatal period appeared to be an independent predictor of later risk of persistent inattention/hyperactivity. This may explain why some VPT born children without obvious evidence of early white matter injury also exhibit ADHD symptoms and/or attention problems. These findings concur with a DTI study of 11-year-old VPT born children without ultrasound evidence of perinatal cystic periventricular leukomalacia or intraventricular hemorrhage (Nagy, et al., 2003). They found significant associations between attention problems and lower fractional anisotropy values in the corpus callosum and internal capsules suggesting disturbances in cerebral organization. Links between reduced cerebral tissue volume and ADHD symptoms have also been reported in the general population, with 7 out of 12 studies that were included in a recent review showing that children and adolescents at risk of ADHD have a 3%–5% reduction in total cerebral volume compared to control children without ADHD (Seidman, et al., 2005).
Regional analysis of neonatal cerebral tissue volume showed that VPT born children with persistent attention/hyperactivity problems had reduced cerebral tissue volume within the dorsal prefrontal, orbitofrontal, premotor, sensorimotor, and parieto-occipital regions at term equivalent. Several general population studies have shown frontal and parietal lobe involvement in the development of ADHD symptoms (Castellanos, et al., 2002; Seidman, et al., 2005). For example, a recent review found smaller frontal lobe volume was consistently documented in children with ADHD, with 9 out of 12 studies reporting significant volumetric reduction in the dorsolateral prefrontal cortex (Seidman, et al., 2005). Although the potential role of the parietal cortex in the pathogenesis of attention problems is not well understood, a few studies have shown reduced parietal lobe volume in children with ADHD (Castellanos, et al., 2002; Seidman, et al., 2005). From a theoretical perspective, the frontoparietal network, which is thought to have a key role in alerting, orienting, and executive attention networks, may have relevance for the pathophysiology of attention/hyperactivity problems in VPT born children (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). Although the occipital lobe has not generally been associated with ADHD/attention problems, this neuroanatomical region may be of interest due to its involvement in visual information processing.
Study findings emphasize the importance of cerebral growth and maturation prior to term, including the time spent in the Neonatal Intensive Care Unit, as a vulnerable period for alterations in cerebral structural development that may place VPT born children at risk of ADHD symptomatology and/or inattention/hyperactivity during early/middle childhood. Apart from one study linking reduced neonatal hippocampal volume with parent reported inattention/hyperactivity at age 5 years (Rogers, et al., 2012), all previous studies have been based on neuroimaging data from VPT born adolescent samples.
Finally, study limitations need to be acknowledged while interpreting these findings. First, regional cerebral parcellation in this study was strictly based on anatomical localization with the commissure making it difficult to delineate regions based on their functional relevance. Given the paucity of longitudinal studies examining neonatal neurological correlates of later attention problems in this high-risk population, study findings provide useful insights, however, further replication is warranted. Second, no anthropometric data was collected on the day of infant MRI scan making it difficult to tease out the associations between infant’s height, head size, cerebral tissue volumes, and later risk. Finally, the child behavior screening measure used in this study was limited by its inability to distinguish between inattention and hyperactivity symptoms. In light of increasing evidence to suggest that VPT born children may be at risk of a “pure” form of attention problems characterized by inattention rather than hyperactivity, extension of this study with more specific attention and hyperactivity measures will be important.
Future studies need to address the persistence of attention/hyperactivity problems in VPT born children using a continuum rather than a dichotomous approach as it may be likely that a few children have chronic problems but may not be reaching the stringent clinical cut-points. Furthermore, it will also be important to assess the extent to which VPT born children with transient inattention/hyperactivity may differ from those with no and persistent problems in terms of neonatal clinical and neurological risk factors. However, from a methodological perspective, caution is warranted regarding classification of children in the transient attention/hyperactivity problems group based on parent or teacher ratings on behavior screening measures due to the risk of over/under-estimation of child problems.
In conclusion, study findings suggest that VPT born children are at an increased risk of persistent attention/hyperactivity problems from early to middle childhood. However, rates of persistent problems are considerably lower than rates estimated based on cross-sectional data. VPT born children’s increased risk of persistent attention/hyperactivity problems appears to at least in part, reflect neonatal disturbances in cerebral structural growth and maturation, particularly in the dorsal prefrontal, and to a lesser extent, orbitofrontal, premotor, sensorimotor, and parieto-occipital regions.
KEY POINTS.
VPT born children are at an increased risk of attention problems, but little is known about the persistence of these problems over time or the neonatal neurological factors that place these children at risk.
