Abstract
Alternatives to convenience sampling (CS) are needed for HIV/STI surveillance of most-at-risk populations in Latin America. We compared CS, time space sampling (TSS), and respondent driven sampling (RDS) for recruitment of men who have sex with men (MSM) and transgender women (TW) in Lima, Peru. During concurrent 60-day periods from June–August, 2011, we recruited MSM/TW for epidemiologic surveillance using CS, TSS, and RDS. A total of 748 participants were recruited through CS, 233 through TSS, and 127 through RDS. The TSS sample included the largest proportion of TW (30.7 %) and the lowest percentage of subjects who had previously participated in HIV/STI research (14.9 %). The prevalence of newly diagnosed HIV infection, according to participants’ self-reported previous HIV diagnosis, was highest among TSS recruits (17.9 %) compared with RDS (12.6 %) and CS (10.2 %). TSS identified diverse populations of MSM/TW with higher prevalences of HIV/STIs not accessed by other methods.
Keywords: MSM, Latin America, Respondent-driven sampling, Time space sampling
Introduction
Routine epidemiologic surveillance of HIV and other sexually transmitted infections (STIs) is a central component of public health efforts to control the spread of infection. Effective surveillance can help determine patterns of disease transmission, identify developing epidemiologic trends, and inform decisions regarding the allocation of limited resources. Although random population sampling is the gold standard for epidemiologic estimates, use of this method to sample minority subpopulations is difficult, costly, and time-consuming and requires a detailed knowledge of the population’s parameters. Assessment and refinement of new surveillance methods to describe the HIV epidemics in most-at-risk populations in developing country contexts is a central challenge for epidemiologic research [1–3].
Epidemiologic studies in Peru have consistently identified men who have sex with men (MSM) and transgender women (TW) as populations at high risk for HIV/STI acquisition, with the prevalence of HIV estimated between 10–24 % in these groups [4–9]. Since 1996, periodic surveillance of MSM/TW in Peru has been conducted using convenience sampling (CS) methods where peer outreach workers recruit participants from community venues, including bars, discos, saunas, volleyball courts, and public parks frequented by MSM/TW [4]. These studies have provided key information on patterns of HIV/STI prevalence in the population, but the generalizability of their findings has been limited by a lack of systematic, random selection methods or statistical measures to adjust prevalence estimates.
Alternative sampling methods, including time space sampling (TSS) and respondent-driven sampling (RDS) have been suggested as potential epidemiologic surveillance tools for MSM/TW in Latin America, but have not been thoroughly evaluated for use in this context. Time space sampling methodology includes a preliminary ethnographic mapping process in which MSM/TW-associated venues and patterns of attendance are detailed [10–12]. Time intervals when a minimum number of potential participants can be found at each venue are then divided into discrete venue-date-time (VDT) units and randomly selected to assemble a recruitment schedule during which a random selection of visitors are invited to participate. In addition to the multistage, random selection of VDT units and individual visitors in each VDT, TSS estimates can be weighted according to the sampling frame of VDTs randomized and the sampling fraction of potential participants counted and actual participants enrolled at each VDT. Barriers to use of TSS for surveillance have been both practical, due to the high cost and infrastructure requirements of TSS, and methodological, including the substantial design effect requiring the enrollment of a large number of subjects in comparison to simple random sampling in order to obtain accurate and precise estimates [3, 13]. Statistically, TSS estimates are based on the assumptions that all potential socialization venues are included in the sampling frame and that identification and enrollment of subjects during each VDT is truly random [13–15]. Particularly important for surveillance of MSM/TW populations, TSS also does not account for members of “hidden” subpopulations that do not attend public socialization venues (e.g., non-gay identified MSM) [16–19].
In contrast to the location-based recruitment of TSS, RDS uses samples derived from participants’ social networks to calculate population-scale estimates of risk behavior and disease prevalence [20–22]. Also following an ethnographic mapping process, socially well-connected “seed” participants are recruited and asked to invite eligible contacts from their social networks to enroll. Participants recruited by seeds are then asked to recruit additional individuals from their networks in successive “waves.” To satisfy RDS assumptions, participant recruitment requires several key elements: (1) Participants and their recruits have a pre-existing relationship; (2) The size of each participant’s social network (the potential sampling frame) is documented; and (3) The total number of recruitment coupons distributed to each participant is limited. By including these conditions, RDS analysis will ideally enroll a large enough number of participants in a sufficient number of recruitment waves to achieve equilibrium (where the enrollment of additional participants does not substantially alter the sample’s population estimates) and produce prevalence estimates representative of the entire population of interest [11, 15, 23–27]. Unlike TSS, RDS methods require minimal infrastructure and personnel involvement and can be easily implemented in limited resource settings. As with TSS, RDS analysis has been found to have a substantial design effect when compared to simple random sampling [28–31]. In addition, factors including geographic patterns of socialization, social and sexual network formation, and power dynamics based on social and economic status are also likely to influence peer recruitment and bias data collection [32, 33]. If unsuccessful in overcoming these limitations, RDS estimates run the risk of characterizing individual social networks rather than the population as a whole [34–36].
CS, TSS, and RDS have all been used for epidemiologic surveillance in Latin America, but evidence directly comparing the methods for MSM/TW recruitment is limited [37–48]. Researchers in Fortaleza, Brazil compared samples of MSM recruited using TSS and RDS during chronologically distinct recruitment periods (TSS in 2002 and RDS in 2005), finding that RDS recruited more participants from lower socioeconomic strata faster and at a lower cost than TSS [42]. An analysis of black MSM in San Francisco also found that RDS recruitment led to a more diverse sample with a greater prevalence of behavioral risk factors for HIV infection than TSS [49]. In a recently published simultaneous comparison of recruitment methods for MSM in Guatemala, RDS was less costly than TSS and more successful in recruiting subjects from difficult-to-access populations, including heterosexual-identified MSM, men who reported sex with men and women (MSMW), and male sex workers [19]. However, participants recruited through TSS represented more diverse geographic areas and were less likely to have participated in previous HIV prevention activities. In order to optimize HIV/STI surveillance of MSM/TW populations in Latin America, and before allocating significant public health resources to any specific sampling technique, each method’s operational and recruitment characteristics should be assessed within local social, epidemiologic, and infrastructural contexts. In order to provide an empiric comparison of representative sampling methods and inform future HIV/STI surveillance methods in Lima, Peru, we conducted a pilot evaluation of CS, TSS, and RDS for recruitment of MSM/TW.
