Abstract
β-blockers are used for a wide range of diseases from hypertension to glaucoma. In some diseases/conditions all β-blockers are effective, while in others only certain subgroups are therapeutically beneficial. The best-documented example for only a subset of β-blockers showing clinical efficacy is in heart failure, where members of the class have ranged from completely ineffective, to drugs of choice for treating the disease.. Similarly, β-blockers were tested in murine asthma models and two pilot clinical studies. A different subset was found to be effective for this clinical indication. These findings call into question the current system of classifying these drugs. To consider “β-blockers”, as a single class is misleading when considering their rigorous pharmacological definition and their appropriate clinical application.
Introduction
Asthma is a chronic inflammation of the airways characterized by inflammatory cell infiltration of the airways, an increase in mucus production and secretion, and airway hyperresponsiveness (AHR). A variety of different mediators and receptors regulate the development and exacerbation of asthma. Mainstays of asthma therapy are inhaled glucocorticosteroids and β2-adrenoceptor (β2AR) agonists. The latter class of drugs comprises the most effective bronchodilators ever discovered, and is first line therapy for rescue during an asthma attack [1]. However, chronic use of long-acting β2AR agonists has been associated with loss of asthma control in murine and human studies, and a small, but significant increase in mortality in human studies [2-4]. Also, studies in murine models of asthma suggest β2AR signaling pathways play an essential permissive role in the development of the asthma phenotype. These data include the finding that β2AR knockout mice have an attenuated asthma phenotype [5], and that administration of 5 different β-blockers, including the selective β2AR inverse agonist, ICI-118551, results in an attenuation of the murine asthma phenotype [6,7]. However, administration of some βAR antagonists like alprenolol did not attenuate the asthma phenotype in the same model, and inhibited the beneficial effect of nadolol [5,7]. These results highlight the importance of β2AR, its signaling profiles and the need to understand its regulation in the development or attenuation of the murine asthma phenotype. This review will explore the pharmacological basis of the different signaling profiles of the various β2AR ligands, and suggest their roles in asthma therapy. Finally, we will discuss the limitations and practical possibilities of screening desired β2AR ligands based on a novel holistic cellular label-free impedance assay.
The evolution of receptor theory
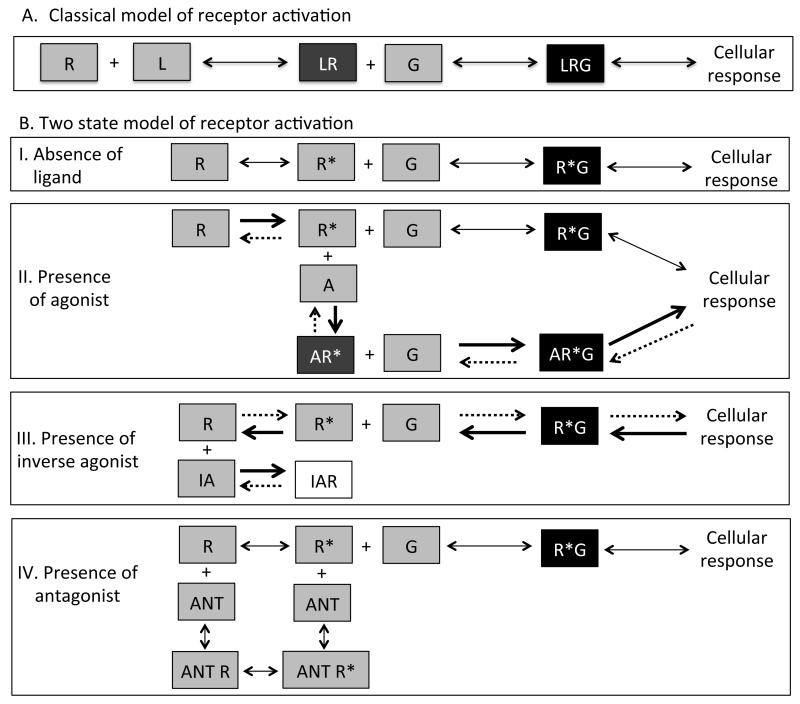
Established theory for the activation of G protein-coupled receptors assumes a receptor in an inactive state ‘R’, which binds to the ligand ‘L’ and produces a binary complex (LR). If the binary complex has affinity for downstream effectors (like G proteins), the ligand is an agonist and leads to a cellular response (Figure 1A). If ligand binding to the receptor produced a binary complex with no affinity for downstream effectors it is termed an antagonist. With the discovery of constitutively or spontaneously active conformations of receptors, it became necessary to include another conformation of the receptor ‘R*’ which was capable of signaling in the absence of the ligand ‘A’ [8].
