Abstract
Reactive astrogliosis, characterized by cellular hypertrophy and various alterations in gene expressionand proliferative phenotypes, is considered to contribute to brain injuries and diseases as diverse as trauma, neurodegeneration, and ischemia. KCa3.1, a potassium channel protein, has been reported to be up-regulated in reactive astrocytes after spinal cord injury in vivo. However, little is known regarding the exact role of KCa3.1 in reactive astrogliosis. To elucidate the role of KCa3.1 in regulating reactive astrogliosis, we investigated the effects of either blocking or knockout of KCa3.1 channels on the production of astrogliosis and astrocytic proliferation in response to transforming growth factor (TGF)-β in primary cultures of mouse astrocytes. We found that TGF-β increased KCa3.1 protein expression in astrocytes, with a concomitant marked increase in the expression of reactive astrogliosis, including glial fibrillary acidic protein (GFAP) and chondroitin sulfate proteoglycans (CSPGs). These changes were significantly attenuated by the KCa3.1 inhibitor TRAM-34. Similarly, the increase in GFAP and CSPGs in response to TGF-β was attenuated in KCa3.1−/− astrocytes. TRAM-34 also suppressed astrocytic proliferation. Additionally, the TGF-β-induced phosphorylation of Smad2 and Smad3 proteins was reduced with either inhibition of KCa3.1 with TRAM-34 or in KCa3.1−/− astrocytes. These findings highlight a novel role for the KCa3.1 channel in reactive astrogliosis phenotypic modulation and provide a potential target for therapeutic intervention for brain injuries.
Keywords: astrocyte, GFAP, chondroitin sulfate proteoglycans, mouse, culture
Astrocytes, the predominant glial cell population in the mammalian central nervous system (CNS), play essential roles not only in physiological functions, such as synaptic plasticity (Dallerac et al. 2013) and hemodynamic responses (Petzold and Murthy 2011),but also in pathophysiological conditions. In response to brain injuries and diseases as diverse as trauma, neurodegeneration, and ischemia, astrocytes undergo characteristic phenotypic modulation known as reactive astrogliosis (Silver and Miller 2004, Sofroniew 2009). Gliosis normally involves cellular hypertrophy and various alterations in gene expression and can include astrocyte proliferation after particularly severe insults (Sofroniew 2005). Glial fibrillary acidic protein (GFAP) expression by astrocytes is a prototypic marker of reactive astrogliosis (Bignami and Dahl 1974, Bignami et al. 1972) and a characteristic response to inflammation after CNS injury. In addition, reactive astrogliosis generates increased expression of extracellular matrix (ECM) molecules, including chondroitin sulfate proteoglycans (CSPGs), a class of glycol-conjugates (McKeon et al., 1999). CSPG overexpression is linked to glial scar formation, which impedes axonal regeneration and outgrowth (Fitch and Silver 1997, Snow et al. 1990). Despite the importance of this process, the molecular mechanisms governing reactive astrogliosis and the role of reactive astrocytes require further clarification.
The intermediate-conductance calcium-activated potassium channel, composed of four KCa3.1 subunits and 4 calmodulin molecules, is expressed in T cells, macrophages, mast cells, epithelium, fibroblasts, and both normal and asthmatic human airway smooth muscle cells (Toyama et al. 2008, Yu et al. 2013b), where they can communicate directly between Ca2+ signaling pathways and changes in membrane potential required for various cellular processes, such as activation, proliferation, and migration (Yu et al. 2013a). Small molecules and peptide toxins such as triarylmethanes (TRAM-34) have been explored as specific selective KCa3.1 blockers. They inhibit airway smooth muscle cell proliferation, fibrocyte migration, macrophage function and T cell activation (Huang et al. 2013, Di et al. 2010). KCa3.1 is a potential molecular target for pharmacological intervention in vascular restenosis, asthma, prostate cancer, and autoimmune disease (Toyama et al. 2008, Bradding and Wulff 2009).
Recently, Bouhy et al. (2011) have reported that KCa3.1 was up-regulated at the mRNA and protein levels after spinal cord injury (SCI), and reactive astrocytes were the main cell type with increased KCa3.1. Furthermore, blockade of KCa3.1 reduced tissue and axonal loss, and improved neuronal survival and locomotor recovery (Bouhy et al. 2011). KCa3.1 blockers also decreased astrogliosis in the brains of glioblastoma multiforme-xenografted mice (D’Alessandro et al. 2013). We thus hypothesized that KCa3.1 might be involved in regulating reactive astrogliosis.
Transforming growth factor (TGF)-β is rapidly up-regulated after CNS injury in vivo and is important both as a soluble regulator of ECM formation and in inducing reactive astrogliosis (Logan et al. 1992, Logan et al. 1994, Wang et al. 2008). Emerging evidence has shown that the primary signaling pathway mediated by TGF-β is the Smad pathway (Derynck and Zhang 2003). TGF-β binds to a heteromeric TGF-β receptor complex consisting of two type I and two type II serine/threonine kinase receptors (TβRI/TβR II), and then the activated type I receptor subsequently phosphorylates Smads, complex with the co-Smad Smad4 and translocate to the nucleus to regulate the downstream transcription factors (Ross and Hill 2008). TGF-β can activate many other pathways including the MAPK and PI3 kinase pathways in a Smad-independent manner (Moustakas and Heldin 2005). It has been shown that TGF-β induction of CSPG expression in astrocytes is Smad2 and Smad3 dependent in vitro (Susarla et al. 2011).
