Abstract
Objective
Premutation and intermediate CGG repeat length at the Fragile X Mental Retardation (FMR1) locus has been associated with premature ovarian failure. We tested whether intermediate length is associated with indicators of ovarian age in a sample of fertile women. Our primary measures of ovarian age were anti-Müllerian hormone (AMH) and follicle stimulating hormone (FSH).
Methods
The cross-sectional sample comprised 258 women with karyotyped spontaneous abortions (140 trisomic, 118 chromosomally normal or with anomalies other than trisomy) and 325 women with recent live births (LBs). We analyzed data from the total sample and data from LBs only. We defined CGG repeat length by the length, both continuous and categorical, on the longer allele.
Results
CGG repeat length was not significantly associated with either hormone measure. A repeat length of 35–54 CGG, versus the modal category of 30 CGG, was associated with approximately a 7% increase in median AMH and a 3% increase in median FSH. Results were unaltered when analyses were limited to LBs. Analyses of hormone levels using cutpoints to define older ovarian age showed no associations with repeat length. Among ten women with repeat lengths of 35–54 CGG analyzed for AGG sequences, the uninterrupted CGG length was not significantly longer among women with hormonal indicators of “old” versus “young” ovarian age.
Conclusions
Our data do not support an association of intermediate CGG repeat length with levels of AMH or FSH among fertile women.
Keywords: Epidemiology, Fragile X, FMR1, ovarian age, AMH, FSH
INTRODUCTION
CGG repeat length at the FMR1 locus is related to premature ovarian failure (POF) and possibly to other manifestations of accelerated ovarian aging. FMR1, a gene on the X chromosome that underlies the Fragile X mental retardation syndrome, codes for a heterogeneous nuclear ribonucleoprotein, FMRP, that shuttles between the nucleus and the cytoplasm of neuronal cells. FMRP plays a subtle, but critical, role in the translation of mRNA. The 5' untranslated region of the FMR1 gene has a polymorphic CGG repeat. Individuals with length ≈ 55–200 are considered premutation carriers because repeats of this length have a propensity to expand to the full mutation in the subsequent generation1. Length between the high end of normal and premutation is considered “intermediate.” While current practice guidelines for fragile X testing in the US define intermediate length as 45–54 CGG1, definitions of intermediate length have included 35–60 CGG.
Premutation carriers are at increased risk of POF, usually defined as menopause before age 40 or two elevated levels of follicle stimulating hormone (FSH) one month apart. Several studies (reviewed in Wittenberger2, Sullivan3; Karimov4) show associations, among women treated for infertility, with other indicators of premature ovarian aging, including menopause before age 45, elevated FSH and decreased anti-Müllerian hormone (AMH). Most studies5–10 support an association of CGG length with POF; risk ratios range from 5.8 to infinity. Among POF women with normal karyotypes, 2–10% are premutation carriers7, 9–11 compared with <1% of the general population.
The most likely mechanism for an association between CGG length and POF is that FMR1 mRNA has a toxic gain of function, leading to accelerated follicular atresia and, consequently, a smaller follicular pool at any given age3, 12, 13. This mechanism may be relevant to associations of intermediate length with POF. In males with intermediate length (41–60 CGG), increased mRNA transcriptional activity was reported14, suggesting RNA “gain-of-function” toxicity even for larger normal alleles.
Some studies suggest that the association of length with POF is also present among women with length in the intermediate range: 41–588, 35–5415, 43–539 (reviewed in Kline16). Odds ratios (OR) range from 2.4–5.5. A study in England10 was interpreted to show no association with intermediate length. We disagree with this interpretation because cases with POF and controls were analyzed differently: each case contributed two chromosomes to the analysis, whereas each control contributed only one. The published data are not sufficiently detailed to limit the analysis to one chromosome per case to compare with one chromosome per control. However, assuming that cases contributed one intermediate-length allele each, we estimate ORs of 1.8 for length 35–54 and 2.6 for length 41–58.
If the association of intermediate length with POF is causal, we expect that intermediate length is also associated with indicators, specifically, low AMH and high FSH, of advanced ovarian age. AMH, which is expressed by the granulosa cells, is detectable in some primary and secondary follicles and in most preantral and small (<6 mm) antral follicles17, 18. FSH, a gonadotropin under negative feedback of inhibin B and estradiol19, 20, reflects the quantity or quality of the antral follicles; it may also provide an indirect measure of characteristics of the underlying oocyte pool21, 22. In a sample of 42 ovaries23, the age-adjusted correlation of ln(number of primordial follicles) with serum AMH was stronger than that the correlation with serum FSH, suggesting that AMH is the better indicator of the size of the oocyte pool. Data from two sites, analyzed together24, show no association of intermediate length (n=49), defined as 35–45 or 46–55, with decreased AMH.
We drew on data from fertile women, unselected for family history of Fragile X disorders, to test whether intermediate CGG length is associated with AMH or FSH. We also assessed associations with inhibin B and estradiol.
METHODS
The analyses draw on data from two studies (New York, New Jersey) designed to test whether indicators of ovarian age or possible causes of a decreased oocyte pool are associated with trisomic spontaneous abortion (SA). The design and protocols of the two studies are similar. Both samples include women with karyotyped SAs and women with chromosomally normal live births (LBs). Previous analyses indicate: (1) trisomic SA is associated with elevated FSH, but not with changes in AMH, inhibin B or estradiol25; (2) trisomic SA is unrelated to highly skewed X inactivation26; and (3) trisomic SA is unrelated to intermediate CGG length16.
New York (NY) study
The NY study (Kline25, 27), was designed to test the hypothesis that the oocyte pools of women with trisomic pregnancies are smaller than those of women with pregnancies of other types.
From 1998–2001, we ascertained a consecutive series of SAs at one hospital. We attempted to karyotype all singleton prefetal (developmental age < nine weeks) SAs to women 18+ years. If a woman’s loss was successfully karyotyped, we asked her to complete a short telephone interview to determine her eligibility for hormone studies. The principal exclusion criteria were hormonal contraceptive use, pregnancy (SAs) or breastfeeding (LBs). Blood was collected on day 1–4 of each woman’s second or later menstrual cycle.
Women with trisomic SAs constituted the case group. Women with non-trisomic chromosomally abnormal SAs and chromosomally normal SAs constituted two of the comparison groups; women with LBs constituted the third. For each case who completed the study, we selected an age-matched (±6 months) control with a chromosomally and anatomically normal LB ≥1800 grams during the 7–13 months prior to selection, no pregnancy loss since the index pregnancy and no known trisomic pregnancy. Table S1a (Supplemental Digital Content 1) sets out the number of women identified and their eligibility for the present analysis.
New Jersey (NJ) study
The NJ study (Warburton26) was designed to examine the relation of highly skewed X-chromosome inactivation to trisomy. We collected sera in anticipation of analyses to examine the relation of hormonal indicators of ovarian age to trisomy25.
From 2003–2005, we ascertained a consecutive series of SAs at one hospital. The NJ study differed from the NY study in the following ways: (i) it included women with singleton SAs <18 weeks (rather than <9 weeks) developmental age; (ii) it included women ineligible for hormone measures (hormones were irrelevant to the primary aim); (iii) age-matched women with LBs were selected for all women with SAs (rather than only for women with trisomic SAs); (iv) we drew blood on days 2–4 (rather than days 1–4); (v) in the event that a woman with an SA was eligible for hormone studies but her first LB control was not, we enrolled a second LB control who was eligible for hormone studies; (vi) LB controls delivered 6 12 months (rather than 7–13 months) preceding the date of their selection. Table S1b (Supplemental Digital Content 2) sets out the number of women identified and their eligibility for the present analysis.