Methods
We staged a simultaneous evaluation of CS, RDS, and TSS between June and August, 2011. The three recruitment methods were initiated simultaneously and continued concurrently until the end of the 8-week study period. Using data from previous surveillance studies in Peru and assuming 3 % precision to estimate a 22 % prevalence of HIV infection in the CS arm, enrollment in any individual recruitment arm was limited to a pre-specified maximum of 750 participants. Due to the absence of previous data using TSS or RDS methods to sample MSM populations in Peru, the sample size necessary to assess statistical differences between recruitment arms was not pre-determined. Eligibility was limited to persons born anatomically male who reported oral or anal intercourse with a male or transgender partner in the previous 12 months. Participants were enrolled at one of three clinic sites during daytime operating hours (for CS, RDS, and TSS) or in a mobile counseling and testing unit available according to a randomly generated, venue-based recruitment schedule (for TSS only). All participants received 10 Nuevos soles (approximately $4.00 USD) as compensation.
Participants completed a 40-question survey addressing demographic data, sexual identity, sexual behavior, previous HIV/STI testing, and prior participation in HIV/STI research or surveillance studies. Participants who enrolled at clinic sites completed the survey using a computer-assisted self-interviewing (CASI) system. Due to security concerns regarding computer use at field venues, participants enrolled in the mobile unit were interviewed by study staff using a paper survey.
All participants received rapid testing for HIV (Determine HIV-1/2 Rapid Antibody Test; Abbott, USA) and syphilis (Bioline Syphilis 3.0; Standard Diagnostics, Korea). Preliminary test results were provided in conjunction with post-test counseling after completing the study survey. Confirmatory HIV (Genetic Systems Western Blot; Biorad, USA) and/or syphilis (MHA-TP; Organon Teknika, USA) test results were available at clinic sites within two weeks. Samples from individuals with confirmed HIV infection were tested for recent HIV acquisition using a “detuned” EIA (Vironostika, Organon Teknika, USA) with an optical density cut-off of 0.75 used to define recent infection. Participants were defined as having newly diagnosed HIV infection if they had laboratory-confirmed evidence of HIV infection and denied previously testing positive for HIV. Participants newly diagnosed with HIV infection were referred to Ministry of Health treatment programs. Participants with untreated syphilis infection received antibiotic therapy according to Ministry of Health guidelines.
The study protocol and consent forms were approved by the ethics committees of Asociación Civil Impacta Salud y Educación, Asociación Civil Vía Libre, and University of California, Los Angeles in compliance with all international regulations regarding the protection of human subjects.
Ethnographic Mapping
Prior to initiating enrollment, an ethnographic mapping process was completed to document venues frequented by MSM/TW in Lima, including bars, discos, saunas, pornographic movie theaters, commercial sex zones, and public spaces; characterize patterns of socialization, including venue attendance by MSM/TW from different sexual identity sub-groups, including TW, gay-identified MSM, and non-gay identified MSM; and identify popular opinion leaders in local MSM/TW communities, through participant observation and informal interviewing techniques. Five mapping teams composed of five surveyors and one supervisor each were assigned to each of five major geographic zones of Lima-Callao (Lima Norte, Lima Este, Lima Sur, Lima Ciudad, and Callao). Using information collected during previous surveillance efforts as a baseline and incorporating word-of-mouth notification of new or emerging venues, mapping teams collected updated ethnographic information on MSM/TW socialization venues and networks.
Information from the ethnographic mapping process was used in all three of the sampling methods. An initial survey in July, 2010 confirmed the continued operation of 478 venues identified during previous mapping and located 263 previously unreported venues. In a follow-up survey completed between May and June, 2011 all 741 sites named in the 2010 map were re-visited by a single team to confirm continued operation of the site and to determine the 4-h time intervals during which a minimum of 20 different MSM/TW visited the venue.
Venue mapping data was used to inform CS by community-based peer outreach workers, though no systematic recruitment or sampling methods were specified for this arm. For the RDS arm, information on popular opinion leaders in local communities was used to help select socially well-connected seeds from diverse sub-populations of MSM/TW (gay-identified MSM, non-gay-identified MSM, TW, and male sex workers). However, no specific data on social network patterns of interaction (network density, frequency of interaction between network members) or influence among MSM/TW (popular opinion leaders) was collected prior to the study. For the TSS arm, specific information on location of MSM/TW venues and attendance patterns was used to define the sampling frame.
Convenience Sampling
Convenience sampling procedures were identical to those used during previous epidemiologic surveillance studies in Peru [4]. Community-based outreach workers were provided with information from the ethnographic mapping procedure to help them identify potential sites for participant recruitment, though specific recruitment sites or hours were not defined. Outreach workers screened potential participants for eligibility and either referred eligible subjects to one of three geographically distributed clinic sites or, most often, accompanied participants to the site for enrollment. Outreach workers were compensated on a sliding scale, with compensation ranging from 20 Soles ($8 USD) per participant if less than 5 participants were enrolled to 40 soles per participant ($16 USD) for workers who enrolled more than 15 participants.
Time Space Sampling
TSS recruitment was based on a two-stage, random selection of MSM/TW visitors during randomly selected 4-h VDT units. The sampling frame for VDT selection was limited to venues and time intervals where a minimum of 20 MSM/TW visitors had been observed during the ethnographic mapping process and stratified by geographic area: (1) Downtown Lima (Lima Cercado) and (2) Outer Districts of Lima. A non-random recruitment event was also scheduled to coincide with Lima’s Marcha de Orgullo Gay (Gay Pride Parade). Prior to each field recruitment session, a member of the study staff re-visited the scheduled site to confirm minimum attendance and participant availability at the selected time interval and to secure cooperation of the owner (if the VDT involved a commercial venue). If the VDT did not meet minimum attendance criteria, if the owner declined permission for recruitment, or if the site was otherwise deemed unsuitable, an alternative VDT from the same geographic area was randomly selected and the confirmation process repeated.