In this study, we present evidence that the KCa3.1 channels are required for reactive astrogliosis in response to TGF-β stimuli. We found that TGF-β increased the expression of KCa3.1 channels with a concomitant marked increase in the expression of GFAP and CSPGs, as well as increased astrocyte proliferation. These changes in response to TGF-β were reduced by pharmacological blockade or gene knockout (KO) of KCa3.1. In addition, blockade of KCa3.1 suppressed astrogliosis by inhibiting TGF-β-induced Smad2 and Smad3 activation.
Materials and Methods
Materials
Recombinant human TGF-β and TRAM-34 were purchased from RandD Systems, Inc (Minneapolis, MN, USA). The following primary antibodies were used: phospho-Smad2/Smad2 and phospho-Smad3/Smad3 (12747, Cell Signaling Technology, Danvers, MA); CS-56 (C8035, Sigma-Aldrich; St Louis, MO); β-actin (A5316, Sigma-Aldrich); GFAP (Z0334, Dako, Glostrup, Denmark); KCa3.1 (ab83740, Abcam, USA); Ki67 (ab16667, Abcam, USA).
Cell culture
All animal care and procedures were approved by the Institutional Animal Care and Use Committee at the National Heart, Lung, and Blood Institute and performed in accordance with their guidelines. WT C57Bl/6 mice were procured from Harlan Laboratories. KCa3.1−/− mice were provided by Dr. James Melvin (National Institute of Dental and Craniofacial Research, Bethesda, MD). Primary cortical astrocyte cultures were prepared from neonatal (1-3 day old) wild type or KCa3.1−/− C57BL/6 mice brain as described previously (Wang et al. 2008). Briefly, the cerebral cortices were dissected out and dissociated into single cell suspension. Dissociated cells were seeded into T75 flasks and grown in DMEM supplemented with 10% FBS, at 37°C and 5% CO2 atmosphere. When cells grew to confluence (10-14 days later), flasks were shaken overnight (200 rpm, 37°C) to deplete microglia and oligodendrocytes from the more adherent astrocytes. The purified astrocytes were plated onto 6-well plates or 12-well plates in serum containing medium. After once again reaching confluence, astrocytes were incubated with serum-free media for 24 h, and were then treated with 10 ng/ml TGF-β for different time periods before harvest. In some cases, the cells were pretreated with the KCa3.1 channel blocker TRAM-34 1 h before TGF-β was added.
Preparation of conditioned media and cell lysates
Astrocyte conditioned medium was placed into a tube containing complete protease inhibitors (Cocktail Set V, Calbiochem, La Jolla, CA). The conditioned medium was then centrifuged at 2000 g for 10 min to remove cell debris and concentrated with a Centricon 100 (Millipore). To compare CSPG levels in conditioned medium, the concentrated medium was made up to a final volume of 50 μl and equal volumes of the solution were loaded onto the gel. To extract cell lysates, cells were washed twice in ice cold PBS and lysed with 2x SDS sample buffer. For cell lysates, equal amounts of protein were loaded. All protein samples were stored at −80°C until needed.
Western blot analysis
Protein lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a PVDF membrane. Membranes were blocked and incubated, according to the instructions provided, with the primary antibody. Protein bands were identified through a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence reagents (Pierce, Rockford, IL). The following primary antibodies were used for Western blots: phospho-Smad2 (Ser465/467) #3108, Smad2 #5339, phospho-Smad3 (Ser423/425) #9520, and Smad3 #9523 (each 1:1000, Cell Signaling Technology); CS-56 (1:500, C8035, Sigma-Aldrich); β-actin (1:3000, A5316, Sigma-Aldrich); GFAP (1:1000, Z0334, Dako); KCa3.1 (1:500, ab83740, Abcam). The following secondary antibodies were used: HRP-linked anti-rabbit IgG (1:2000, NA9340V, GE Healthcare); HRP-linked anti-mouse IgG (1:2000, NA9310V, GE Healthcare). Immunoreactivity for each protein was quantified using Image J software. Blots of cell lysate were reprobed with β-actin as a loading control.
Immunocytochemistry
Astrocytes were fixed with 4% paraformaldehyde at room temperature for 20 min, washed with PBS, and incubated with 10% normal goat serum and 0.1% Triton X-100 in PBS for 30 min. Next, cells were incubated overnight at 4°C with the primary antibody, washed with PBS, and then incubated with Alexa Fluor 488- or 568-conjugated secondary antibody (1:1000, Invitrogen) for 1 h at room temperature. Cells were then washed, mounted onto microscope slides in Vectashield mounting medium containing the nuclear dye, 4,6-diamidino-2-phenylindole (DAPI) (Vector laboratories, Burlingame, CA). The following primary antibodies were used: phospho-Smad2 #3108 and phospho-Smad3 #9520 (each 1:100, Cell Signaling); GFAP (1:400, Z0334, Dako); KCa3.1 (1:100, ab83740, Abcam); Ki67 (1:50, ab16667, Abcam).