Study of repeat structure
At the conclusion of the analyses reported here, we selected five matched pairs from the NJ study to examine the relation of the length of the longest uninterrupted CGG repeat to hormone level. (We selected women from the NJ study because we have more stored DNA from the NJ participants.) From among the 72 women with allele 2 intermediate repeat length (35–54), we selected cases with “older ovarian age”, the five with the lowest AMH (0.071–0.187 ng/ml). Each AMH value was < the 5th percentile for the woman’s five-year age group. Although not a selection criterion, all women had FSH levels in the upper 5th percentile for their five-year age groups.
From the remaining 67 women with allele 2 intermediate repeat length, we sought five matched controls with “younger ovarian age.” We eliminated potential controls with allele 1 of intermediate length (n=2) because no case had an allele 1 of intermediate length. We also eliminated women with AMH levels ≥ 7.08 ng/ml (n=5) to exclude possible undetected cases of polycystic ovary syndrome. Among the remaining 60 women, we defined as potential controls women with, for their five-year age group: (1) AMH ≥ the median and (2) FSH < the 75th percentile. Twenty-five women met these criteria for “younger ovarian age.” From these 25, we selected five controls matched as closely as possible to each case for allele 2 repeat length and age (Table 5, footnote).
Both studies
Each study was approved by the Institutional Review Boards of the study hospital and of our institution. Mean maternal age is younger for the NY sample (Table 1). The majority of women were white, non-Hispanic. The distribution of CGG length, classified in five categories based on the length of allele 2, did not differ between sites; 14.9% had intermediate-length repeats. The proportion of LBs differs between sites because we selected LB controls for all SAs in the NJ study but only for trisomic SAs in the NY study.
Table 1.
Selected characteristics of women in the analytic sample by site
| New York sample | New Jersey sample | |
|---|---|---|
| Number of women | 113 | 470 |
| Age (years) at the blood drawa (Mean (sd)) | 34.1 (5.9) | 35.4 (4.8) |
| Ethnicity: White, non-Hispanicb (%) | 94.7 | 87.0 |
| CGG repeat length at the FMR1 locus on allele 2c (%) | ||
| 20–24 | 3.5 | 6.0 |
| 25–29 | 9.7 | 8.7 |
| 30 | 46.0 | 42.3 |
| 31–34 | 27.4 | 27.7 |
| 35–54 | 13.3 | 15.3 |
| Outcome of the index pregnancy | ||
| Trisomic loss | 36 | 104 |
| Other loss | 27 | 91 |
| Live birthd | 50 | 275 |
Mean age is significantly younger (p=0.01) in the New York sample.
The proportion white, non-Hispanic is significantly higher (p=0.02) in the New York sample.
The distribution of CGG repeat length did not vary significantly (p=0.81) between sites.
The lower proportion of live birth controls in the New York sample reflects the differing study designs: in New York, live birth controls were selected for trisomy cases only; in New Jersey, live birth controls were selected for all women with losses.
Hormonal indicators of ovarian age
Blood samples were processed in a refrigerated centrifuge and, after separation, sera were frozen at° 25°C (NY) or 20°C (NJ) at the study hospital prior to delivery to New York City, where they were stored at 20°C. AMH and inhibin B were measured by enzyme-linked immunosorbent assays (DSL Inc., Webster, TX). FSH and estradiol were measured by solid-phase chemiluminescent enzyme immunoassays (Immulite) (Diagnostic Products Co, Los Angeles, CA). For AMH, sensitivity was 0.05 ng/ml; intra- and inter-assay coefficients of variation (CoV) were 2.3% and 8.9%, respectively. For FSH, sensitivity was 0.1 mIU/ml; intra- and inter-assay CoV were 1.9% and 5.0%. For inhibin B, sensitivity was 15 pg/ml; intra- and inter-assay CoV were 3.9% and 6.1%. For estradiol, sensitivity was 20 pg/ml; intra- and inter-assay CoV were 9.3% and 10.5%.
Among all women with hormone measures25, chronologic age is related inversely to ln(AMH), positively to ln(FSH) and ln(estradiol), and unrelated to ln(inhibin B). The correlation with estradiol is compatible with observations that levels may be elevated during the menopausal transition28, 29.
FMR1 CGG repeat length
We previously described our measures of CGG length16. Length was determined by PCR and capillary electrophoresis (CE)30 using prototype FMR1 PCR reagents obtained from Asuragen, Inc. Length was measured using Peak Scanner software after a comparison of PCR product length to a ladder of ROX-labeled size standards. All assay runs included a pooled mixture of five alleles ranging from 20 to 120 CGG repeats for which length had been previously verified by sequencing. We used these process controls to estimate length in the sample.
Randomly ordered samples were run in 30 batches. We estimated length from batch-specific linear regression equations relating mobility of peaks from the CE analysis to true length of the process controls. The computation yields a non-integer estimate of length; we rounded these values to the nearest integer for analysis. A second DNA sample from five matched pairs was submitted for analysis of the structure of the CGG repeat. We used three PCR assays to identify the presence and location of interrupting AGG sequences on each allele31. Uninterrupted CGG length was defined as the number of CGG repeats following the last AGG, if present, on that allele. All laboratory staff were blind to participant characteristics.
We defined CGG length three ways: length on allele 1, length on allele 2 and biallelic mean. In accordance with the predominant approach in the POF literature8, we consider length on allele 2 the primary measure.
Figure S1 (Supplemental Digital Content 3) shows the distribution of length for alleles 1 and 2. We analyzed each of the four measures as a categorical variable defined, approximately, by percentiles -- lower 5th, 5-<25th, 25-<75th, 75-<95th, upper 5th -- among LB controls. We defined the middle category as the reference group. The upper 5th percentile for allele 2 ranged from 35–54 repeats. (Analyses excluded six women with premutations.) For the biallelic mean, the lower bound of the upper 5th percentile was 35.5. Because one study32 suggests that FMRP expression is low for short alleles, we examined associations with short length on allele 1; the lower 5th percentile for allele 1 ranged from 9–19 repeats.
Statistical analyses
First, we used site-stratified multiple linear regression models to test the null hypothesis that intermediate CGG length is associated with any hormone. All analyses adjusted for karyotype group, age at blood draw, day of blood draw, duration of time in storage and smoking history (Table 3). We adjusted for these covariates because each was associated (p<0.05) independently with one or more hormones in at least one sample. We estimated the magnitude of the associations between each category of length and hormone and set 95% confidence intervals (CIs) around the estimates. We used a logarithmic transformation for each hormone to meet the normal error assumption of least squares regression. Thus, differences correspond approximately to percentage change in hormone level. To facilitate interpretation, we present the geometric means of hormone levels. The geometric mean corresponds to the median when the logarithmic hormone values have a symmetrical distribution, as they do in our data. The estimated percent change in median hormone level is equal to exp(difference) 1. We combined the evidence from the two sites, weighting the regression coefficients by the inverse of their squared standard errors. We repeated the analyses (1) limiting the sample to LBs and (2) limiting the sample to white, non-Hispanic women.