Each VDT unit was staffed by one Counter, three Recruiters, and two Interviewers in a mobile unit containing two separate interviewing/testing spaces. The Counter enumerated all men and TW who crossed a predetermined point of entry to the venue during the 4-h interval. Recruiters approached potential participants, screened for eligibility, and escorted participants to the mobile unit to complete study procedures. If both counseling and testing spaces were occupied, potential participants were provided with a recruitment card containing information about the study, the address and operating hours of the clinic enrollment sites, and an invitation to visit one of the sites for enrollment. Recruitment cards included a numeric code to allow tracking of participant enrollment by VDT.
Respondent Driven Sampling
Based on data obtained during ethnographic mapping, 12 socially well-connected seed participants were purposively selected to obtain diverse representation of major MSM/ TW subgroups identified during previous research (3 heterosexual/bisexual MSM, 3 homosexual MSM, 3 TW, and 3 non-transgender male sex workers). Due to slow initial recruitment in the RDS arm, an additional 12 seeds with similar characteristics were enrolled in Week 3. Seed participants and recruits received brief instruction on RDS recruitment and were provided with 5 recruitment coupons to distribute to MSM/TW in their social network. Respondent driven sampling participants were paid a flat fee of 15 Nuevos soles ($6.00 USD) for each of their recruits who enrolled. Respondent driven sampling coupons were identical to the cards administered during TSS recruitment and included a numeric code to allow tracking of enrollment by participant referral networks.
Data Analysis
For the comparison of participant characteristics between recruitment samples, data from CS and RDS recruitment arms were analyzed as crude, or unweighted, estimates while data from the TSS arm was analyzed as a weighted sample. For CS and RDS arms, prevalence estimates were calculated as percentages with 95 % confidence intervals. For the TSS arm, analyses were stratified by venue district (Downtown Lima vs. Outer Districts) and adjusted for clustering by recruitment venue using the svyset command in Stata 11.0 (Stata Corporation, College Station, TX, USA). Using the VDT as the primary sampling unit, TSS estimates were weighted according to the probability of recruiting an individual participant from a specific VDT. Sampling estimates were weighted according to the size of the VDT sampling frame (Number of VDTsSelected/Number of VDTsSampling Frame), and the number of visitors to each VDT as a fraction of visitors counted across all VDTs (Number of VisitorsVDT/Number VisitorsAll VDTs) divided by the number of participants enrolled at each VDT as a fraction of all participants enrolled (Number EnrolledVDT/Number EnrolledAll VDTs) [12]. No adjustments were made to account for participant refusal or for attendance at multiple VDTs by a single individual. Due to the small size and limited number of recruitment waves in the RDS sample, no adjusted RDS analysis was performed [47].
Differences between participants in the three recruitment arms (socio-demographic variables, sexual identity, sexual risk behavior, and HIV/STI prevalence) were explored using contingency tables. Differences in categorical variables including demographics, sexual risk behavior, and HIV/STI prevalence were compared between the CS and TSS arms using unpooled Z-tests (calculated as the difference between the two sample estimates divided by the square root of the sum of their variances). RDS data was not included in the statistical comparisons due to the small number of participants recruited through this method.
Results
A total of 748 participants were recruited through CS, 233 through TSS, and 127 through RDS. In the TSS sample, a total of 20,062 potential participants were enumerated during 40 recruitment visits to 34 different venues (11 Public Areas, 9 Discos, 5 Commercial Sex Venues, 3 Saunas, 3 Video/Theaters, 3 Restaurants/Bars, and 1 Special Event). A total of 1,207 men or TW were approached by recruiters, of whom 1,096 met eligibility criteria and 203 were enrolled in the mobile unit. An additional 466 eligible contacts were provided with recruitment coupons, of whom 30 subsequently presented to clinic sites for enrollment. In the RDS sample, 24 seed participants recruited a total of 103 participants, with a median of 1 recruitment wave (range 0–6) and 3 participants enrolled (range 1–42) per recruitment chain.
Characteristics of participants’ age, education, sexual identity/role, sexual behavior, and HIV testing history are described in Table 1. Qualitatively, the TSS sample included larger proportions of both TW and heterosexual-identified MSM compared with the CS and RDS samples, which included greater proportions of gay- and bisexual-identified MSM. Statistically, the TSS sample, when compared with the CS group, had significantly larger proportions of both TW and of participants who recently provided sex in exchange for money or goods, though not of those who self-identified as sex workers. The prevalence of self-reported unprotected insertive (though not receptive) anal intercourse was significantly lower among participants in the TSS sample compared with the CS arm.
Table 1.