Proliferation assays
A total of 5x104 cells per well were plated in 48-well plates. In these experiments, confluent astrocytes were incubated with serum-free media for 24 h to induce quiescence, and then the cells were pretreated with the KCa3.1 channel blocker TRAM-34 for 1 h before 10% FBS was added. To determine the proportion of astrocytes undergoing mitotic cell division, cells were stained with anti-Ki67 antibody and with DAPI dye to label cell nuclei. Images were taken from 10 fields per well and analyzed using Image J software (Schneider et al. 2012). Quantitative output measures were used to calculate proliferation rate indices where the proportion of astrocytes positive for Ki67 was determined as a function of total cell number per field (DAPI). For cell counting, astrocytes were plated in 24-well plate at a density of 1 × 105 cells per well in 1 ml culture medium. Following 96 h culture, the cells were detached with a solution containing 0.25% trypsin-EDTA, and counted using a standard hemocytometer.
Intracellular free calcium measurement
A confocal laser scanning microscope (Zeiss LSM 510 Meta) was used to evaluate relative changes in intracellular calcium concentration ([Ca2+]i) by monitoring Fluo-4 fluorescence. Cells were loaded with 3 μM Fluo-4 AM (F-14201, Invitrogen) for 30 min in an incubator and were then washed three times with 0.01 M PBS. 300 μM UTP was added to the cells at 60 s. TRAM-34 (1 μM) was added 1 h before the experiment. Fluo-4 fluorescence was measured at 510 nm with excitation at 488 nm. Confocal images were taken and stored every 5 s for 1000 s. Data were obtained by evaluating the fluorescence from selected whole areas of cell bodies, and then taking the average of 10-20 cells.
Statistical analysis
All data are presented as means ± SEM. For multiple comparisons, one-way ANOVA was used, followed by the Dunnett’s test for selected pairs if appropriate. For individual comparisons, the statistical analysis was performed using the Student t test. Data analysis was performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). The threshold for statistical significance was set at p < 0.05.
Results
Up-regulation of KCa3.1 in TGF-β-induced reactive astrogliosis
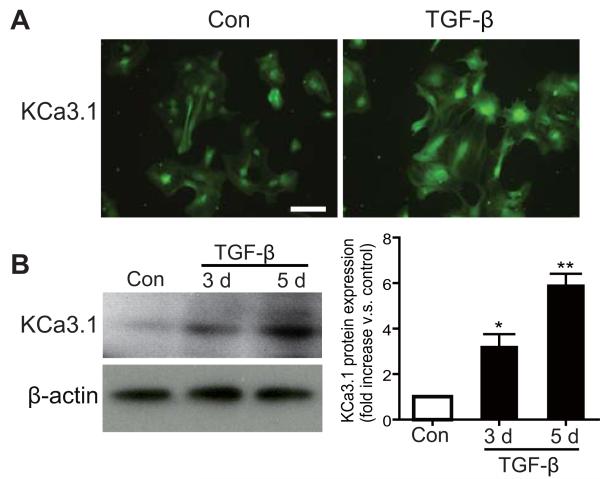
TGF-β is rapidly up-regulated after CNS injury in vivo and is important both as a soluble regulator of ECM formation and in inducing reactive astrogliosis. To study the role of KCa3.1 in the process of reactive astrogliosis, we stimulated confluent cultures of astrocytes with TGF-β (10 ng/ml) for 3 or 5 days to induce reactive astrogliosis (Wang et al. 2008). As shown in Figure 1, TGF-β induced a time-dependent increase in KCa3.1 protein expression (Fig. 1A, B and C), as well as the expression of the reactive astrogliosis marker protein GFAP (Fig. 2A) and the production of CSPGs (Fig. 3A). This suggests that an increase in KCa3.1 occurs concomitant with up-regulation of other signs of reactive astrogliosis as compared to quiescent cells.
Fig. 1.
Up-regulation of KCa3.1 in TGF-β-induced reactive astrogliosis. (A) Stimulation with 10 ng/ml TGF-β for 72 h increased KCa3.1 expression in astrocytes. Scale bar = 50 μm. (B) Stimulation with 10 ng/ml TGF-β increased KCa3.1 protein expression in astrocytes in a time-dependent fashion (20 μg whole-cell lysates, n = 3). Data are presented as means ± SEM. *p < 0.05, **p < 0.01 versus control (TGF-β stimulation time 0 h).
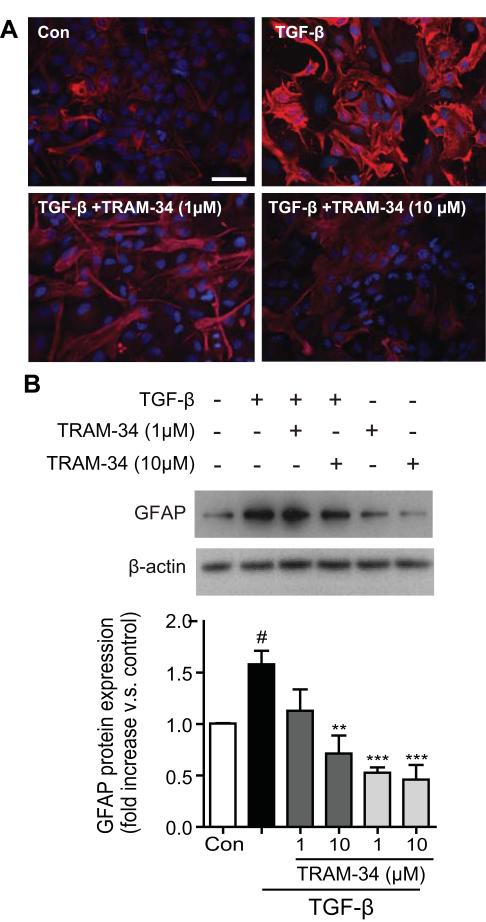
Fig. 2.