Second, we examined associations, for each site separately, of CGG length, defined by the length on allele 2 (35+ versus 20 34), with hormones defined categorically. For AMH and inhibin B, we dichotomized as closely as possible to the fifth percentile among LBs to define older ovarian age (AMH ≤0.186 ng/ml, Inhibin B ≤15 pg/ml). For FSH, we dichotomized at ≥10 mIU/ml, a level (for the Immulite assay) used by several studies33–35 to define older ovarian age. This level corresponds to approximately the top 5% of LBs. For estradiol, where either high or low levels might indicate older ovarian age, we used three categories: ≤20 pg/ml, the limit of detection (≈11th percentile), ≥85 pg/ml (95th percentile) and, as the reference group, >20-<85 pg/ml. Because the number of women with hormone levels indicating “older” ovarian age was small, we adjusted only for site to estimate ORs for the two sites combined (using the Mantel-Haenszel statistic). The majority of women with low AMH (32 of 38) or high FSH (37 of 40) were age 35+. We repeated the analyses limiting the sample to women age 35+. For FSH, which is elevated among women with trisomic losses, we repeated the analysis adjusting also for karyotype group and age (continuous).
RESULTS
Hormones continuous
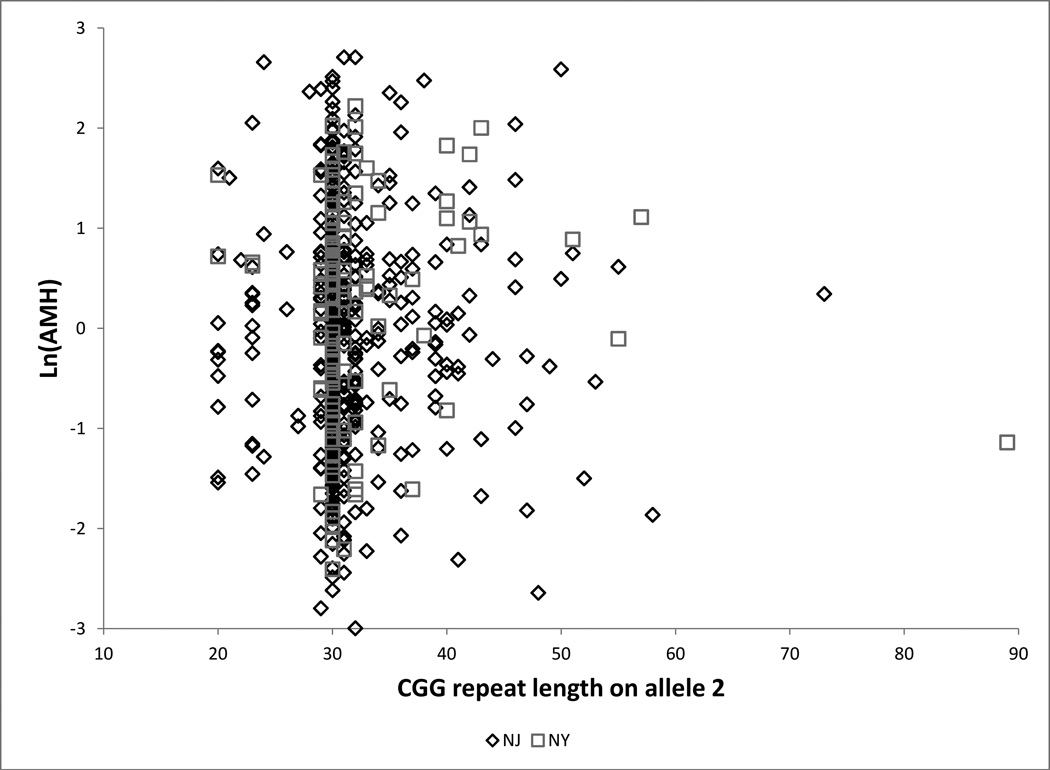
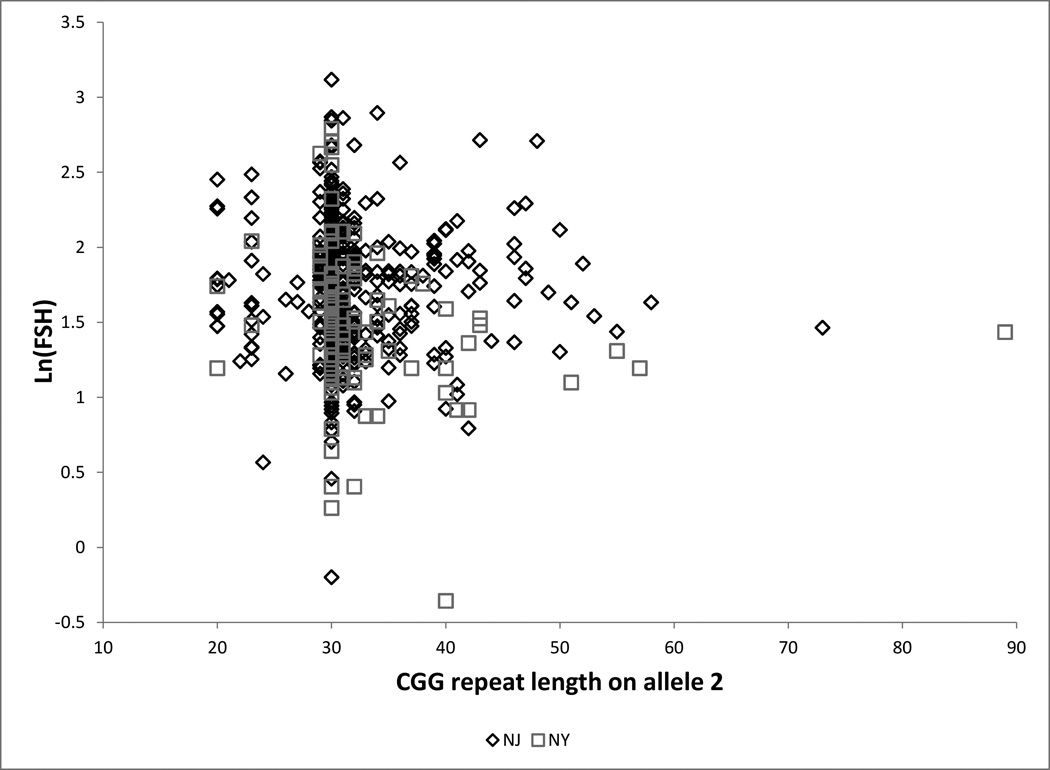
(Table 2, Table 3, Figure 1): Among women with allele 2 length 35–54 compared with women with length 30, estimated ln(AMH) is increased by 0.065 units in the total sample and decreased by 0.135 units among LBs. Length 35–54 is associated with an increase in ln(FSH) of 0.025 units (total sample) to 0.087 units (LBs). For the total sample, the point estimates correspond to an estimated change in the median of approximately 7% for AMH and 3% for FSH. All point estimates are compatible with zero. Length 35–54 is unrelated to inhibin B or estradiol. Results were unchanged when we limited analyses to white, non-Hispanic women (n=516): adjusted differences were 0.132 (95% CI −0.13,0.39) for ln(AMH) and 0.003 (95% CI −0.10,0.10) for ln(FSH) (data not shown).