Estimates of demographic characteristics and risk behavior prevalence of MSM/TW samples recruited using CS, TSS, and RDS; Lima, Peru 2011
| Convenience sampling (CS) (N = 748) | Time space sampling (TSS) (N = 233) | Respondent driven sampling (RDS) (N = 127) | p valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Unweighted (%) | 95 % CI | Weighted (%) | 95 % CI | Unweighted (%) | 95 % CI | Unweighted (%) | 95 % CI | ||
| Age (median, IQR) | 26 (21–33) | 25 (21–31) | 28 (23–36) | ||||||
| Completed secondary education | 88.4 | 85.6–90.7 | 82.4 | 70.0–90.4 | 79.8 | 74.2–84.5 | 75.2 | 66.3–82.4 | 0.78 |
| Sexual identity | |||||||||
| Heterosexual | 12.8 | 10.6–15.4 | 15.3 | 9.9–23.1 | 15.5 | 11.4–20.7 | 12.6 | 7.9–19.5 | 0.50 |
| Bisexual | 29.0 | 25.8–32.3 | 25.7 | 26.2–38.3 | 23.7 | 18.7–29.6 | 37.8 | 29.8–46.5 | 0.57 |
| Homosexual | 43.4 | 39.9–47.0 | 28.9 | 29.6–39.0 | 28.4 | 23.0–34.6 | 34.6 | 26.9–43.3 | 0.10 |
| Trans | 14.8 | 12.4–17.6 | 30.7 | 19.1–45.3 | 32.3 | 26.6–38.6 | 15.0 | 9.8–22.2 | 0.02 |
| Sexual role | |||||||||
| Activo (insertive) | 37.3 | 33.9–40.9 | 29.1 | 20.4–39.7 | 30.2 | 24.6–36.4 | 43.3 | 35.0–52.0 | 0.33 |
| Pasivo (receptive) | 26.8 | 23.8–30.1 | 39.4 | 27.3–53.0 | 37.1 | 31.1–43.4 | 29.9 | 22.6–38.4 | 0.11 |
| Moderno (versatile) | 35.8 | 32.5–39.4 | 31.5 | 22.2–42.5 | 32.8 | 27.0–39.0 | 26.8 | 19.8–35.0 | 0.61 |
| Any unprotected insertive anal intercourse (3 months) | 46.1 | 42.5–49.7 | 20.1 | 14.4–27.2 | 24.7 | 19.6–30.6 | 44.1 | 35.7–52.8 | 0.01 |
| Any unprotected receptive anal intercourse (3 months) | 41.6 | 38.1–45.2 | 31.1 | 21.8–42.2 | 32.5 | 26.8–38.8 | 40.2 | 32.0–48.9 | 0.27 |
| Median number of male sexual partners (3 months) | 2 (1–6) | 3 (1–60) | 4 (1–10) | ||||||
| Median number of female sexual partners (3 months) | 0 (0–1) | 0 (0–1) | 0 (0–2) | ||||||
| Received compensation for sex (6 months) | 37.7 | 34.3–41.3 | 58.6 | 42.2–73.3 | 51.5 | 45.1–57.9 | 44.1 | 35.7–52.8 | 0.05 |
| Self–identify as “sex worker” | 25.6 | 22.6–28.9 | 35.7 | 24.5–48.8 | 35.1 | 29.2–41.4 | 36.2 | 28.4–44.9 | 0.18 |
| Prior HIV testing | 69.4 | 66.0–72.6 | 69.7 | 55.9–80.6 | 69.7 | 63.5–75.2 | 67.7 | 59.1–75.2 | 0.99 |
| Prior HIV diagnosis | 3.2 | 2.2–4.7 | 2.2 | 0.8–6.3 | 2.2 | 1.0–5.0 | 7.9 | 4.4–13.9 | 0.48 |
| HIV infection | 13.4 | 11.1–16.0 | 20.1 | 14.5–27.1 | 21.5 | 16.7–27.2 | 20.5 | 14.4–28.3 | 0.06 |
| Newly diagnosed HIV infection | 10.2 | 8.1–12.5 | 17.9 | 13.4–23.6 | 19.3 | 14.8–24.9 | 12.6 | 7.9–19.5 | < 0.01 |
| Recent HIV infection (detuned EIA) | 1.1 | 0.5–2.0 | 2.8 | 0.9–7.7 | 2.2 | 0.9–4.9 | 1.6 | 0.5–5.5 | 0.33 |
| Syphilis infection (any RPR) | 19.5 | 16.7–22.2 | 26.3 | 16.4–39.3 | 26.2 | 21.0–32.2 | 19.7 | 13.7–27.5 | 0.12 |
| Syphilis infection (RPR > 1:8) | 7.2 | 5.6–9.3 | 8.7 | 4.8–15.0 | 10.3 | 7.6–14.9 | 7.9 | 4.4–13.9 | 0.57 |
Adjusted comparison of CS and weighted TSS estimates only (RDS estimates excluded from comparisons due to limited sample size)
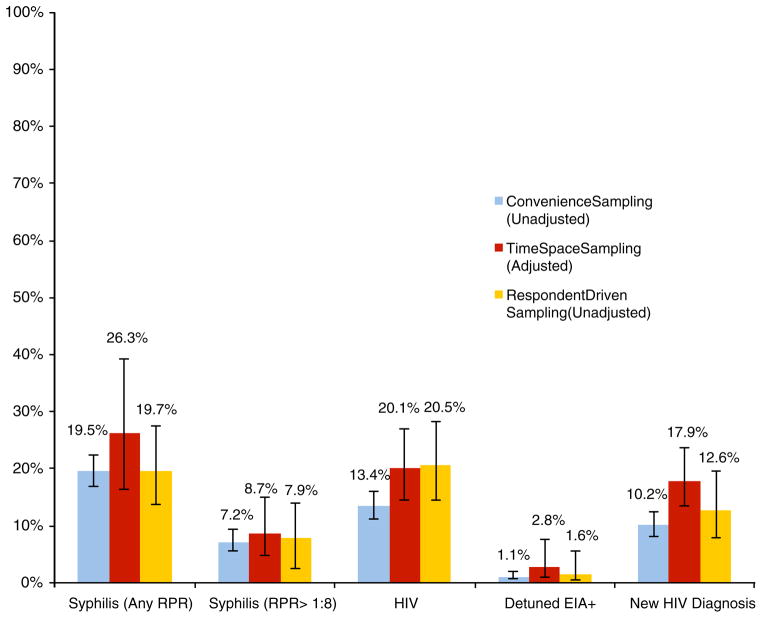
The size of participants’ MSM/TW social networks, frequency of venue attendance, previous involvement with LGBT community or political organizations, and prior participation in HIV/STI surveillance and/or research studies are reported in Table 2. Participants in the TSS sample more frequently reported attending an MSM/TW venue at least once a month (e.g., bars, discos, saunas, private gatherings, or public cruising areas associated with MSM/TW), but less commonly described previous participation in HIV/STI-related research than participants in the other arms, all of which were statistically significant in comparisons between the CS and weighted TSS estimates. The prevalence of newly diagnosed HIV infection (according to participants’ self-report of a previous HIV diagnosis) was significantly higher in the weighted TSS estimate compared with the CS sample (17.9 % in TSS and 10.2 % in CS; p < 0.01), and the difference in overall HIV prevalence between the TSS and CS samples had a trend toward statistical significance (20.1 and 13.4 %, respectively; p = 0.06). A qualitative, though not statistically significant, difference in the prevalence of recently acquired HIV infection (according to detuned EIA) was also observed (2.8 % in TSS versus 1.1 % in CS; p = 0.33) (Fig. 1). No significant differences in syphilis infection were noted, with a high prevalence of disease observed across all recruitment arms.
Table 2.