Blockade of KCa3.1 channel attenuates TGF-β-induced GFAP protein expression. (A) Representative immunocytochemistry showing GFAP expression in cultured astrocytes treated with TGF-β (10 ng/ml) for 5 days in the presence of TRAM-34 (1, 10μM). Scale bar = 50 μm. Con: control. (B) Representative western blot showing GFAP expression in cultured astrocytes treated with TGF-β (10 ng/ml) for 5 days in the presence of TRAM-34 (1, 10μM). Quantification of western for GFAP immunoreactivity (n = 3). Data are presented as means ± SEM. #p < 0.05 versus control, **p < 0.01, ***p < 0.001 versus TGF-β alone.
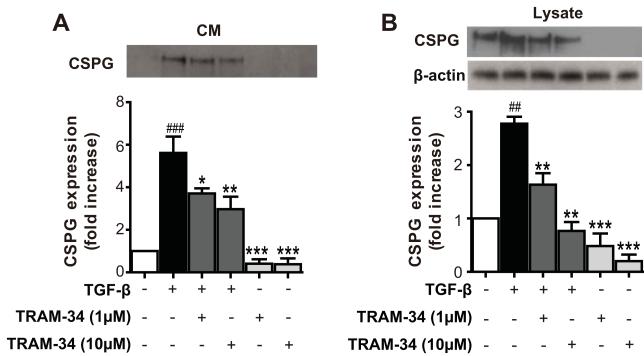
Fig. 3.
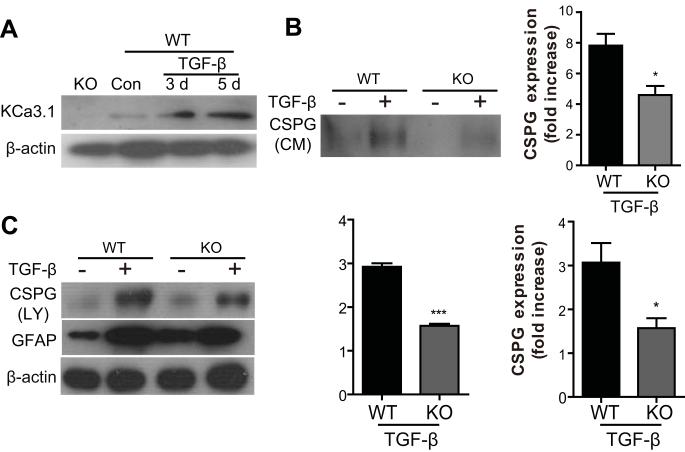
Blockade of KCa3.1 inhibits TGF-β-induced CSPG expression. (A) Representative western blot showing CSPG expression in conditioned medium (CM) harvested from astrocytes treated with TGF-β (10 ng/ml) for 5 days in the presence of TRAM-34 (1, 10μM). (B) Representative western blot showing CSPG expression in cell lysates (LY) harvested from astrocytes treated with TGF-β (10 ng/ml) for 5 days in the presence of TRAM-34 (1, 10μM). Quantification of western blot for CS-56 immunoreactivity (n = 3). Data are presented as means ± SEM. ## p < 0.01, ### p < 0.001 versus control, * p < 0.05, ** p < 0.01, *** p < 0.001 versus TGF-β alone. Con: control.
Blockade of KCa3.1 attenuated TGF-β-induced up-regulation of GFAP and CSPG in astrocytes
To evaluate whether the up-regulation of KCa3.1 was involved in the production of reactive astrogliosis, astrocytes in culture were exposed to TGF-β (10 ng/ml) in the presence or absence of TRAM-34 (1, 10 μM) for 5 days. Western blot analysis was performed using the GFAP and CS-56 monoclonal antibodies. The exact epitope recognized by CS-56 is not known, but CS-56 monoclonal antibody raised against the disaccharide repeat composed of both C-4-S and C-6-S, which are found in most side chains of CSPG (Ito et al. 2005). Compare to the control, astrocytes treated with TGF-β had a significant increase in GFAP (Fig. 2A, B), and CSPGs, both in the conditioned medium (Fig. 3A) and in cell lysates (Fig. 3B). TRAM-34 treatment reduced the actions of TGF-β: the amount of CSPG secreted into the culture medium and in the lysates of reactive astrocytes was reduced in a dose-dependent manner by TRAM-34 (p < 0.01; Fig. 3A, B), and TRAM-34 significantly suppressed the effect of TGF-β on GFAP production (p < 0.01; Fig. 2A, B). Thus, blockade of KCa3.1 reduces the TGF-β-induced expression of GFAP and CSPGs in reactive astrocytes.