Table 2.
Observed means for anti-Müllerian hormone (AMH), follicle stimulating hormone (FSH), inhibin B and estradiol by CGG repeat length at the FMR1 locus on allele 2, by site, for (1) the total sample and (2) women with live births (LBs)
| AMH (ng/ml) | FSH (mIU/ml) | Inhibin B (pg/ml) | Estradiol (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Length of repeat on allele 2 | Number | Ln (sd)a | Geometricb | Ln (sd)a | Geometricb | Ln (sd)a | Geometricb | Ln (sd)a | Geometricb |
| (1) New York-all women | |||||||||
| 20–24 | 4 | 0.88 (0.43) | 2.42 | 1.61 (0.36) | 5.02 | 3.77 (0.59) | 43.3 | 3.56 (0.25) | 35.1 |
| 25–29 | 11 | 0.05 (0.82) | 1.06 | 1.82 (0.35) | 6.17 | 3.55 (0.63) | 34.9 | 3.37 (0.27) | 29.1 |
| 30 | 52 | −0.08 (1.21) | 0.93 | 1.49 (0.53) | 4.44 | 3.39 (0.46) | 29.7 | 3.52 (0.43) | 34.0 |
| 31–34 | 31 | 0.24 (1.20) | 1.27 | 1.44 (0.39) | 4.23 | 3.65 (0.38) | 38.5 | 3.71 (0.50) | 40.7 |
| 35–54c | 15 | 0.62 (1.03) | 1.86 | 1.23 (0.52) | 3.42 | 3.75 (0.47) | 42.5 | 3.69 (0.50) | 40.0 |
| (1) New Jersey-all women | |||||||||
| 20–24 | 28 | 0.03 (1.08) | 1.03 | 1.74 (0.44) | 5.69 | 3.59 (0.36) | 36.2 | 3.59 (0.42) | 36.1 |
| 25–29 | 41 | 0.08 (1.30) | 1.09 | 1.77 (0.37) | 5.89 | 3.61 (0.43) | 36.9 | 3.64 (0.45) | 38.0 |
| 30 | 199 | −0.03 (1.10) | 0.97 | 1.70 (0.43) | 5.45 | 3.57 (0.49) | 35.6 | 3.77 (0.52) | 43.3 |
| 31–34 | 130 | −0.10 (1.13) | 0.91 | 1.71 (0.38) | 5.54 | 3.54 (0.46) | 34.6 | 3.70 (0.42) | 40.5 |
| 35–54d | 72 | 0.10 (1.13) | 1.10 | 1.71 (0.38) | 5.55 | 3.53 (0.47) | 34.2 | 3.64 (0.44) | 38.1 |
| (2) New York-women with LBs | |||||||||
| 20–24 | 2 | 1.09 (0.62) | 2.99 | 1.61 (0.18) | 5.01 | 3.64 (0.91) | 37.9 | 3.68 (0.35) | 39.8 |
| 25–29 | 6 | 0.21 (0.44) | 1.23 | 1.76 (0.28) | 5.79 | 3.70 (0.63) | 40.3 | 3.46 (0.31) | 31.9 |
| 30 | 21 | −0.24 (1.00) | 0.78 | 1.59 (0.45) | 4.88 | 3.48 (0.40) | 32.4 | 3.52 (0.45) | 33.7 |
| 31–34 | 14 | −0.06 (1.27) | 0.94 | 1.44 (0.32) | 4.22 | 3.68 (0.41) | 39.7 | 3.90 (0.55) | 49.1 |
| 35–54e | 7 | 0.20 (1.23) | 1.22 | 1.34 (0.32) | 3.81 | 3.68 (0.44) | 39.7 | 3.63 (0.40) | 37.7 |
| (2) New Jersey-women with LBs | |||||||||
| 20–24 | 19 | 0.20 (1.14) | 1.22 | 1.74 (0.35) | 5.73 | 3.71 (0.32) | 40.7 | 3.56 (0.46) | 35.2 |
| 25–29 | 26 | 0.41 (1.10) | 1.50 | 1.72 (0.32) | 5.56 | 3.70 (0.37) | 40.5 | 3.62 (0.50) | 37.3 |
| 30 | 116 | 0.10 (1.05) | 1.10 | 1.65 (0.42) | 5.20 | 3.52 (0.46) | 33.8 | 3.67 (0.56) | 39.2 |
| 31–34 | 78 | −0.15 (1.23) | 0.86 | 1.75 (0.40) | 5.76 | 3.57 (0.48) | 35.6 | 3.71 (0.45) | 40.9 |
| 35–54d | 36 | 0.12 (1.28) | 1.13 | 1.70 (0.42) | 5.49 | 3.52 (0.49) | 33.6 | 3.58 (0.41) | 36.0 |
To meet the normality assumption of least squares regression, we used natural logarithmic transformations for each hormone.
The geometric mean is approximately equal to the median. Values are reported in the original scale using the inverse transformation, i.e., exp(mean).
Longest repeat length is 51.
Longest repeat length is 53.
Longest repeat length is 43.
Table 3.
Adjusted mean differences for AMH, FSH, inhibin B, and estradiol by CGG repeat length at the FMR1 locus on allele 2 compared with length 30: (1) the total sample of 583; (2) 325 women with live births
| Adjusted differencea (95% CI) in mean ln(hormone) by repeat length (versus length 30) on allele 2 |
||||
|---|---|---|---|---|
| AMH (ng/ml) | FSH (mIU/ml) | Inhibin B (pg/ml) | Estradiol (pg/ml) | |
| (1) Total sample | ||||
| 20–24 | −0.050 (−0.426, 0.325) | 0.095 (−0.048, 0.238) | 0.064 (−0.106, 0.235) | −0.069 (−0.228, 0.090) |
| 25–29 | 0.021 (−0.280, 0.322) | 0.136 (0.019, 0.252)b | 0.066 (−0.072, 0.204) | −0.091 (−0.220, 0.038) |
| 30 | — | — | — | — |
| 31–34 | 0.041 (−0.158, 0.241) | 0.009 (−0.068, 0.086) | 0.037 (−0.054, 0.129) | −0.032 (−0.117, 0.054) |
| 35–54c | 0.065 (−0.185, 0.315)) | 0.025 (−0.071, 0.121) | 0.046 (−0.068, 0.159) | −0.059 (−0.166, 0.047) |
| (2) Women with live births | ||||
| 20–24 | −0.013 (−0.498, 0.473) | 0.113 (−0.060, 0.286) | 0.183 (−0.025, 0.390) | 0.006 (−0.203, 0.214) |
| 25–29 | 0.296 (−0.109, 0.702) | 0.072 (−0.073, 0.217) | 0.214 (0.040, 0.388)b | 0.042 (−0.133, 0.216) |
| 30 | — | — | — | — |
| 31–34 | −0.046 (−0.325, 0.234) | 0.038 (−0.062, 0.137) | 0.095 (−0.024, 0.215) | 0.063 (−0.057, 0.183) |
| 35–54c | −0.135 (−0.510, 0.241) | 0.087 (−0.047, 0.221) | 0.086 (−0.074, 0.246) | −0.051 (−0.212, 0.111) |
Data from each site were analyzed separately. Within each site, analyses adjust for karyotype group (trisomy, other loss versus live birth), age in single years (linear and quadratic), smoking (former, current versus never), day of blood draw (1–2 versus 3–4) and interval (continuous) between blood draw and assay. Age is associated with all four hormones; trisomy and current smoking are associated with elevated FSH; day of blood draw is associated with inhibin B and estradiol; increasing interval in storage is associated with FSH and estradiol. For the sites combined, the adjusted difference in mean ln(hormone) is a weighted average of the difference for each site, weighting the site-specific estimated regression coefficient by the inverse of its squared standard error.