Participation in social networks/organizations/venues of MSM/TW samples recruited using CS, TSS, and RDS; Lima, Peru 2011
| Convenience sampling (CS) (N = 748) | Time space sampling (TSS) (N = 233) | Respondent driven sampling (RDS) (N = 127) | p valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Unweighted % | 95 % CI | Weighted % | 95 % CI | Unweighted % | 95 % CI | Unweighted % | 95 % CI | ||
| Size of MSM/TW social network (median, IQR) | |||||||||
| Any contact | 10 (3–20) | 20 (10–100) | 16 (7–30) | ||||||
| Contact within previous 6 months | 5 (2–10) | 10 (4–20) | 7 (3–15) | ||||||
| Attendance at MSM/TW venues once a month or more | 59.7 | 56.1–63.2 | 90.6 | 85.5–95.7 | 86.1 | 81.7–90.6 | 72.4 | 64.1–79.5 | 0.02 |
| Prior participation in MSM/TW political or social organization | 15.1 | 12.7–17.9 | 6.5 | 2.2–10.8 | 9.5 | 6.4–14.0 | 13.4 | 8.6–20.4 | < 0.01 |
| Prior participation in HIV/STI research study | |||||||||
| Any participation | 31.6 | 28.3–35.0 | 14.9 | 9.9–21.7 | 14.3 | 10.4–19.4 | 35.4 | 27.6–44.1 | < 0.01 |
| Participation within previous year | 11.7 | 9.6–14.2 | 8.3 | 2.7–13.8 | 6.9 | 4.3–11.0 | 15.7 | 10.4–23.1 | 0.27 |
Adjusted comparison of CS and weighted TSS estimates only (RDS estimates excluded from comparisons due to limited sample size)
Fig. 1.
Differences in HIV and syphilis prevalence among samples of MSM/TW according to recruitment methodology; Lima, Peru 2011
Discussion
Our findings provide empirical data on the operational efficiency and population characteristics of MSM/TW recruited for epidemiologic surveillance using three different sampling methodologies in Lima, Peru. All three methods enrolled a diverse sample of MSM/TW within a limited time period, though with significant differences in both operational and participant recruitment characteristics. Our empiric comparison underlines important differences in the potential use of these methodologies as frameworks for epidemiologic surveillance of MSM/TW in Latin America.
Convenience Sampling was effective at recruiting a large number of participants within a brief period of time with minimal resource requirements. However, the lack of statistical representativeness necessary for population-level estimates of HIV/STI prevalence and associated risk behaviors limits the potential use of this methodology for epidemiologic surveillance. Although continued use of CS recruitment methods could contribute to greater comparability of future surveillance surveys with previous findings, the resulting statistical estimates may not accurately reflect the prevalence of disease or associated risk behaviors in the MSM/TW population as a whole. Accordingly, future epidemiologic surveillance would ideally be based on alternative methods capable of recruiting statistically representative samples of MSM and TW populations in Peru.
In our study, RDS suffered from a low rate of enrollment that affected recruitment efficiency and undermined the potential validity of resulting population estimates. These findings are in striking contrast to other studies that have successfully used RDS for recruitment of MSM/TW in other areas of the world, including several studies in Latin America [19, 25, 42, 47, 50–52]. Specific factors that may have impaired RDS recruitment in our study include the low productivity of the seed participants, the lack of information on the size and interconnectedness of participants’ social networks, the geographic and/or temporal availability of enrollment sites, and the low perceived value of the incentives offered. RDS recruitment suffered from both a large number of non-productive seeds and a small number of recruitment waves achieved: 52.4 % of the non-seed sample was derived from two recruitment chains, and only three out of twenty-four chains achieved at least three waves of non-seed recruitment. Previous studies seeking to define characteristics of “productive” RDS seeds have found that factors including social network density, the strength of ties within the social network, and the number of social contacts with study-relevant behavior (though not the size of the seed participant’s network) are all important for predicting recruitment activity [53]. While our formative research used participant observation to identify well-connected individuals with large social networks of MSM/TW, we did not assess the density or strength of ties either within seed participants’ networks or among Peruvian MSM/TW generally. Other material factors that may have impaired RDS recruitment include the limited availability of enrollment sites and the low value of the enrollment incentives. Although our three enrollment sites were geographically distributed across the city, Lima’s sprawling urban landscape resulted in substantial time and travel requirements for many participants to attend one of the sites during daytime operating hours for a financial incentive that, in light of Peru’s rapidly growing economy, may have been perceived as low [32]. A recent case study of RDS methods in San Francisco has suggested that traditional recruitment procedures may need to be tailored to the specific conditions of the population of interest in order to be effective [54]. Illustrating this point, previous research conducted by members of our team was successful in using RDS to recruit TW in Lima, but was aided by the dense social networks connecting Peruvian TW, and supplemented traditional RDS enrollment sites with field recruitment procedures that removed many of the practical barriers to recruitment discussed here [47]. As a result, we suggest that RDS can be an effective method for sampling discrete populations connected by dense social networks, but may require substantial procedural modifications in order to be effective for epidemiologic surveillance of large, diverse, geographically diffuse populations with varying degrees of social interconnectedness, such as MSM/TW in Peru.
In contrast, TSS was effective in recruiting a large number of participants from previously undersampled populations during a brief time frame, but was also limited by a low rate of participant enrollment at traditional study sites. TSS was most effective during field-based recruitment, where the primary restrictions on enrollment were the limited space available in the mobile unit (two private counseling and testing spaces) and the length of time required for each participant to complete study procedures (45–60 min) within each 4-h VDT unit. Similar to the RDS arm, the percentage of TSS participants invited to participate in the field who later enrolled at one of the study sites was small (6.4 % of all MSM/TW who received coupons, or 12.9 % of the total TSS sample). In this context, strategies to increase interviewing capacity and limit time requirements for field interviewing and testing procedures could expand the number of participants recruited per VDT and substantially increase the efficiency of TSS enrollment.