Study findings showed that VPT born children are at an increased risk of persistent attention/hyperactivity problems from age 4 to 9 years; although this risk is more modest than previously reported based on cross-sectional data.
Increased risk of persistent attention/hyperactivity problems in VPT born children can partly be accounted for by neonatal disturbances in cerebral structural development assessed using MRI. Specific abnormalities include reduction in the proportion of total tissue within the intracranial cavity particularly in the dorsal prefrontal, and to a lesser extent, orbitofrontal, premotor, sensorimotor, and parieto-occipital regions, along with concomitant increase of cerebrospinal fluid volume.
Acknowledgments
The study is supported by the Neurological Foundation of New Zealand (022/PG), the Health Research Council of New Zealand (03/196), the Canterbury Medical Research Foundation (05/01), the Washington University Intellectual and Developmental Disabilities Research Center (NIH/NICHD P30 HD062171), and a New Zealand International Doctoral Research Scholarship (NZIDRS 2008).
Footnotes
Conflict of Interest statement: No conflicts declared.
The authors declare they do not have any potential or competing conflicts of interest.
References
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Abernethy LJ, Palaniappan M, Cooke RW. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Archives of Disease in Childhood. 2002;87(4):279–283. doi: 10.1136/adc.87.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Ball SW, Fried R, Doyle AE, Cohen D, et al. Are cognitive deficits in attention deficit/hyperactivity disorder related to the course of the disorder? A prospective controlled follow-up study of grown up boys with persistent and remitting course. Psychiatry Research. 2009;170(2-3):177–182. doi: 10.1016/j.psychres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Research. 2010;177(3):299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–1492. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Goodman R, Scott S. Comparing the Strengths and Difficulties Questionnaire and the Child Behavior Checklist: is small beautiful? Journal of Abnormal Child Psychology. 1999;27(1):17–24. doi: 10.1023/a:1022658222914. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Annals of Neurology. 1998;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Annals of Neurology. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. Journal of Pediatrics. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Indredavik MS, Skranes JS, Vik T, Heyerdahl S, Romundstad P, Myhr GE, et al. Low-birth-weight adolescents: psychiatric symptoms and cerebral MRI abnormalities. Pediatric Neurology. 2005;33(4):259–266. doi: 10.1016/j.pediatrneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Larroque B, Ancel PY, Marchand-Martin L, Cambonie G, Fresson J, Pierrat V, et al. Special care and school difficulties in 8-year-old very preterm children: the Epipage cohort study. PloS One. 2011;6(7):e21361. doi: 10.1371/journal.pone.0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai J, Anderson P, Bourne A. Comparing psychiatric diagnoses generated by the Strengths and Difficulties Questionnaire with diagnoses made by clinicians. Australian and New Zealand Journal of Psychiatry. 2004;38(8):639–643. doi: 10.1080/j.1440-1614.2004.01428.x. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, et al. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatric Research. 2003;54(5):672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Allin MP, Frangou S, Rifkin L, Murray RM. Hyperactivity in adolescents born very preterm is associated with decreased caudate volume. Biological Psychiatry. 2005;57(6):661–666. doi: 10.1016/j.biopsych.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5 Pt 1):939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, et al. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Shah DK, Guinane C, August P, Austin NC, Woodward LJ, Thompson DK, et al. Reduced occipital regional volumes at term predict impaired visual function in early childhood in very low birth weight infants. Investigative Ophthalmology & Visual Science. 2006;47(8):3366–3373. doi: 10.1167/iovs.05-0811. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(Pt 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Cheong J, Doyle LW, Roberts G, Lee KJ, Lim J, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Developmental Medicine and Child Neurology. 2011;53(11):1000–1006. doi: 10.1111/j.1469-8749.2011.04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics New Zealand. 2001 Census: Regional Summary. [May 2008];2001 Available from: www.stats.govt.nz/Census/2001-census-data.aspx.
- Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(Pt 3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Annals of Neurology. 2008;63(5):642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- Warfield SK, Kaus M, Jolesz FA, Kikinis R. Adaptive, template moderated, spatially varying statistical classification. Medical Image Analysis. 2000;4(1):43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Archives of General Psychiatry. 2011;68(7):742–752. doi: 10.1001/archgenpsychiatry.2011.62. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, et al. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Archives of General Psychiatry. 1997;54(9):847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PloS one. 2012;7(12):e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Anderson P, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128(Pt 11):2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]