Figure 1.
A. Classical model of GPCR activation. Receptor ‘R’ when activated by a ligand L, forms a binary complex LR, that has high affinity for signaling molecules like G proteins. The LRG complex can activate downstream signaling pathways eliciting cellular responses. Antagonist-bound receptor has low or no affinity for G and prevents downstream signaling.
B. Two-state model of receptor theory. I. Receptors can exist in two conformations, the inactive conformation ‘R’ and the active conformation ‘R*’. The active conformation R* has high affinity for G and can form R*G complexes to stimulate cellular responses in the absence of a ligand, referred to as constitutive signaling. These conformations exist in equilibrium in a system until a stimulus disturbs the equilibrium. II. In the presence of an agonist ‘A’, which has higher affinity for R* than R, the equilibrium shifts towards R*. The binary complex of AR* has higher affinity for G and stimulates downstream signaling. III. In the presence of an inverse agonist ‘IA’ with higher affinity for the inactive conformation R than R*, the equilibrium shifts towards R once the IAR complex is formed. This further shifts the equilibrium away from R*G and reduces the constitutively active R* cellular response. IV. In the presence of an antagonist with similar affinities for R and R* to form ANT R and ANT R* complexes, the equilibrium is maintained and the constitutive activation of cellular responses by R*G is not affected because the equilibrium does not shift in either direction.
The two-state model of receptor activation proposes that receptors exist in two conformations, R (the inactive state) and R* (the active state) and both states exist in equilibrium. This two-state model of receptor activation allows the classification of ligands as agonists, antagonists,or inverse agonists, on the basis of their relative affinities for the inactive (R) and the active (R*) receptor conformations [9-13]. As shown in Figure 1B, an agonist (A) has more affinity for the active state, it binds to the R* conformation forming AR* and shifts the equilibrium towards R*. Conversely, an inverse agonist (IA) has more affinity for the inactive conformation R, and forms IAR shifting the equilibrium towards R. This results in a reduction in the constitutive (basal) activity of the system by reducing the number of constitutively active receptors. An antagonist has relatively equal affinity for both conformations R and R* and does not alter the equilibrium. An antagonist, sometimes referred to as a ‘neutral antagonist’ for added emphasis, cannot “block” or antagonize the constitutive activity like an IA; but antagonists block the effects of both agonists and inverse agonists [9,14-16]. A partial agonist (not shown in figure), has a relatively higher affinity for R* as compared to R, but the differential affinity for R* relative to R is lower than that of a full agonist. Similarly, a partial inverse agonist (not shown in figure) has a relatively higher affinity for R than R* conformation but again with lower differences in the affinity for R relative to R* when compared to a full inverse agonist.
Role of constitutive versus ligand-activated receptor in asthma
Based on the two-state receptor activation theory, β2AR signaling can result from either a ligand or by the constitutively-active receptor in the absence of a ligand [11]. In our murine asthma models, the inverse agonist nadolol but not the antagonist alprenolol was able to attenuate the asthma phenotype [6,7]. These data suggested that constitutive activation of β2AR was involved in the development of murine asthma phenotype. However, in experiments to test this hypothesis, removal of the endogenous ligand for the β2AR, epinephrine, by genetic or pharmacological means resulted in a loss of the asthma phenotype [17]. These results indicated that while an inverse agonist like nadolol, but not the antagonist alprenolol, attenuated the murine asthma phenotype, constitutive activity was not the key signaling factor in the development of the asthma phenotype. These results left the question of why inverse agonists, but not the antagonists, were beneficial in murine asthma models. The finding that inverse agonism per se was not the key property was also consistent with the initial 2004 study where carvedilol, another inverse agonist for the β2AR, was not as effective as nadolol in attenuating the asthma phenotype in mice [7]. While carvedilol did produce a decrease in peak airway resistance it differed from nadolol in that carvedilol caused a leftward shift in the methacholine dose-response curve (increased AHR) [7]. In addition, while nadolol has been shown to reduce AHR in mild-asthmatics in pilot clinical studies [18,19], propranolol, another βAR inverse agonist with a similar signaling profile as carvedilol (discussed further in the section ‘biased signaling’), did not have a similar beneficial effect [20,21]. All of these studies suggest a complexity beyond the two-state model that warrants further understanding of the signaling pathways of the β2AR, the ligands that act upon them, and their role in asthma.