From an epidemiologic standpoint, TSS succeeded in identifying a population sample distinct from those identified through CS, with greater representation of previously hidden subpopulations including TW, more extensive connections to venues and social networks associated with MSM/TW, a lower degree of involvement in prior HIV/STI research, and a higher prevalence of previously undiagnosed HIV infection (due to the small number of participants recruited through RDS, epidemiologic comparisons are limited to the CS and TSS samples). In the TSS arm, 14.9 % of participants reported prior participation in HIV/ STI-related research, compared with 31.6 % of the CS arm (p < 0.01). At the same time, individuals recruited through TSS reported more frequent attendance at MSM/TW venues than CS-recruited participants (attendance at least once per month by 90.6 % of TSS sample, compared with 59.7 % of CS sample; p = 0.02) and larger social networks of MSM/TW (Median number of recent social contacts = 10 in TSS sample, 5 in CS). Participants in the TSS sample also maintained a trend towards a higher prevalence of HIV infection than in the CS arm (p = 0.06), and a significantly higher prevalence of previously undiagnosed HIV (p < 0.01). Finally, the TSS sample included larger proportions of traditionally underrepresented subpopulations such as TW (p = 0.02), though the small scale of our study limits definitive statistical comparisons for recruitment of other sexual identity subgroups.
Within this context, potential benefits of continued use of CS for surveillance include consistency with previous HIV/STI monitoring studies and recruitment of a participant sample that better represents the target population of existing prevention efforts (as indicated by the relatively high prevalence of participants reporting previous involvement in HIV/STI research). However, both the large proportion of MSM/TW recruited through TSS who had not participated in previous investigations, and the high prevalence of undiagnosed HIV infection identified in the TSS sample suggest the importance of expanding both education and surveillance efforts to identify alternative MSM/TW populations that have not been reached through current public health surveillance and education systems. The high degree of social connectedness observed among participants in the TSS arm (as measured by their frequency of venue attendance and the size of their MSM/ TW social networks) also suggests that this group is an important component of the MSM/TW population in Peru that should be accounted for in HIV/STI prevention efforts. As a result, weighted analysis of data collected from TSS samples may be the most effective method to accurately define and monitor developments in the diversity of sexual identities, sexual risk behavior, and HIV/STI prevalences of MSM/TW populations in Peru’s local context.
Our analysis includes several limitations that could restrict the generalizability of the findings. The primary objective of the study was to conduct an empiric comparison of operational and population characteristics of different recruitment methods to inform the design of future epidemiologic surveillance efforts with MSM/TW in Peru and Latin America. Our study was not designed to provide accurate, precise estimates of HIV/STI prevalence or associated risk behaviors among MSM/TW in Peru. In the absence of an epidemiologic gold standard to determine the true prevalence of HIV/STIs and associated risk behaviors (e.g., truly random population surveys or census of the entire MSM/TW population), we are not able to verify the accuracy of the estimates obtained by the different sampling methods. Due to the small size of our RDS and TSS samples, clustering of participant recruitment within specific social network chains or venues may have biased the observed participant characteristics. In addition, the short time frame for recruitment and the relatively small sizes of the samples may not reflect the characteristics of the three methodologies as accurately as if recruitment had been allowed to continue over a longer period and enroll a greater number of participants. Finally, the use of different methods for survey completion (CASI for participants at clinic sites and paper surveys for participants at field venues) is likely to have influenced reporting of behavioral data, and may underlie the lower prevalence of unprotected anal intercourse observed among TSS participants. A comparison of participants in the TSS arm who completed paper surveys at field venues with those who completed CASI surveys at clinic sites found no substantial qualitative or statistically significant differences in self-reported risk behavior (p > 0.1 for all comparisons). Despite these limitations, our findings provide a unique source of empiric data comparing commonly used methods for epidemiologic surveillance in developing countries and will help inform Latin America’s future HIV/STI surveillance agenda.
Our findings provide data from a simultaneous, empiric comparison of CS, TSS, and RDS methods, highlighting the methodological and epidemiological issues involved in use of these strategies for epidemiologic surveillance of MSM/TW in Peru. While CS was effective in recruitment of a large sample of the target population within a limited period of time, there remain important questions concerning how well the sample recruited represents the larger MSM/TW population. In contrast to previous research, RDS was not effective for subject recruitment in our study, though none of the recruitment strategies that depended on potential participants visiting a study site for enrollment were as effective as those where participants were escorted to the site by recruiters or enrolled in mobile field units. TSS recruitment enrolled a sample with the greatest diversity of gender/sexual identity, lowest levels of self-reported sexual risk behavior, highest prevalences of undiagnosed HIV infection, and lowest frequency of participation in prior HIV/STI research. Decisions regarding which sampling method to use for future surveillance of MSM/TW in Latin America will depend on local contextual factors, including available resources, state of the regional HIV epidemic, and characteristics of MSM/TW social networks and community formations.
Acknowledgments
This work was supported by The Global Fund to Fight AIDS, Tuberculosis and Malaria and CARE Peru (PER-506-G03-H and PER-607-G05-H); the National Institutes of Health (T32 MH080634, P30 MH58107, P30 AI028697, UL1 TR000124, and K23 MH08461); and discretionary core funds from Asociación Civil Impacta Educación y Salud.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the views of the Global Fund, CARE, or the National Institutes of Health.