Requirement of KCa3.1 for astrocyte proliferation
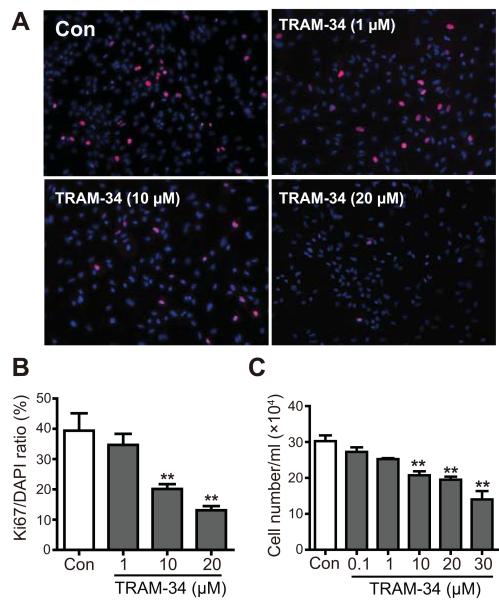
Besides changes in gene expression, the basic process of reactive astrogliosis involves cell proliferation after particular insults. To elucidate whether KCa3.1 expression was important in controlling cell proliferation, we tested the effect TRAM-34, on astrocyte proliferation in response to serum. Astrocytes were first incubated with serum-free media for 24 h to induce quiescence, and then exposed to 10 % FBS. After 96 h in FBS, proliferation was measured by Ki67 staining and cell counting. TRAM-34 (1-30 μM) reduced the proliferation of astrocytes measured by Ki67 staining (p < 0.01, Fig. 4A, B) and cell counting (p < 0.01, Fig. 4C) in dose-dependent manner. These results suggest that blockade of KCa3.1 reduces the proliferation of astrocytes in response to the mitogens in FBS.
Fig. 4.
Blockade of KCa3.1 channels inhibits astrocyte proliferation. Cells were trypsinized and seeded for 48 h, then treated for 96 h with or without TRAM-34 (1-30 μM) in culture medium. Cell proliferation was then measured as described in Materials and Methods. After treating cells with serum-free medium for 24 h, proliferation was induced by 10% FBS in the absence or presence of TRAM-34 (1, 10 and 20 μM) for 96 h, and then assayed by measuring Ki67 (A, B) or counting cells (C) at the indicated times (n = 4). Data are presented as means ± SEM. ** p < 0.01 versus control.
Knockout of KCa3.1 inhibited TGF-β-induced up-regulation of GFAP and CSPG in reactive astrogliosis
To provide direct evidence that KCa3.1 channels are indeed involved in the process of reactive astrogliosis, we cultured astrocytes from KCa3.1−/− mice and evaluated their response to TGF-β. The absence of KCa3.1 in cultured astrocytes was confirmed by examining the levels of KCa3.1 protein by Western blot (Fig. 5A). Similar to TRAM-34, astrocytes from KCa3.1−/− mice exhibit a decrease in GFAP (p < 0.05, Fig. 5C) or CSPGs, in either conditioned medium (p < 0.05, Fig. 5B) or cell lysates (p < 0.001, Fig. 5C) as compared to wt astrocytes.
Fig. 5.
KCa3.1 gene knockout inhibits TGF-β-induced GFAP and CSPG protein expression. (A) The absence of KCa3.1 expression in astrocytes of KCa3.1−/− mice was confirmed by Western blot. (B-C) Astrocytes from KCa3.1−/− mice prevented the TGF-β-induced increase in GFAP (p < 0.05, C) and CSPG conditioned medium (CM) (p < 0.05, B) and CSPG lysates (LY) (p < 0.001, C) protein expression responses to TGF-β compared with astrocytes from WT mice (n = 3-4). * p < 0.05, *** p < 0.05 versus WT. WT: wild type, KO: knockout, Con: control.
Involvement of KCa3.1 in TGF-β-induced reactive astrogliosis through the Smad2 and Smad3 signaling pathways
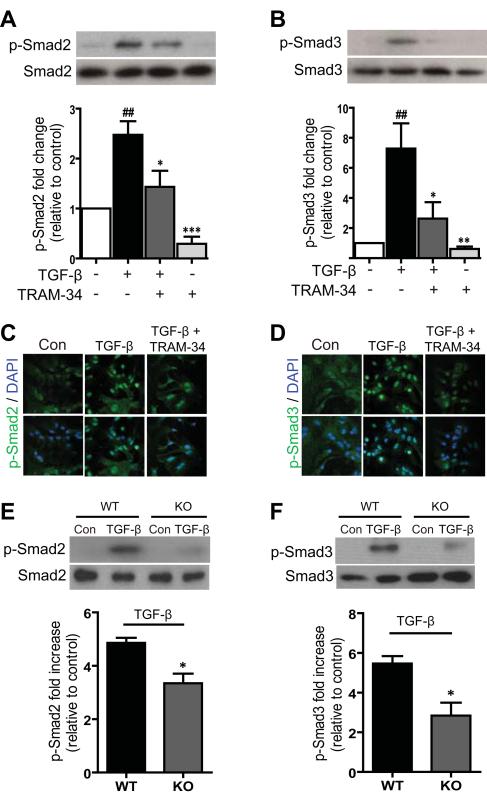
To understand the mechanisms by which blockade of KCa3.1 inhibits TGF-β-stimulated reactive astrogliosis, we examined the effects of KCa3.1 on the TGF-β signal transduction pathways, Smad2 and Smad3. The phosphorylation of Smad2 and Smad3 were examined by Western blot analysis. In these experiments, astrocytes were incubated with serum-free media for 24 h. Exposure of astrocytes to TGF-β for 1 h resulted in significantly increased phosphorylation of both Smad2 (p < 0.01, Fig. 6A, C) and Smad3 (p < 0.01, Fig. 6B, D). Pre-treatment 1 h of astrocytes with 10 μM TRAM-34 significantly inhibited the phosphorylation of Smad2 (p < 0.05, Fig. 6A) and Smad3 (p < 0.05, Fig. 6B) in response to TGF-β stimulation. Gene disruption studies also support a role for KCa3.1 in regulating TGF-β-stimulated reactive astrogliosis. Astrocytes from KCa3.1−/− mice exhibited reduced phosphorylation of Smad2 (p < 0.05, Fig. 6E) and Smad3 (p < 0.05, Fig. 6F) in response to TGF-β as compared with astrocytes from wild type (WT) mice. These results indicate that KCa3.1 regulates TGF-β-induced astrogliosis through the Smad2 and Smad3 signaling pathways.