Alternate signaling pathways
“The illiterate of the 21st century will not be those who cannot read and write, but those who cannot learn, unlearn, and relearn. ” –Alvin Toffler
While activation of multiple signaling pathways of a receptor by a ligand has been recently described as ‘proof’ for the existence of multiple active receptor conformations, it is important to recall that the classical model of receptor activation is sufficient to explain this observation [22,23]. For example, a ligand may activate its preferred canonical pathway, but if the ligand is of very high efficacy and forms excess active receptors than the canonical pathway can handle, the ‘overflow’ of active receptors could now activate a second pathway. Kenakin termed this type of dual pathway activation as resulting from the ‘strength of signal’ of the agonist [23]. Using GPCRs as an example, in this scenario the majority of ligands would have efficacies that would keep the number of activated receptors at a level where there would be sufficient G proteins of the receptor’s canonical pathway to only cause one signaling cascade. However, if the number of activated receptors exceeded the available G proteins of the canonical pathway, the activated receptors would now begin to bind to a second G protein subtype or other signal transduction component.
However, it became necessary to challenge the ‘strength of signal’ argument when studies began to appear that showed reversal of agonist potencies dependent for two pathways activated by the same receptor. A study by Berg et al, using CHO-1C19 and CHO-FA4 cells stably expressing 5-HT2C and 5-HT2A receptors demonstrated that agonists for 5-HT2A and 5-HT2C receptors were able to activate two downstream pathways with different rank order of potencies [24]. With the discovery that the potency of agonists could depend on the signaling pathway being measured, it eliminated classical ligand efficacy as a possible explanation. Reversal of agonist potencies for two pathways originating from a single receptor is not incorporated into traditional theories of receptor activation, including the two-state model, and necessitated extension of the earlier models to include at least a second active receptor conformation.
Biased signaling
The canonical signaling pathway of β2AR activation leads to accumulation of cyclic AMP (cAMP) and downstream signaling molecules via activation of adenylyl cyclase by the Gαs subunit of the G protein. This signaling is “turned off” by phosphorylation of the receptor by G protein-coupled receptor kinases (GRKs) and arrestins. However, studies over the last 10 to 15 years have unveiled novel signaling pathways for the β2AR. Apart from the canonical signaling, another signaling pathway has been identified that involves activation of downstream mitogen-activated protein kinases (MAPK) like ERK1/2, JNK and/or p38 by arrestin. Thus, there can exist at least two distinct signaling pathways that are initiated upon activation of β2AR [25,26].
As described above for the 5-HT system, different ligands can have different activation profiles for the different signaling pathways via the β2AR [24,27-29]. For instance compounds such as propranolol that are inverse agonists on the cAMP signaling pathway were found to be agonists for the MAPK cascade clearly demonstrating the need for at least two distinct active conformations [30]. More recently, studies showed that epinephrine and formoterol are full agonists at the β2AR for the G protein-signaling and the arrestin-mediated signaling pathway, whereas nadolol and ICI-118,551 are inverse agonists at both pathways for β2AR [27-29] but carvedilol and propranolol are inverse agonists at the canonical G protein pathway but partial agonists for ERK1/2 activation [27] demonstrating the existence of distinct class of β-blockers. This form of signaling profile of a ligand is referred to as ligand-directed trafficking of receptor stimulus, biased signaling or functional selectivity, to indicate the preference of a ligand towards activation of a particular signaling pathway as opposed to another [23,31]. Table 1 describes the different types of ligands and their differential effects on downstream signaling pathways via the β2AR
Table 1.
Ligand bias for activation of the two major β2AR downstream signaling pathways (27-29)
| Ligand group |
G protein-mediated pathway (cellular response-1) |
Arrestin-mediated pathway (cellular response-2) |
Receptor conformation affinities |
Example |
|---|---|---|---|---|
| 1* | Activates | Activates | R*~R**>>>R | Epinephrine |
| 2 | Partially activates | Partially activates | R*~R**>>R | Albuterol |
| 3# | Partially activates | Partially activates | R*~R**>R | Alprenolol |
| 4 | Inactivates | Inactivates | R>>>R*~R** | ICI-118,551 |
| 5 | Inactivates | Partially activates | R**>R>>>R* | Carvedilol |
| 6 | Activates or partially activates |
Inactivates | R*>R>>>R** | unknown |
All ligands are compared to the endogenous ligand epinephrine, and the activation or inactivation of pathways is relative to the activation by epinephrine.