95% confidence intervals exclude a difference of zero in ln(hormone). Given the number of comparisons carried out, we consider that these two associations likely reflect type 1 error.
In our sample, the longest CGG repeat length on allele 2 is 53.
Figure 1.
a Ln(AMH) by CGG repeat length at the FMR1 locus on allele 2 for each site
Caption: □ = New York (n = 116), ♢ = New Jersey (n = 473). Figure includes six women with premutations (repeat length ≥ 55)
b Ln(FSH) by CGG repeat length at the FMR1 locus on allele 2 for each site
Caption: □ = New York (n = 116), ♢ = New Jersey (n = 473). Figure includes six women with premutations (repeat length ≥ 55)
Similar analyses for the total sample show no significant associations between long biallelic mean or short allele 1 length and any hormone (data not shown). For short allele 1 length, in the total sample, adjusted differences were 0.162 (95% CI −0.28,0.60) for ln(AMH) and 0.077 (95% CI −0.09,0.25) for ln(FSH).
Hormones categorical
(Table 4): The proportion of women with hormone levels indicative of older ovarian age did not vary significantly with length. For the longest category versus the remainder, ORs (adjusted for site) were 0.7 for low AMH and 0.4 for high FSH. Results were essentially unchanged when we limited analyses to LBs. When we repeated the analyses among women age 35+ (n=315), site-adjusted ORs ratios relating the longest category to low AMH and high FSH were 0.5 (95% CI 0.1,2.2) and 0.4 (95% CI 0.1,1.8). For FSH (which is associated with trisomy), adjusting also for karyotype and age (linear); the adjusted OR relating length 35 54 to elevated FSH was 0.6 (95% CI 0.2,2.0).
Table 4.
Proportion with low AMH (≤ 0.186 ng/ml), high FSH (≥ 10 mIU/ml), low inhibin B (≤ 15 pg/ml), low estradiol (≤ 20 pg/ml), and high estradiol (≥ 85 pg/ml) among women with CGG repeat length at the FMR1 locus of 35+ versus <35, and odds ratios relating repeat length to hormone level, for the total sample and for women with live births (LBs): (1) the New York sample, (2) the New Jersey sample, (3) the samples combined.
| Allele 2 20–34 | Allele 2 35–54 | Odds ratio (95% CIa) | |
|---|---|---|---|
| (1) New York-all women (N) | 98 | 15 | |
| Low AMH | 7.1 | 0.0 | 0.0 (0.0, 4.0) |
| High FSH | 6.1 | 0.0 | 0.0 (0.0, 5.1) |
| Low inhibin B | 10.2 | 0.0 | 0.0 (0.0, 3.0) |
| Low estradiol | 9.2 | 0.0 | 0.0 (0.0, 2.9) |
| High estradiol | 5.1 | 6.7 | 1.2 (0.0, 10.4) |
| (2) New Jersey-all women (N) | 398 | 72 | |
| Low AMH | 6.8 | 5.6 | 0.8 (0.3, 2.4) |
| High FSH | 7.8 | 4.2 | 0.7 (0.2, 2.0) |
| Low inhibin B | 4.5 | 4.2 | 0.9 (0.2, 3.3) |
| Low estradiol | 11.3 | 11.1 | 1.0 (0.4, 2.2) |
| High estradiol | 5.0 | 5.6 | 1.1 (0.3, 3.3) |
| (3) Both sites-all womenb,c | |||
| Low AMH | 0.7 (0.2, 1.8) | ||
| High FSH | 0.4 (0.1, 1.4) | ||
| Low inhibin B | 0.6 (0.2, 1.9) | ||
| Low estradiol | 0.8 (0.3, 1.8) | ||
| High estradiol | 1.1 (0.4, 3.0) |
| Allele 2 20–34 | Allele 2 35–54 | Odds ratio (95% CI) | |
|---|---|---|---|
| (1) New York-women with LBs (N) | 43 | 7 | |
| Low AMH | 2.3 | 0.0 | 0.0 (0.0, 116.7) |
| High FSH | 4.7 | 0.0 | 0.0 (0.0, 22.3) |
| Low inhibin B | 4.7 | 0.0 | 0.0 (0.0, 22.3) |
| Low estradiol | 7.0 | 0.0 | 0.0 (0.0, 10.4) |
| High estradiol | 7.0 | 0.0 | 0.0 (0.0, 10.4) |
| (2) New Jersey-women with LBs (N) | 239 | 36 | |
| Low AMH | 4.6 | 5.6 | 1.2 (0.2, 5.5) |
| High FSH | 6.3 | 5.6 | 0.9 (0.1, 4.0) |
| Low inhibin B | 5.4 | 8.3 | 1.6 (0.4, 6.1) |
| Low estradiol | 13.4 | 11.1 | 0.8 (0.3, 2.4) |
| High estradiol | 5.4 | 5.6 | 1.0 (0.2, 4.2) |
| (3) Both sites-women with LBs (N)c | 282 | 43 | |
| Low AMH | 4.3 | 4.7 | 1.1 (0.2, 4.7) |
| High FSH | 6.0 | 4.7 | 0.8 (0.1, 3.3) |
| Low inhibin B | 5.3 | 7.0 | 1.3 (0.3, 4.7) |
| Low estradiol | 12.4 | 9.3 | 0.7 (0.2, 2.2) |
| High estradiol | 5.7 | 4.7 | 0.8 (0.1, 3.5) |
CI = confidence interval.
We do not sum the samples because (a) the ratio of trisomy cases to live birth controls differs between the sites and (b) trisomy is associated with elevated FSH.
Odds ratio from a Mantel-Haenszel analysis that stratified by site.
Repeat structure
(Table 5): The CGG repeat was interrupted by AGG repeats in all instances; only one woman, a control, showed an uninterrupted repeat in the intermediate range. The mean length of the longest uninterrupted CGG repeat was significantly longer among women with younger ovarian age than among women with older ovarian age.
Table 5.