Contributor Information
J. L. Clark, Email: jlclark@mednet.ucla.edu, Division of Infectious Diseases and Program in Global Health, Department of Medicine, UCLA Geffen School of Medicine, 10833 Leconte Avenue, CHS 37-121, Los Angeles, CA 90095, USA
K. A. Konda, Division of Infectious Diseases and Program in Global Health, Department of Medicine, UCLA Geffen School of Medicine, 10833 Leconte Avenue, CHS 37-121, Los Angeles, CA 90095, USA
A. Silva-Santisteban, Unidad de Estudios de Sexualidad y Desarollo Humano, Universidad Peruana Cayetano Heredia, Lima, Peru
J. Peinado, Asociación Civil Impacta Salud y Educación, Lima, Peru
J. R. Lama, Asociación Civil Impacta Salud y Educación, Lima, Peru
L. Kusunoki, Asociación Civil Impacta Salud y Educación, Lima, Peru
A. Perez-Brumer, Division of Infectious Diseases and Program in Global Health, Department of Medicine, UCLA Geffen School of Medicine, 10833 Leconte Avenue, CHS 37-121, Los Angeles, CA 90095, USA
M. Pun, Dirección General de Epidemiología, Ministerio de Salud, Lima, Peru
R. Cabello, Asociación Civil Via Libre, Lima, Peru
J. L. Sebastian, Estrategia Nacional para el Control de VIH e ITS, Ministerio de Salud, Lima, Peru
L. Suarez-Ognio, Dirección General de Epidemiología, Ministerio de Salud, Lima, Peru
J. Sanchez, Asociación Civil Impacta Salud y Educación, Lima, Peru
References
- 1.Bastos FI, Caceres C, Galvao J, Veras MA, Castilho EA. AIDS in Latin America: assessing the current status of the epidemic and the ongoing response. Int J Epidemiol. 2008;37(4):729–37. doi: 10.1093/ije/dyn127. [DOI] [PubMed] [Google Scholar]
- 2.Calleja JM, Walker N, Cuchi P, Lazzari S, Ghys PD, Zacarias F. Status of the HIV/AIDS epidemic and methods to monitor it in the Latin America and caribbean region. AIDS. 2002;16(Suppl 3):S3–12. doi: 10.1097/00002030-200212003-00002. [DOI] [PubMed] [Google Scholar]
- 3.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez J, Lama JR, Kusunoki L, Manrique H, Goicochea P, Lucchetti A, et al. HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immune Defic Syndr. 2007;44(5):578–85. doi: 10.1097/QAI.0b013e318033ff82. [DOI] [PubMed] [Google Scholar]
- 5.Tabet S, Sanchez J, Lama J, Goicochea P, Campos P, Rouillon M, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16(9):1271–7. doi: 10.1097/00002030-200206140-00010. [DOI] [PubMed] [Google Scholar]
- 6.Montano SM, Sanchez JL, Laguna-Torres A, Cuchi P, Avila MM, Weissenbacher M, et al. Prevalences, genotypes, and risk factors for HIV transmission in South America. J Acquir Immune Defic Syndr. 2005;40(1):57–64. doi: 10.1097/01.qai.0000159667.72584.8b. [DOI] [PubMed] [Google Scholar]
- 7.Miller WM, Buckingham L, Sanchez-Dominguez MS, Morales-Miranda S, Paz-Bailey G. Systematic review of HIV prevalence studies among key populations in Latin America and the Caribbean. Salud Publica Mex. 2013;55(Suppl 1):S65–78. doi: 10.21149/spm.v55s1.5099. [DOI] [PubMed] [Google Scholar]
- 8.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista CT, Sanchez JL, Montano SM, Laguna-Torres VA, Lama JR, Kusunoki L, et al. Seroprevalence of and risk factors for HIV-1 infection among South American men who have sex with men. Sex Transm Infect. 2004;80(6):498–504. doi: 10.1136/sti.2004.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond HF, Rebchook G, Curotto A, Vaudrey J, Amsden M, Levine D, et al. Comparing internet-based and venue-based methods to sample MSM in the San Francisco Bay Area. AIDS Behav. 2010;14(1):218–24. doi: 10.1007/s10461-009-9521-6. [DOI] [PubMed] [Google Scholar]
- 11.Kalton G. Methods for oversampling rare populations in social surveys. Surv Method. 2009;35(2):125–41. [Google Scholar]
- 12.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The young men’s survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep. 1996;111(Suppl 1):138–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Karon JM, Wejnert C. Statistical methods for the analysis of time-location sampling data. J Urban Health. 2012;89(3):565–86. doi: 10.1007/s11524-012-9676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack LM, Osmond DH, Paul JP, Catania JA. Evaluation of the center for disease control and prevention’s HIV behavioral surveillance of men who have sex with men: sampling issues. Sex Transm Dis. 2005;32(9):581–9. doi: 10.1097/01.olq.0000175419.02839.d6. [DOI] [PubMed] [Google Scholar]
- 15.Semaan S. Time-space sampling and respondent-driven sampling with hard-to-reach populations. Method Innov Online. 2010;5(2):60–75. [Google Scholar]
- 16.MacKellar DA, Gallagher KM, Finlayson T, Sanchez T, Lansky A, Sullivan PS. Surveillance of HIV risk and prevention behaviors of men who have sex with men—a national application of venue-based, time-space sampling. Public Health Rep. 2007;122(Suppl 1):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valleroy LA, MacKellar DA, Karon JM, Rosen DH, McFarland W, Shehan DA, et al. HIV prevalence and associated risks in young men who have sex with men. JAMA. 2000;284(2):198–204. doi: 10.1001/jama.284.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, et al. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116(Suppl 1):216–22. doi: 10.1093/phr/116.S1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Bailey G, Miller W, Shiraishi RW, Jacobson JO, Abimbola TO, Chen SY. Reaching men who have sex with men: a comparison of respondent-driven sampling and time-location sampling in Guatemala City. AIDS Behav. 2013;17:3081–90. doi: 10.1007/s10461-013-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Prob. 1997;44(2):175–99. [Google Scholar]
- 21.Heckathorn D. Respondent-Driven Sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Prob. 2002;49(1):11–34. [Google Scholar]
- 22.Salganik M, Heckathorn D. Sampling and estimation in hidden populations using respondent-driven sampling. Soc Method. 2004;34:193–239. [Google Scholar]
- 23.Malekinejad M, Johnston LG, Kendall C, Kerr LR, Rifkin MR, Rutherford GW. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav. 2008;12(4 Suppl):S105–30. doi: 10.1007/s10461-008-9421-1. [DOI] [PubMed] [Google Scholar]