This group refers to ligands that have weak agonist properties at G protein-mediated pathway or also known as ‘β-blockers with intrinsic sympathomimetic activity’
How ligand bias changed receptor theory
“Plurality is never to be posited without necessity.”
-William of Occam (Occam’s razor)
The ability of one receptor to activate more than one downstream signaling pathway independently has opened up a plethora of new possibilities. The two-state model of receptor activation cannot accommodate or explain the additional independent signaling pathway through one active state of the receptor. This required the addition of another active state of the receptor ‘R**’ and the postulation of the three-state model for receptor activation [32]. It is important to emphasize that just as the discovery of inverse agonism necessitated the existence of constitutively active receptors, the discovery of biased ligands necessitated the existence of more than one active conformational state of the receptor.
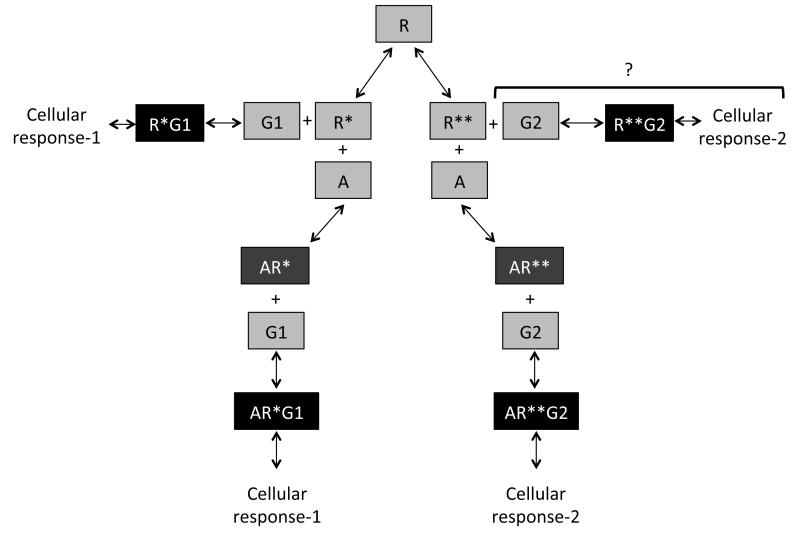
The three-state model of receptor activation has similar principles as the two-state model, an IA preferentially binds to the inactive conformation and an antagonist binds with equal affinity to all conformations. However, in the three-state model, one agonist has more affinity for R* over R** and another agonist has more affinity for R** than R*. These affinities would translate into observations of activation of one or the other pathway depending on the agonist in consideration. The possibility of an agonist having similar affinities for both the active conformations also arises [32]. This can be deduced not only theoretically but also practically as was seen in the preceding section (biased signaling) with epinephrine. In addition, there are ligands that activate one pathway and inhibit another pathway as discussed above in biased signaling of ligands. Figure 2 provides a schematic to understand how the three-state model of receptor activation can correlate to biased signaling of ligands. The three-state model is probably sufficient to explain most phenomenon that have been published so far, but there is emerging data indicating that in addition to the ligand bias between G protein and arrestin there is also ligand-receptor bias for different G proteins. This would probably argue in favor of multiple active states that may be in part driven by the selective effectors to which the receptor couples. While, as is discussed below, the majority of current in vivo data can be explained using three receptor states, current in vitro and structural studies suggest the existence of more than two active states [33,34]. Therefore, the existence of multiple states is highly probable to also eventually be extrapolated to in vivo studies.
Figure 2. Biased signaling of ligands based on the three-state model of receptor activation.
Receptors can exist in three different conformations based on the three-state model of receptor activation, inactive (R), two active states (R* and R**). R* and R** can activate two different downstream cellular responses based on the second messenger systems they activate. For our purposes, let R* activate cellular response-1 or G protein-mediated signaling pathway via G1 second messenger and R** activate cellular response-2 or arrestin-mediated signaling pathway via G2 second messenger. R* can activate cellular response-1 constitutively as was discussed in the two-- receptor theory model (left lateral arm of schematic). Theoretically, R** can also activate cellular response-2 (arrestin-mediated pathway) constitutively (right lateral arm of schematic), however, this has not been shown experimentally and hence denoted with ‘?’. As discussed in the two-state model of receptor activation, ligand ‘A’ can bind to R* to form AR* and activate cellular response-1 via binding to G1 (left descending arm of schematic). ‘A’ can also bind to the other active conformation R** and form AR** that can activate cellular response-2 via binding to G2 (right descending arm of schematic). Biased ligands will have different affinities for R* and R** and will result in differences in the responses observed.