Fig. 6.
Involvement of KCa3.1 in TGF-β-induced reactive astrogliosis through the Smad2 and Smad3 signaling pathways. (A) Representative images of total Smad2 and phosphorylated Smad2 (p-Smad2) in astrocytes in control, treated with TGF-β (10 ng/ml) and TGF-β plus 10 μM TRAM-34. Mean values of p-Smad2 relative to total Smad2 (n = 3). (B) Representative images of total Smad3 and phosphorylated Smad3 (p-Smad3) in astrocytes in control, treated with 10 ng/ml TGF-β and TGF-β plus 10 μM TRAM-34. Mean values of p-Smad3 relative to total Smad3 protein (n = 3). Data are presented as means ± SEM. ## p < 0.01 versus control, * p < 0.05, ** p < 0.01, *** p < 0.001 versus TGF-β alone. (C-D) Astrocytes were grown on glass coverslips in medium with 10% FBS, starved overnight, and treated with 10 ng/ml TGF-β in the absence or presence of TRAM-34 for 1 h. Cells were fixed, permeabilized, and incubated with antibody to phospho-Smad2 (C) and phospho-Smad3 (D). Nuclei were stained with DAPI. Results are representative of three separate experiments. Confocal images were obtained at an original magnification of x60. (E) Representative images of total Smad2 and phosphorylated Smad2 (p-Smad2) in astrocytes from KCa3.1−/− mice, responses to TGF-β compared with astrocytes from KCa3.1+/+ mice. Mean values of p-Smad2 relative to total Smad2 (n = 3). (F) Representative images of total Smad3 and phosphorylated Smad3 (p-Smad3) in astrocytes from KCa3.1−/− mice, responses to TGF-β compared with astrocytes from WT mice. Mean values of p-Smad3 relative to total Smad3 (n = 3). Data are presented as means ± SEM. * p < 0.05 versus WT phosphorylated Smad2 or Smad3 alone. WT: wild type, Con: control, KO: knockout.
Blockade of KCa3.1 inhibited UTP-Induced of Ca2+ influx in astrocytes
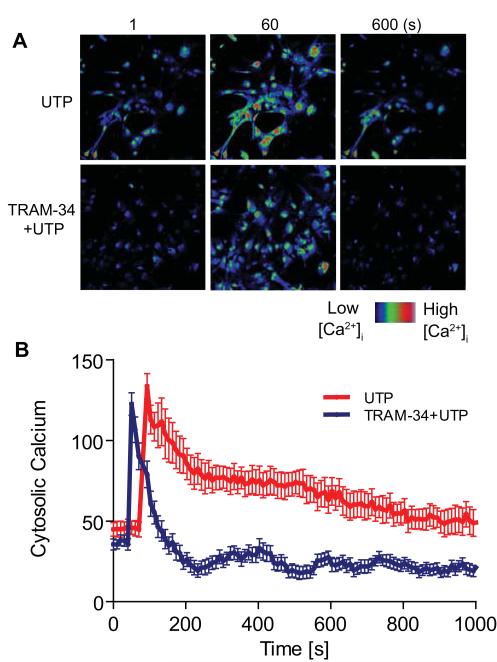
Intracellular Ca2+ is thought to play a crucial role in almost all cellular processes, including regulation of reactive astrogliosis (Sofroniew and Vinters 2010). Activation of KCa3.1 channels is thought to maintain a negative membrane potential which serves as a driving force for subsequent Ca2+ influx. Astrocytes express a number of P2Y receptors, including the ATP/UTP receptor P2Y2 (Franke et al. 2012), and activation of purinergic receptors may produce astrogliosis. We therefore assessed Ca2+ signaling in astrocytes treated with UTP. Astrocytes were loaded with the Ca2+ binding fluorescent dye Fluo-4 AM. Application of 300 μM UTP caused a large, rapid increase in [Ca2+]i, which decayed gradually towards baseline, such that [Ca2+]i was still increased after 15 min. When astrocytes were pre-treated with 1 μM TRAM-34, 300 μM UTP also caused a rapid increase in [Ca2+]i, but decayed much more rapidly, bringing [Ca2+]i below baseline within 3 min (Fig. 7A, B). These results suggest that the KCa3.1 channel plays an important role in reactive astrogliosis by regulating intracellular Ca2+.