Genotype and length of the longest uninterrupted CGG repeat in women with hormonal indicators of older ovarian age (case)a and younger ovarian age (control) in the New Jersey sample
| Pair | Case/ Control |
Repeat number |
Genotype | Uninterrupted CGG length |
Difference (case – control) in uninterrupted length |
|
|---|---|---|---|---|---|---|
| 1 | Case | 48 | 9CGG+AGG+9CGG+AGG+28CGG | 28 | 2 | |
| Control | 46 | 9CGG+AGG+9CGG+26CGG | 26 | |||
| 2 | Case | 41 | 9CGG+AGG+9CGG+AGG+11CGG+AGG+9CGG | 11 | –11 | |
| Control | 42 | 9CGG+AGG+9CGG+AGG+22CGG | 22 | |||
| 3 | Case | 36 | 9CGG+AGG+9CGG+AGG+16CGG | 16 | –9 | |
| Control | 36 | 10CGG+AGG+25CGG | 25 | |||
| 4 | Case | 47 | 9CGG+AGG+9CGG+AGG+27CGG | 27 | –13 | |
| Control | 51 | 10CGG+AGG+40CGG | 40 | |||
| 5 | Case | 43 | 9CGG+AGG+10CGG+AGG+12CGG+AGG+9CGG | 12 | –18 | |
| Control | 40 | 9CGG+AGG+30CGG | 30 | |||
| Mean (95% CI) | –9.8 (–19.0, –0.62) | |||||
Cases are the five women with allele 2 CGG repeat length 35–54 with the lowest AMH levels. AMH level ranged from 0.071 ng/ml to 0.187 ng/ml; FSH levels ranged from 8.8 mIU/ml to 15.1 mIU/ml. Controls are women with allele 2 repeat length 35–54 and younger ovarian age. We defined younger ovarian age by (1) AMH level in the 50th−<95th percentile for the five-year age group and (2) FSH level <75th percentile for the five-year age group. Controls were matched to cases as closely as possible for age and repeat length. Among the five matched pairs, the case-control differences range from: years −1.6 to 2.3; allele 2 repeat length −4 to zero.
DISCUSSION
Our data do not support an association of intermediate CGG repeat length at the FMR1 locus with two indicators of ovarian aging. For allele 2 length 35–54, estimated changes in median AMH and FSH are approximately 7% and 3%. Our data rule out decreases in median AMH of 17+% and increases in median FSH of 13+%. When we categorized hormone level, intermediate length was unrelated to either low AMH (OR=0.7) or high FSH (OR=0.4). Our data rule out associations of ≥ 1.8-fold for AMH and ≥ 1.4-fold for FSH. Results were essentially unchanged when we: limited analyses to LBs; limited analyses to white, non-Hispanic women; or defined length by the biallelic mean. Statistical power (0.80, α=0.05, two-tailed) was sufficient to detect a 30% decrease in median AMH and a 15% increase in median FSH. For AMH and FSH defined categorically, detectable ORs are 3.2 and 3.0.
Short (9–19) length on allele 1 is unrelated to either hormone. Decreases in median AMH of 24+% are unlikely.
We examined the structure of the CGG repeat to test whether or not our null result derives from misclassification, i.e., our measure of CGG length did not take account of AGG interruptions. Contrary to our hypothesis, cases (older ovarian age) did not show longer uninterrupted CGG length. On the contrary, the uninterrupted repeat was significantly longer among controls -- (probably because of type I error). In all instances, the repeat was interrupted by AGG sequences, a result consistent with a previous observation31.
The strengths of our study include: excellent assay validity and reliability; laboratory technicians blind to all characteristics of the woman; random ordering of samples to prevent potential confounding by assay batch; a sample unselected for history of Fragile X syndrome; face validity.
The unselected sample allows us to generalize our findings to most women rather than only to women from families in which the premutation has demonstrated the capacity to expand -- important because one study36 indicates that the risk of expansion for length 55 59 to the full mutation in a single generation is lower for unselected women than for women from Fragile X families. On the other hand, because our sample over-represents SAs, findings might not be generalizable to all fertile women. To address this issue, we repeated all analyses limiting the sample to LBs. The findings for LBs are consistent with the findings for the total sample.
With respect to face validity, our rates of premutation are comparable to those observed in other samples37–43 of women unselected for a family history of Fragile X syndrome or developmental problems. Our rate of repeat length >40 is similar to the rate observed in a survey8 of 2,781 unselected women8. In contrast, two studies9, 10 of POF which defined intermediate length as 41–58 report lower rates among controls.
For intermediate (35–54) length, our sample is larger than all but one reported series. Our rate of intermediate length (14.8%) is higher than those observed in most other studies. In a study10 of POF, the untransmitted X was analyzed among 2,779 women screened because their sons had learning difficulties; 8.0% had intermediate length. Among smaller samples of fertile or unselected controls from one other study15 of POF, two studies44, 45 of occult POF among infertile women and one study42 of an unselected sample, rates of intermediate length range from 6.2%42 to 13.0%15 (our computations). (All studies except one10, which analyzed only one chromosome per control, report rates for alleles rather than for women. To estimate the above rates, we assumed that no woman had two alleles with intermediate length.)
Disagreement between studies may reflect ethnic variation, small sample size or different assays. Our data suggest that the proportion of women with intermediate length among a predominantly white non-Hispanic sample is higher than most prior studies report.
To our knowledge, our study is the first to assess the association between intermediate length and FSH in a sample unselected for infertility. With respect to AMH, our data are consistent with the one other study24 in which women were not selected for infertility. In infertility treatment settings, several studies raise the possibility that intermediate length is associated with occult POF, for which elevated FSH is among the criteria. These data are difficult to interpret: (1) elevated basal FSH is only one of several criteria defining occult POF4, 44, 46; (2) small samples limit statistical power45; (3) samples referred for FMR1 testing are vulnerable to potential selection bias44.
We anticipated that AMH, because of its stronger correlation with the size of the primordial pool23, would measure ovarian age better than would FSH. The upper 95% confidence limits for low AMH and high FSH were 1.8 and 1.4. We thus reject the hypothesis that risk ratios for AMH or FSH are in the range (1.8–5.5 (our computations)) reported for intermediate length in relation to POF. We consider four explanations.
Observations linking intermediate length to POF are in error. We note two methodologic issues of concern. (a) Except for one study15, rates of intermediate (35–54) length among controls from studies of POF10 or occult POF44, 45 are about half the rate observed in our sample. While these variations may reflect true differences among samples, they may also reflect differences in assays. If the latter, the potential for misclassification limits inference. (b) It is unclear whether or not samples from cases and controls were assayed in the same batches. Our study, which randomly ordered samples for assay, eliminates the potential for confounding by technical differences between batches.
A one-time hormone measure may be insufficient to estimate ovarian age. Evidence of cycle-to-cycle variability stems mainly from studies of infertile women. No study of women unselected for infertility uses the interclass correlation coefficient to evaluate variability, hindering their interpretability. For AMH, one study47 shows similar means for two cycles. For follicular phase FSH, two studies47, 48 show similar means for two cycles; two others49, 50 indicate that variability may be greater for older women than for younger women. Three series support the use of a single measure of AMH51–53 or FSH51, 54 to predict time to menopause. These findings derive mainly from women aged 35–40 years at the beginning of follow-up; implications for measures during the reproductive years are unknown. There is no reason to think that measurement error in hormone measures is related to CGG length. Nonetheless, since non-differential measurement error attenuates associations, it is possible that we underestimated associations.
Processes leading to accelerated ovarian aging are different from processes which lead to POF; that is, POF does not represent the tail of the distribution of rates of ovarian aging. If so, since most women aged 15–40 are fertile, our findings indicate that for most women CGG length is not associated with accelerated ovarian aging.
The attributable risk for intermediate CGG length as a cause of POF is too low to produce detectable associations with hormone levels. Among 45 women with length 41–588, only one (2.2%) had POF. This observation, coupled with our results, suggests that few women with intermediate-length repeats experience detrimental effects on the ovary. The toxicity of intermediate-length repeats may be determined by characteristics such as the length of the 3' pure CGG repeat and the number of AGG interspersions55, 56. Among women with length 41–589, the CGG repeat was uninterrupted in nine POF cases but interrupted with AGG repeats in two controls. In contrast, in our sample the CGG repeat was interrupted in all ten women studied.