Note: This schematic is only a representation of the intact system in the three-state model of receptor activation. The three-state model has two modes of operation, the first where all equilibria are interconnected (intact) and the second where they are disconnected (isolated), and to explain the experimental data we needed to use the isolated model. Also, the equilibria change based on the ligands added to the system. Therefore, the schematic is not an accurate representation of the dynamic nature of the equilibria between the different states of the receptors and their binary or ternary complexes. Also, there is no experimental evidence of the existence of a direct equilibrium between R* and R** and is therefore excluded from this schematic.
Biased-signaling in pathophysiology
Based on the present understanding of the three-state model of receptor activation and the biased signaling properties of ligands, we can see if this knowledge helps explain the differential effects of different ligands (β-blockers) in pathophysiological conditions like asthma. Studies have shown that chronic nadolol or ICI-118,551 treatment attenuated the asthma phenotype in murine asthma models, conversely carvedilol caused a leftward shift in AHR, and alprenolol did not attenuate the asthma phenotype in mice [6,7]. Similarly, in pilot clinical trials, nadolol reduced AHR in patients with mild asthma whereas propranolol did not [18-20]. In classic receptor theory, nadolol, carvedilol, propranolol ICI-118,551 and alprenolol are grouped as ‘β-blockers’. In the two-state model these can be further separated into inverse agonists (nadolol, carvedilol, propranolol, and ICI-118,551), and antagonists (alprenolol). However, neither of those classifications can explain the results in asthma. Even with the two-state model we are left with the fact that the inverse agonist carvedilol caused a leftward shift in AHR in murine models, and propranolol provides no benefit in AHR compared to nadolol in clinical studies [7,18-20]. It could therefore be hypothesized that a better understanding of the molecular mechanisms controlling the activation of multiple signaling pathways by β2AR biased-signaling, may help in subdividing “β-blockers” in a manner that predicts the outcomes of treatment in asthma. Indeed, when ‘β-blockers’ are viewed as a wide array of ligands with distinct signaling profiles, the discrepant observations described above becomes clearer. For instance, studies have shown that nadolol and ICI-118,551 are inverse agonists at the β2AR for both G protein-mediated and arrestin-mediated pathways; and while carvedilol and propranolol share their inverse agonist effect on the canonical G protein-signaling cascade, they activate ERK 1/2 [27,29]. In fact, the same studies show that of all the ‘β-blockers’ classified as inverse agonists, only carvedilol and propranolol activate ERK 1/2. Therefore classifying nadolol and ICI-118,551 with carvedilol and propranolol as similar ligands based on the fact that they are inverse agonists is an incomplete characterization.
The importance of biased-signaling in disease conditions extends beyond asthma. The use of beta-blockers in congestive heart failure (CHF), was clinically contraindicated for years, on the basis of “logic” that using a beta-blocker in a failing heart would further decrease cardiac output. However, carvedilol and metoprolol now have FDA approval as first-line therapy in the treatment of CHF but it is important to note that not all β-blockers are beneficial for the therapy of CHF [35-38]. Even adding bisoprolol which is approved for CHF in Europe, it is a small percentage of β-blockers that are effective in CHF. Furthermore, several β-blockers such as bucindolol, celiprolol, and nebivolol have been shown to be ineffective in the management of CHF [39-42]. Indeed the CHF story is the most extensive and well-documented evidence that not all β-blockers have equal or even similar therapeutic efficacy in specific disease conditions, and remains the best clinical data as the example of the dangers of grouping “β-blockers” as drugs with a class effect. The evidence now suggests we must classify βAR ligands on the basis of several factors that more formally describe their signaling profiles.