Fig. 7.
Involvement of KCa3.1 in UTP-induced intracellular calcium increase in astrocytes. Astrocytes were loaded with the Ca2+-sensitive dye Fluo-4 AM at 37°C for 30 min and changes in [Ca2+]i were monitored by confocal microscopy. (A) Representative fields of cells treated with UTP or TRAM-34 plus UTP. (B) Changes in [Ca2+]i as averages in changes of fluorescent intensities. 300 μM UTP, a metabotropic purinergic receptor agonist, was added to astrocytes at 60 s. In the presence of 1 μM TRAM-34, UTP caused rapidly decaying Ca2+ influx. Signal acquisition lasted for 1000 s. Data are presented as means ± SEM (n = 20 cells).
Discussion
The major finding in our studies is that KCa3.1 expression is increased in parallel with other markers of astrogliosis after TGF-β stimulation. Subsequently, with administration of the KCa3.1 inhibitor TRAM-34, a highly selective inhibitor of KCa3.1, we provided evidence that pharmacological inhibition of KCa3.1 was effective in mitigating the development of reactive astrogliosis after TGF-β stimulation, which implies an important role for KCa3.1 in the pathogenesis of CNS injury disease. This was confirmed by the attenuated GFAP and CSPG protein expression in astrocytes from KCa3.1−/− mice. Furthermore, our results suggest that blockade or KO of KCa3.1 inhibited TGF-β-induced astrogliosis through Smad2 and Smad3-dependent pathways.
A Ca2+-activated potassium channel, later identified as KCa3.1, was first detected in human erythrocytes (Gardos 1958). KCa3.1 channels are voltage-independent, gated by a Ca2+/calmodulin complex that binds to the C-terminal domain of the channel. Opening of the channel increases K+ efflux that maintains the membrane potential serving as the driving force for subsequent Ca2+ influx (Maylie et al. 2004). There is accumulating evidence that KCa3.1 plays a major role in diseases characterized by excessive cell activation and proliferation, such as pulmonary fibrosis (Roach et al. 2013), renal fibrosis (Huang et al. 2013), and asthma (Yu et al. 2013b). However, the role of KCa3.1 in the nervous system is not yet clear.
In the normal mouse brain, there is a relatively low level of expression of KCa3.1 channels. However, after spinal cord injury, reactive astrocytes express KCa3.1 channels (Bouhy et al. 2011). Recovery of locomotor function and the extent of tissue damage were both improved by the infusion of TRAM-34, supporting a role for KCa3.1 channels. The migration of xenografted glioblastoma cells as well as the astrocytic reactivity to the graft were both reduced by TRAM-34 (D’Alessandro et al. 2013) but the reduction in reactivity may well have been due to the indirect effect on tumor cell migration. Whether this recovery is mediated through inhibition of the TGF-β/Smad pathway will require further investigations.
Microglial activation and macrophage invasion are amongst the earliest responses to CNS injury. These activated cells then release a host of cytokines and growth factors, including TGF-β, basic fibroblast growth factor, interleukin 6, and interleukin 1 (Hausmann 2003). TGF-β regulates the expression of CSPGs in several cell types, such as reactive astrocytes (Wang et al. 2008), smooth muscle cells (Schonherr et al. 1991) and fibroblasts (Heimer et al. 1995). TGF-β levels are increased after CNS injury (Logan et al. 1992), and neutralization of TGF-β within a brain injury site greatly reduced the extent of the gliotic scar (Logan et al. 1999). Whether KCa3.1 channels are involved in the response of macrophages and microglia is not certain: Bouhy et al. (2011) did not find any evidence of KCa3.1 channels in macrophages/microglia after spinal cord injury, but did find the channels in primary cultures of activated macrophages/microglia. In contrast, Chen et al. (2011) did find KCa3.1 channels in activated microglia in an experimental model of stroke, and recovery was improved with TRAM-34. Most recently, KCa3.1 channels were implicated in the activation of cultured microglia by purinergic channels (Ferreira et al. 2013). Thus, TRAM-34 may reduce astrocyte reactivity in vivo both directly and indirectly through limiting inflammation.
Inhibition of KCa3.1 may elicit an anti-astrogliosis effect by multiple mechanisms. TGF-β induces a reactive phenotype of astrocytes that is accompanied by up-regulation of reactive astrogliosis marker proteins. We have previously reported that TGF-β signaling in astrocytes is mediated through Smads (Susarla et al. 2011). TGF-β binds to a transmembrane Ser/Thr kinase receptor (TGF-β type I receptor) and then phosphorylates Smad2 and Smad3, which regulate transcription of downstream target genes (Weiss and Attisano 2013) and subsequently contribute to reactive astrogliosis (Smith and Strunz 2005). However, blockade of KCa3.1 channels has been shown to inhibit several different signaling pathways downstream of TGF-β (Huang et al. 2013). We found that blockade or KO of KCa3.1 successfully inhibited TGF-β-induced reactive astrogliosis, including GFAP and CSPG expression, through the Smad2 and Smad3 pathways. Thus, the anti-astrogliosis effects of KCa3.1 are at least partly mediated by the suppression of TGF-β signaling.