CONCLUSIONS
The majority of women with intermediate CGG repeat length are not at increased risk of accelerated ovarian aging. The discordance of this finding from findings related to POF suggests that the structure of the repeat determines its potential to affect the ovary. We favor efforts to determine whether the association between intermediate length on the FMR1 gene and POF is limited to repeats with particular structures or patterns.
Supplementary Material
Fig. S1 Distribution of CGG repeat length at the FMR1 locus
Caption:  = Allele 1,
= Allele 1,  = Allele 2. Figure includes six women with premutations (repeat length ≥ 55)
= Allele 2. Figure includes six women with premutations (repeat length ≥ 55)
Fig. S1 Data Frequency of CGG repeat lengths for alleles 1 and 2 among 589 women
Acknowledgments
We thank Dr. Amalia Kelly who collaborated in the design of both studies; Dr. Michel Ferin and colleagues who carried out the hormone assays; Karen Oppenheimer who collaborated in the design of the CGG repeat length laboratory analyses, carried out the assays and collaborated in their interpretation; Judy Chih-Yu who prepared the DNA. We thank Gary Latham at Asuragen, Inc. for providing the reagents for this study and Stela Filipovic-Sadic and Eliot Blatt for performing the analyses related to repeat structure.
For the New York study, we thank Dr. Grace Jorgensen and her colleagues for their help in providing access to their patients; Dr. Lynne Reuss, co-investigator; Maria Bautista, Jennifer Cassin, Terry Fox, the late Kris Keough, and Donna West, who facilitated our work at the study hospital; Megan Meldrum who carried out the fieldwork of the study; Renee Davenport who assisted in data processing and checking; Antonio Sobrino, who prepared the samples for karyotyping.
For the New Jersey study, we thank Dr. Martin Hochberg and his colleagues for providing access to their patients. We especially thank Dr. Arthur Christiano in Pathology, who facilitated our work and advised on diagnostic issues. We thank Larry Bologna, Denise Campbell, Gina Chavez, Lois Deyo, Cheryl Dulaff, Diane Gerardi, Nancy Librera, Deborah Manente, Mary Reiner, Louis Rizzo, Donna Rochette and Marriett Trentacoste, who facilitated our work at the study hospital; Richard Buchsbaum, whose programming and data management expertise facilitated both the day-to-day fieldwork and the statistical analysis; Project Director L. Perry Brothers, Fieldworkers Melissa Bielecki, Kathleen Carstens and Beth Fishner, and Renee Davenport, who assisted in tasks too countless to list.
Neither study would have been possible without the help of the women who participated to further understanding of the causes of reproductive loss.
Funding: Data collection for the New York study was supported by a grant (R01 AG 15386) from the National Institutes on Aging. Data collection for the New Jersey study was supported by a grant (R01 HD 42725) from the National Institutes on Child Health and Development. The work for this paper, including the CGG repeat length and hormone assays, was supported by a grant (R01 HD 053814-01A2) from the National Institutes on Child Health and Human Development. Funding for the re-agents for the assay was supported in part by a grant (R43 HD 060450) to Asuragen, Inc. from the National Institutes on Child Health and Human Development.
Footnotes
Conflict of interest: None
Authors’ roles
J.K. designed the study and analysis and wrote the manuscript. A.K. collaborated in the design of the study and analysis, carried out the statistical programming and helped write the manuscript. B.L. collaborated in the design of the study and the analysis and helped write the manuscript. S.B. collaborated in the design of the laboratory analyses and assessment of validity and reliability, oversaw the laboratory analyses for CGG repeat length and helped write the manuscript. A.H. collaborated in the application of laboratory methods for assessing repeat length, the assessment of validity and reliability of the assay, oversaw the analyses related to the structure of the repeat and helped write the manuscript. D.W. collaborated in the design of the study, oversaw the laboratory that karyotyped spontaneous abortion specimens and helped write the manuscript.
List of Supplemental Digital Content
Supplemental Digital Content 1.pdf
Supplemental Digital Content 2.pdf
Supplemental Digital Content 3.tif
Supplemental Digital Content 4.pdf
References
- 1.Monaghan KG, Lyon E, Spector EB. ACMG Standards and Guidelines for fragile X testing: a revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med. 2013;15(7):575–586. doi: 10.1038/gim.2013.61. [DOI] [PubMed] [Google Scholar]
- 2.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29(4):299–307. doi: 10.1055/s-0031-1280915. [DOI] [PubMed] [Google Scholar]
- 4.Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, Ginsburg E, Thornton KL, Welt CK. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum Reprod. 2011;26(8):2077–2083. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- 5.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, Jenkins EC, Brown WT, Howard-Peebles PN, Becchi C, Cummings E, Fallon L, Seitz S, Black SH, Vianna-Morgante AM, Costa SS, Otto PA, Mingroni-Netto RC, Murray A, Webb J, Vieri F, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study- -preliminary data. Am J Med Genet. 1999;83(4):322–325. [PMC free article] [PubMed] [Google Scholar]
- 6.Hundscheid RD, Smits AP, Thomas CM, Kiemeney LA, Braat DD. Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet A. 2003;117A(1):6–9. doi: 10.1002/ajmg.a.10862. [DOI] [PubMed] [Google Scholar]
- 7.Bussani C, Papi L, Sestini R, Baldinotti F, Bucciantini S, Bruni V, Scarselli G. Premature ovarian failure and fragile X premutation: a study on 45 women. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):189–191. doi: 10.1016/j.ejogrb.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 9.Bodega B, Bione S, Dalpra L, Toniolo D, Ornaghi F, Vegetti W, Ginelli E, Marozzi A. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21(4):952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25(5):1335–1338. doi: 10.1093/humrep/deq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13(5):1184–1187. doi: 10.1093/humrep/13.5.1184. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GE, Le WW, Entezam A, Otsuka N, Tong ZB, Nelson L, Flaws JA, McDonald JH, Jafar S, Usdin K. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J Histochem Cytochem. 2012;60(6):439–456. doi: 10.1369/0022155412441002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21(23):5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007;44(3):200–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117(4):376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 16.Kline J, Kinney A, Brown S, Levin B, Oppenheimer K, Warburton D. Trisomic pregnancy and intermediate CGG repeat length at the FMR1 locus. Hum Reprod. 2012;27(7):2224–2232. doi: 10.1093/humrep/des098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 18.Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S. Anti-Mullerian Hormone (AMH) protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90(10):5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- 19.Burger HG. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition--an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol. 1994;130(1):38–42. doi: 10.1530/eje.0.1300038. [DOI] [PubMed] [Google Scholar]