To try and understand why nadolol and ICI-118,551 but not alprenolol or carvedilol attenuated asthma phenotype in murine models and why nadolol but not propranolol reduced AHR in human studies, future studies need to take into consideration the characteristics and differences of these β2AR ligands in activation of downstream signaling pathways [20,21,43-45]. The beneficial effect of nadolol and ICI-118,551 in AHR compared to the failure of carvedilol and propranolol is likely related to the ability of the latter, but not the former, to activate ERK 1/2 signaling. These findings form a hypothesis that using ligands that block the arrestin-mediated signaling via β2AR may be beneficial in asthma [46]. This hypothesis is supported by the finding that knocking out arrestin-3 in mice resulted in an attenuated asthma phenotype [46,47]. However, further studies using different ligands with distinct biased-profiles are warranted to test this hypothesis.
Searching for ligands with preferred bias or the needle in a haystack?
With indications that biased-signaling may play an important role in determining therapeutic application of ligands for various pathophysiological conditions, the major concern is to be able to quantitatively and qualitatively determine the bias of ligands for signaling pathways. We need to know the properties of desired ligands that make them possibly viable for therapy as soon and expeditiously as possible. A variety of assays are available on the market to help quantify the ability and extent of activation of the β2AR-stimulated G protein-mediated signaling pathway by quantifying the amount of cAMP accumulated and the arrestin-mediated pathways by quantifying the MAPK activation [28,48]. In addition, the MAPK activation results from both G protein- and arrestin-mediated signaling complicating the interpretation. This has led many investigators to directly monitor arrestin engagement and activation using protein complementation assays (Path-Hunter- Discoverex) or BRET-based assays.
Although these individual assays are useful, to dissect mechanisms or profile compounds, for high-throughput screening, running these individual assays for each ligand under study may prove to be a time and resource-consuming practice. Also, individually measuring these different pathways does not take into account the high level of signal integration that gives rise to the ultimate biological responses. It follows that high throughput individual in vitro assays tend to trade convenience for a potential loss in physiologic relevance. Using a more holistic assays that capture the global responses to different ligands may provide a useful tool to better classify compounds based on a their overall signaling profiles. Measurements of cellular impedance may represent such a label-free assay that could offer predictive value on the therapeutic potential of molecules [49].
The value of any high-throughput screen lies solely in its predictive value for the development of an appropriate drug for the disease under investigation. Thus, the screening assay of choice is likely to be, at least in part, disease-specific. Cell impedance measurement (xCELLigence, ACEA Biosciences) may represent such a predictive assay for asthma drug efficacy. Stallaert et al. used this assay in HEK 293S cells overexpressing the β2AR and treated with various β ligands for 100 minutes [49]. Following cluster analysis, the resulting impedance signatures produced 5 distinct groups of β-AR ligands. Each group has a characteristic pattern of cellular impedance that is used for the classification. Interestingly, while we have no logical explanation or hypothesis of why the cellular impedance signature would be predictive, this holistic readout correlates to the signaling profiles of compounds, such that the members of each group (as classified by Stallaert et al) have similar effects on the G protein-mediated and/or ERK 1/2 activation pathways as shown by other studies [27-29,49].
The cellular impedance readout further classifies inverse agonists into two groups, group 4 and group 5 ligands. Group 4 includes carvedilol and propranolol and group 5 includes ICI-118,551 and metoprolol. As discussed, carvedilol had limited effects in attenuation of murine asthma phenotype and propranolol did not reduce AHR in asthma patients [7,20]. Whereas, ICI-118,551 and metoprolol attenuated murine asthma phenotype and nadolol reduced AHR in mild asthmatics [6,7,18,50]. (Please note that nadolol was also tested in the cellular impedance assay and it is also a Group 5 ligand, unpublished data). For reasons that are not understood, the cellular impedance data correlates very well to the in vivo therapeutic efficacy in asthma models and could provide a method to identify compounds that could then be tested in chronic murine asthma models and possibly human studies.
Even though the cellular impedance is not a result of direct interaction to the endogenous signaling molecules, it is a global read-out of the signaling pathways occurring inside the cell and can help predict how a ligand will behave in its activation profile of a receptor, at least with regards to the asthma models. The fact that in this and other assays, the ligands that inhibit the asthma phenotype or AHR in asthmatics consistently block ERK 1/2 signaling, suggests this is an essential property of developing a successful ligand for asthma therapy. Further studies are needed to define the exact role of the canonical Gs pathway.