In summary, we report that blockade or elimination of KCa3.1 channels prevented astrogliosis in response to TGF-β in cultured astrocytes, through the Smad2/3 pathway. Therefore, inhibition of KCa3.1 signaling may provide a therapeutic approach to attenuate reactive astrogliosis after brain injury.
Acknowledgements
This work was supported by National Basic Research Program of China grant 2010CB529806, and National Natural Science Foundation of China grant 81102491 and the Division of Intramural Research of the National Heart, Lung, and Blood Institute of NIH. The assistance of the Light Microscopy Core Facility of the National Heart, Lung, and Blood Institute, NIH is greatly appreciated. We thank Dr. James Melvin and Dr. Marcelo A. Catalan Gaete (National Institute of Dental and Craniofacial Research, NIH) for kindly providing the KCa3.1−/− mice. The authors have no conflict of interest to declare.
This work was funded by National Basic Research Program of China (grant number 2010CB529806): This information is usually included already, but please add to the Acknowledgments if not.
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Abbreviations
- KCa3.1
intermediate-conductance calcium activated potassium channel
- TRAM-34
(1-((2-chlorophenyl) (diphenyl)methyl)-1H-pyrazole)
- CSPG
chondroitin sulfate proteoglycan
- GFAP
glial fibrillary acidic protein
- TGF-β
Transforming growth factor (TGF)-β
- ECM
extracellular matrix
- TβRI/TβRII
transforming growth factor beta receptor-I/II
Footnotes
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
References
- Bignami A, Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J. Comp. Neurol. 1974;153:27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bouhy D, Ghasemlou N, Lively S, Redensek A, Rathore KI, Schlichter LC, David S. Inhibition of the Ca2+-dependent K+ channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. J. Neurosci. 2011;31:16298–16308. doi: 10.1523/JNEUROSCI.0047-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradding P, Wulff H. The K+ channels KCa3.1 and Kv1.3 as novel targets for asthma therapy. Br. J. Pharmacol. 2009;157:1330–1339. doi: 10.1111/j.1476-5381.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Raman G, Bodendiek S, O’Donnell ME, Wulff H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J Cereb Blood. Flow Metab. 2011;31:2363–2374. doi: 10.1038/jcbfm.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro G, Catalano M, Sciaccaluga M, Chece G, Cipriani R, Rosito M, Grimaldi A, Lauro C, Cantore G, Santoro A, Fioretti B, Franciolini F, Wulff H, Limatola C. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell. Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallerac G, Chever O, Rouach N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front. Cell. Neurosci. 2013;7:159. doi: 10.3389/fncel.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc. Natl. Acad. Sci. USA. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Schlichter LC. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca2+ signaling, store refilling and migration of rat microglial cells. PLoS One. 2013;8:e62345. doi: 10.1371/journal.pone.0062345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys. Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J. Neurosci. 1989;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Heimer R, Bashey RI, Kyle J, Jimenez SA. TGF-β modulates the synthesis of proteoglycans by myocardial fibroblasts in culture. J. Mol. Cell Cardiol. 1995;27:2191–2198. doi: 10.1016/s0022-2828(95)91479-x. [DOI] [PubMed] [Google Scholar]
- Huang C, Shen S, Ma Q, Chen J, Gill A, Pollock CA, Chen XM. Blockade of KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway in diabetic mice. Diabetes. 2013;62:2923–2934. doi: 10.2337/db13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology. 2005;15:593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- Logan A, Baird A, Berry M. Decorin attenuates gliotic scar formation in the rat cerebral hemisphere. Exp. Neurol. 1999;159:504–510. doi: 10.1006/exnr.1999.7180. [DOI] [PubMed] [Google Scholar]
- Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A. Effects of transforming growth factor β1 on scar production in the injured central nervous system of the rat. Eur. J. Neurosci. 1994;6:355–363. doi: 10.1111/j.1460-9568.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Logan A, Frautschy SA, Gonzalez AM, Sporn MB, Baird A. Enhanced expression of transforming growth factor β1 in the rat brain after a localized cerebral injury. Brain. Res. 1992;587:216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-β signals. J. Cell. Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Roach KM, Duffy SM, Coward W, Feghali-Bostwick C, Wulff H, Bradding P. The K+ Channel KCa3.1 as a Novel Target for Idiopathic Pulmonary Fibrosis. PLoS One. 2013;8:e85244. doi: 10.1371/journal.pone.0085244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Hill CS. How the Smads regulate transcription. Int. J. Biochem. Cell Biol. 2008;40:383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-β1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J. Biol. Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta. Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-β-mediated induction of chondroitin sulfate proteoglycans. J. Neurochem. 2011;119:868–878. doi: 10.1111/j.1471-4159.2011.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J. Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- Yu ZH, Wang YX, Song Y, Lu HZ, Hou LN, Cui YY, Chen HZ. Up-regulation of KCa3.1 promotes human airway smooth muscle cell phenotypic modulation. Pharmacol. Res. 2013a;77:30–38. doi: 10.1016/j.phrs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Yu ZH, Xu JR, Wang YX, Xu GN, Xu ZP, Yang K, Wu DZ, Cui YY, Chen HZ. Targeted inhibition of KCa3.1 channel attenuates airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell. Mol. Biol. 2013b;48:685–693. doi: 10.1165/rcmb.2012-0236OC. [DOI] [PubMed] [Google Scholar]