- 20.Burger HG. Inhibin and reproductive aging. Exp Gerontol. 2000;35(1):33–39. doi: 10.1016/s0531-5565(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg RL, Grodin JM, Rodbard D, Ross GT. Gonadotropins in women with amenorrhea. The use of plasma follicle-stimulating hormone to differentiate women with and without ovarian follicles. Am J Obstet Gynecol. 1973;116(7):1003–1012. doi: 10.1016/s0002-9378(16)33850-9. [DOI] [PubMed] [Google Scholar]
- 22.Klein NA, Harper AJ, Houmard BS, Sluss PM, Soules MR. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J Clin Endocrinol Metab. 2002;87(12):5746–5750. doi: 10.1210/jc.2002-020622. [DOI] [PubMed] [Google Scholar]
- 23.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2010;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, van Kessel AG, Braat DD, Sherman SL, Thomas CM. Intra-individual stability over time of standardized anti-Mullerian hormone in FMR1 premutation carriers. Hum Reprod. 2011;26(8):2185–2191. doi: 10.1093/humrep/der146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kline JK, Kinney AM, Levin B, Kelly AC, Ferin M, Warburton D. Trisomic pregnancy and elevated FSH: implications for the oocyte pool hypothesis. Hum Reprod. 2011;26(6):1537–1550. doi: 10.1093/humrep/der091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warburton D, Kline J, Kinney A, Yu CY, Levin B, Brown S. Skewed X chromosome inactivation and trisomic spontaneous abortion: no association. Am J Hum Genet. 2009;85(2):179–193. doi: 10.1016/j.ajhg.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M, Warburton D. Trisomic pregnancy and the oocyte pool. Hum Reprod. 2004;19(7):1633–1643. doi: 10.1093/humrep/deh310. [DOI] [PubMed] [Google Scholar]
- 28.Klein NA, Battaglia DE, Miller PB, Branigan EF, Giudice LC, Soules MR. Ovarian follicular development and the follicular fluid hormones and growth factors in normal women of advanced reproductive age. J Clin Endocrinol Metab. 1996;81(5):1946–1951. doi: 10.1210/jcem.81.5.8626862. [DOI] [PubMed] [Google Scholar]
- 29.Klein NA, Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremner WJ, Soules MR. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clin Endocrinol Metab. 2000;85(12):4520–4525. doi: 10.1210/jcem.85.12.7056. [DOI] [PubMed] [Google Scholar]
- 30.Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, Hagerman PJ, Stenzel TT, Hadd AG, Latham GJ, Tassone F. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolin SL, Sah S, Glicksman A, Sherman SL, Allen E, Berry-Kravis E, Tassone F, Yrigollen C, Cronister A, Jodah M, Ersalesi N, Dobkin C, Brown WT, Shroff R, Latham GJ, Hadd AG. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. Am J Med Genet A. 2013;161(4):771–778. doi: 10.1002/ajmg.a.35833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5' untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12(23):3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- 33.Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril. 1999;71(4):715–718. doi: 10.1016/s0015-0282(98)00525-1. [DOI] [PubMed] [Google Scholar]
- 34.Trout SW, Seifer DB. Do women with unexplained recurrent pregnancy loss have higher day 3 serum FSH and estradiol values? Fertil Steril. 2000;74(2):335–337. doi: 10.1016/s0015-0282(00)00625-7. [DOI] [PubMed] [Google Scholar]
- 35.Gurbuz B, Yalti S, Ozden S, Ficicioglu C. High basal estradiol level and FSH/LH ratio in unexplained recurrent pregnancy loss. Arch Gynecol Obstet. 2004;270(1):37–39. doi: 10.1007/s00404-003-0490-0. [DOI] [PubMed] [Google Scholar]
- 36.Nolin SL, Glicksman A, Ding X, Ersalesi N, Brown WT, Sherman SL, Dobkin C. Fragile X analysis of 1112 prenatal samples from 1991 to 2010. Prenat Diagn. 2011;31(10):925–931. doi: 10.1002/pd.2815. [DOI] [PubMed] [Google Scholar]
- 37.Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57(5):1006–1018. [PMC free article] [PubMed] [Google Scholar]
- 38.Spence WC, Black SH, Fallon L, Maddalena A, Cummings E, Menapace-Drew G, Bick DP, Levinson G, Schulman JD, Howard-Peebles PN. Molecular fragile X screening in normal populations. Am J Med Genet. 1996;64(1):181–183. doi: 10.1002/(SICI)1096-8628(19960712)64:1<181::AID-AJMG31>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Toledano-Alhadef H, Basel-Vanagaite L, Magal N, Davidov B, Ehrlich S, Drasinover V, Taub E, Halpern GJ, Ginott N, Shohat M. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69(2):351–360. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G. Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn. 2007;27(11):991–994. doi: 10.1002/pd.1815. [DOI] [PubMed] [Google Scholar]
- 41.Cronister A, Teicher J, Rohlfs EM, Donnenfeld A, Hallam S. Prevalence and instability of fragile X alleles: implications for offering fragile X prenatal diagnosis. Obstet Gynecol. 2008;111(3):596–601. doi: 10.1097/AOG.0b013e318163be0b. [DOI] [PubMed] [Google Scholar]
- 42.Otsuka S, Sakamoto Y, Siomi H, Itakura M, Yamamoto K, Matumoto H, Sasaki T, Kato N, Nanba E. Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain Dev. 2010;32(2):110–114. doi: 10.1016/j.braindev.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):589–597. doi: 10.1002/ajmg.b.32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92(2):464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, Perez C, Criado B, Arrieta I. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene. 2013;521(1):145–149. doi: 10.1016/j.gene.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 46.Pastore LM, Young SL, Baker VL, Karns LB, Williams CD, Silverman LM. Elevated prevalence of 35–44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci. 2012;19(11):1226–1231. doi: 10.1177/1933719112446074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Hansen LM, Batzer FR, Gutmann JN, Corson SL, Kelly MP, Gocial B. Evaluating ovarian reserve: follicle stimulating hormone and oestradiol variability during cycle days 2–5. Hum Reprod. 1996;11(3):486–489. doi: 10.1093/humrep/11.3.486. [DOI] [PubMed] [Google Scholar]
- 49.Brown JR, Liu HC, Sewitch KF, Rosenwaks Z, Berkeley AS. Variability of day 3 follicle-stimulating hormone levels in eumenorrheic women. J Reprod Med. 1995;40(9):620–624. [PubMed] [Google Scholar]
- 50.Jain T, Klein NA, Lee DM, Sluss PM, Soules MR. Endocrine assessment of relative reproductive age in normal eumenorrheic younger and older women across multiple cycles. Am J Obstet Gynecol. 2003;189(4):1080–1084. doi: 10.1067/s0002-9378(03)00583-0. [DOI] [PubMed] [Google Scholar]
- 51.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC, Broekmans FJ. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 52.Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimullerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril. 2012;98(5):1254–1259. e1–e2. doi: 10.1016/j.fertnstert.2012.07.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab. 2013;98(2):729–735. doi: 10.1210/jc.2012-3176. [DOI] [PubMed] [Google Scholar]
- 54.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napierala M, Michalowski D, de Mezer M, Krzyzosiak WJ. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res. 2005;33(2):451–463. doi: 10.1093/nar/gki186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zumwalt M, Ludwig A, Hagerman PJ, Dieckmann T. Secondary structure and dynamics of the RCGG) repeat in the mRNA of the fragile X mental retardation 1 (FMR1) gene. RNA Biol. 2007;4(2):93–100. doi: 10.4161/rna.4.2.5039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Distribution of CGG repeat length at the FMR1 locus
Caption:  = Allele 1,
= Allele 1,  = Allele 2. Figure includes six women with premutations (repeat length ≥ 55)
= Allele 2. Figure includes six women with premutations (repeat length ≥ 55)
Fig. S1 Data Frequency of CGG repeat lengths for alleles 1 and 2 among 589 women