Conclusions
“There are no whole truths; all truths are half-truths. It is trying to treat them as whole truths that plays the devil” -Alfred North Whitehead
The importance of understanding biased-signaling and its role in pathophysiology is of growing importance in the development of better therapeutics. Apart from the large body of clinical evidence in CHF, clinical studies using nadolol in mild-asthmatics have reduced AHR [18,19]; whereas, those using propranolol and esmolol have not [20,51]. These results underline the importance of complete characterization of the signaling profiles of different ligands. By recognizing additional signaling pathways we may have opened a Pandora’s box containing an increasing number of new receptor conformations; for example, there is strong evidence the β2AR can also activate the Gi pathway [52]. While this appears to add layers of complexity to our understanding of ligand-receptor interactions, it provides the exciting prospect of designing improved medicines based on increasingly sophisticated principles of pharmacological selectivity.
Highlights.
Despite known differences, β-blockers are often regarded as a single class of drugs
In several diseases only a subset of β-blockers are therapeutically effective
Multiple signaling pathways and ligand bias can explain differences in effectiveness
Results of an in vitro cellular impedance assay correlates with asthma phenotypes
Renewed classification of β-blockers into specific profiles of ligand bias is required
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnes PJ. Drugs for asthma. British journal of pharmacology. 2006;147(Suppl 1):S297–303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. The Cochrane database of systematic reviews. 2012;4:CD006923. doi: 10.1002/14651858.CD006923.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Lin R, Degan S, Theriot BS, Fischer BM, Strachan RT, Liang J, Pierce RA, Sunday ME, Noble PW, Kraft M, et al. Chronic treatment in vivo with beta-adrenoceptor agonists induces dysfunction of airway beta(2) - adrenoceptors and exacerbates lung inflammation in mice. British journal of pharmacology. 2012;165:2365–2377. doi: 10.1111/j.1476-5381.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, Ho SB, Ehre C, Kesimer M, Knoll BJ, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. American journal of respiratory cell and molecular biology. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of beta-adrenergic antagonists. Molecular pharmacology. 1994;45:490–499. [PubMed] [Google Scholar]
- 10.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. The Journal of biological chemistry. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 11.Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends in pharmacological sciences. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- 12.Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. Journal of molecular biology. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 13.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. The Journal of biological chemistry. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 14.Barker EL, Westphal RS, Schmidt D, Sanders-Bush E. Constitutively active 5-hydroxytryptamine2C receptors reveal novel inverse agonist activity of receptor ligands. The Journal of biological chemistry. 1994;269:11687–11690. [PubMed] [Google Scholar]
- 15.Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, Hyek MF, Kenakin TP, Allen LF, et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 16.Leff P. The two-state model of receptor activation. Trends in pharmacological sciences. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- 17.Thanawala VJ, Forkuo GS, Al-Sawalha N, Azzegagh Z, Nguyen LP, Eriksen JL, Tuvim MJ, Lowder TW, Dickey BF, Knoll BJ, et al. beta2-Adrenoceptor agonists are required for development of the asthma phenotype in a murine model. American journal of respiratory cell and molecular biology. 2013;48:220–229. doi: 10.1165/rcmb.2012-0364OC. *This paper rules out the previous hypothesis that inverse agonism was the crucial property needed to produce attenuation of the asthma phenotype in murine models.
- 18.Hanania NA, Singh S, El-Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss S, Shardonofsky F, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulmonary pharmacology & therapeutics. 2008;21:134–141. doi: 10.1016/j.pupt.2007.07.002. **This paper shows how using different members of the class of β-blocker produces clinically different outcomes as compared to ref #20 Short PM et al 2013
- 19.Hanania NA, Mannava B, Franklin AE, Lipworth BJ, Williamson PA, Garner WJ, Dickey BF, Bond RA. Response to salbutamol in patients with mild asthma treated with nadolol. The European respiratory journal. 2010;36:963–965. doi: 10.1183/09031936.00003210. [DOI] [PubMed] [Google Scholar]
- 20.Short PM, Williamson PA, Anderson WJ, Lipworth BJ. Randomized placebo-controlled trial to evaluate chronic dosing effects of propranolol in asthma. American journal of respiratory and critical care medicine. 2013;187:1308–1314. doi: 10.1164/rccm.201212-2206OC. **This paper shows how using different members of the class of β-blocker produces clinically different outcomes as compared to ref #18 Hanania et al 2008
- 21.Kazani S, Israel E. What doesn’t kill may not make you stronger. beta-blockers for asthma. American journal of respiratory and critical care medicine. 2013;187:1281. doi: 10.1164/rccm.201305-0815ED. [DOI] [PubMed] [Google Scholar]
- 22.Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annual review of pharmacology and toxicology. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 23.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends in pharmacological sciences. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 24.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Molecular pharmacology. 1998;54:94–104. [PubMed] [Google Scholar]
- 25.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annual review of pharmacology and toxicology. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. The Journal of biological chemistry. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 27.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Molecular pharmacology. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Molecular pharmacology. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 30.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends in pharmacological sciences. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Leff P, Scaramellini C, Law C, McKechnie K. A three-state receptor model of agonist action. Trends in pharmacological sciences. 1997;18:355–362. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 33.Staus DP, Wingler LM, Strachan RT, Rasmussen SG, Pardon E, Ahn S, Steyaert J, Kobilka BK, Lefkowitz RJ. Regulation of beta2-Adrenergic Receptor Function by Conformationally Selective Single-Domain Intrabodies. Molecular pharmacology. 2014;85:472–481. doi: 10.1124/mol.113.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Westhuizen ET, Breton B, Christopoulos A, Bouvier M. Quantification of Ligand Bias for Clinically Relevant beta2-Adrenergic Receptor Ligands: Implications for Drug Taxonomy. Molecular pharmacology. 2014;85:492–509. doi: 10.1124/mol.113.088880. [DOI] [PubMed] [Google Scholar]
- 35.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 36.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA : the journal of the American Medical Association. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 37.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 38.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. The New England journal of medicine. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 39.A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. The New England journal of medicine. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 40.Mulder BA, van Veldhuisen DJ, Crijns HJ, Bohm M, Cohen-Solal A, Babalis D, Roughton M, Flather MD, Coats AJ, Van Gelder IC. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: insights from SENIORS. European journal of heart failure. 2012;14:1171–1178. doi: 10.1093/eurjhf/hfs100. [DOI] [PubMed] [Google Scholar]
- 41.van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) Journal of the American College of Cardiology. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 42.Witchitz S, Cohen-Solal A, Dartois N, Weisslinger N, Juste K, Darmon JY. Treatment of heart failure with celiprolol, a cardioselective beta blocker with beta-2 agonist vasodilatory properties. The CELICARD Group. The American journal of cardiology. 2000;85:1467–1471. doi: 10.1016/s0002-9149(00)00796-7. [DOI] [PubMed] [Google Scholar]
- 43.Bond RA. The intrinsic bias of generalizations. American journal of respiratory and critical care medicine. 2014;189:359. doi: 10.1164/rccm.201306-1109LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipworth B, Short P, Anderson W, Williamson P. Reply: pharmacological obfuscation of clinical relevance. American journal of respiratory and critical care medicine. 2014;189:360–361. doi: 10.1164/rccm.201306-1166LE. [DOI] [PubMed] [Google Scholar]
- 45.Penn RB. Far from “disappointing”. American journal of respiratory and critical care medicine. 2014;189:360. doi: 10.1164/rccm.201306-1143LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding beta(2) -adrenoceptor ligands in the treatment of asthma. British journal of pharmacology. 2011;163:18–28. doi: 10.1111/j.1476-5381.2010.01178.x. **This paper reviews the different types of β2 adrenergic ligands and highlights the different possibilities of their signaling profiles. This study provides an insight into the probability of ligand bias and the effect it would have on asthma therapy.
- 47.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. The Journal of clinical investigation. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. The Journal of biological chemistry. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 49.Stallaert W, Dorn JF, van der Westhuizen E, Audet M, Bouvier M. Impedance responses reveal beta(2)-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PloS one. 2012;7:e29420. doi: 10.1371/journal.pone.0029420. a cellular impedance assay, the authors demonstrate a novel method to screen different β2 ligands and classify them based on their impedance signatures that have an apparent relevance to their downstream signaling pathway activation. This method allows for a high-throughput screening method for β2 ligands to distinguish their signaling profiles and bias.
- 50.Lin R, Peng H, Nguyen LP, Dudekula NB, Shardonofsky F, Knoll BJ, Parra S, Bond RA. Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulmonary pharmacology & therapeutics. 2008;21:115–124. doi: 10.1016/j.pupt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Short PM, Anderson WJ, Williamson PA, Lipworth BJ. Effects of intravenous and oral beta-blockade in persistent asthmatics controlled on inhaled corticosteroids. Heart. 2013 doi: 10.1136/heartjnl-2013-304769. [DOI] [PubMed] [Google Scholar]
- 52.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]