- 24.Platt L, Wall M, Rhodes T, Judd A, Hickman M, Johnston LG, et al. Methods to recruit hard-to-reach groups: comparing two chain referral sampling methods of recruiting injecting drug users across nine studies in Russia and Estonia. J Urban Health. 2006;83(6 Suppl):i39–53. doi: 10.1007/s11524-006-9101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez-Valles J, Garcia D, Campbell RT, Diaz RM, Heckathorn DD. HIV infection, sexual risk behavior, and substance use among Latino gay and bisexual men and transgender persons. Am J Public Health. 2008;98(6):1036–42. doi: 10.2105/AJPH.2006.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez-Valles J, Heckathorn DD, Vazquez R, Diaz RM, Campbell RT. From networks to populations: the development and application of respondent-driven sampling among IDUs and Latino gay men. AIDS Behav. 2005;9(4):387–402. doi: 10.1007/s10461-005-9012-3. [DOI] [PubMed] [Google Scholar]
- 27.Robinson WT, Risser JM, McGoy S, Becker AB, Rehman H, Jefferson M, et al. Recruiting injection drug users: a three-site comparison of results and experiences with respondent-driven and targeted sampling procedures. J Urban Health. 2006;83(6 Suppl):i29–38. doi: 10.1007/s11524-006-9100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston LG, Chen YH, Silva-Santisteban A, Raymond HF. An empirical examination of respondent driven sampling design effects among HIV risk groups from studies conducted around the world. AIDS Behav. 2013;17(6):2202–10. doi: 10.1007/s10461-012-0394-8. [DOI] [PubMed] [Google Scholar]
- 29.Montealegre JR, Johnston LG, Murrill C, Monterroso E. Respondent driven sampling for HIV biological and behavioral surveillance in Latin America and the Caribbean. AIDS Behav. 2013;17(7):2313–40. doi: 10.1007/s10461-013-0466-4. [DOI] [PubMed] [Google Scholar]
- 30.Salganik MJ. Variance estimation, design effects, and sample size calculations for respondent-driven sampling. J Urban Health. 2006;83(6 Suppl):i98–112. doi: 10.1007/s11524-006-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel S, Salganik MJ. Assessing respondent-driven sampling. Proc Natl Acad Sci USA. 2010;107(15):6743–7. doi: 10.1073/pnas.1000261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCreesh N, Johnston LG, Copas A, Sonnenberg P, Seeley J, Hayes RJ, et al. Evaluation of the role of location and distance in recruitment in respondent-driven sampling. Int J Health Geo. 2011;10:56. doi: 10.1186/1476-072X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paquette D, Bryant J, de Wit J. Respondent-driven sampling and the recruitment of people with small injecting networks. AIDS Behav. 2012;16(4):890–9. doi: 10.1007/s10461-011-0032-x. [DOI] [PubMed] [Google Scholar]
- 34.Heimer R. Critical issues and further questions about respondent-driven sampling: comment on Ramirez-Valles, et al. (2005) AIDS Behav. 2005;9(4):403–8. doi: 10.1007/s10461-005-9030-1. [DOI] [PubMed] [Google Scholar]
- 35.McCreesh N, Frost SD, Seeley J, Katongole J, Tarsh MN, Ndunguse R, et al. Evaluation of respondent-driven sampling. Epidemiology. 2012;23(1):138–47. doi: 10.1097/EDE.0b013e31823ac17c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salganik MJ. Commentary: respondent-driven sampling in the real world. Epidemiology. 2012;23(1):148–50. doi: 10.1097/EDE.0b013e31823b6979. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa A, Jr, Pascom AR, Szwarcwald CL, Kendall C, McFarland W. Transfer of sampling methods for studies on most-at-risk populations (MARPs) in Brazil. Cad Saude Publica. 2011;27(Suppl 1):S36–44. doi: 10.1590/s0102-311x2011001300005. [DOI] [PubMed] [Google Scholar]
- 38.Calleja JM, Marum LH, Carcamo CP, Kaetano L, Muttunga J, Way A. Lessons learned in the conduct, validation, and interpretation of national population based HIV surveys. AIDS. 2005;19(Suppl 2):S9–17. doi: 10.1097/01.aids.0000172872.88347.f3. [DOI] [PubMed] [Google Scholar]
- 39.Carballo-Dieguez A, Balan I, Marone R, Pando MA, Dolezal C, Barreda V, et al. Use of respondent driven sampling (RDS) generates a very diverse sample of men who have sex with men (MSM) in Buenos Aires, Argentina. PLoS ONE. 2011;6(11):e27447. doi: 10.1371/journal.pone.0027447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost SD, Brouwer KC, Firestone Cruz MA, Ramos R, Ramos ME, Lozada RM, et al. Respondent-driven sampling of injection drug users in two U.S.-Mexico border cities: recruitment dynamics and impact on estimates of HIV and syphilis prevalence. J Urban Health. 2006;83(6 Suppl):i83–97. doi: 10.1007/s11524-006-9104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gondim RC, Kerr LR, Werneck GL, Macena RH, Pontes MK, Kendall C. Risky sexual practices among men who have sex with men in Northeast Brazil: results from four sequential surveys. Cad Saude Publica. 2009;25(6):1390–8. doi: 10.1590/s0102-311x2009000600021. [DOI] [PubMed] [Google Scholar]
- 42.Kendall C, Kerr LR, Gondim RC, Werneck GL, Macena RH, Pontes MK, et al. An empirical comparison of respondent-driven sampling, time location sampling, and snowball sampling for behavioral surveillance in men who have sex with men, Fortaleza, Brazil. AIDS Behav. 2008;12(4 Suppl):S97–104. doi: 10.1007/s10461-008-9390-4. [DOI] [PubMed] [Google Scholar]
- 43.Szwarcwald CL, De Souza PR, Jr, Damacena GN, Junior AB, Kendall C. Analysis of data collected by RDS among sex workers in 10 Brazilian cities, 2009: estimation of the prevalence of HIV, variance, and design effect. J Acquir Immune Defic Syndr. 2011;57(Suppl 3):S129–35. doi: 10.1097/QAI.0b013e31821e9a36. [DOI] [PubMed] [Google Scholar]
- 44.Garcia PJ, Holmes KK, Carcamo CP, Garnett GP, Hughes JP, Campos PE, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet. 2012;379 (9821):1120–8. doi: 10.1016/S0140-6736(11)61846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paz-Bailey G, Jacobson JO, Guardado ME, Hernandez FM, Nieto AI, Estrada M, et al. How many men who have sex with men and female sex workers live in El Salvador? Using respondent-driven sampling and capture-recapture to estimate population sizes. Sex Transm Infect. 2011;87(4):279–82. doi: 10.1136/sti.2010.045633. [DOI] [PubMed] [Google Scholar]
- 46.Kerr LR, Mota RS, Kendall C, Pinho AD, Mello MB, Guimaraes MD, et al. HIV among MSM in Brazil. AIDS. 2012 Oct 17; [Google Scholar]
- 47.Silva-Santisteban A, Raymond HF, Salazar X, Villayzan J, Leon S, McFarland W, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav. 2012;16(4):872–81. doi: 10.1007/s10461-011-0053-5. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira LO, de Oliveira ES, Raymond HF, Chen SY, McFarland W. Use of time-location sampling for systematic behavioral surveillance of truck drivers in Brazil. AIDS Behav. 2008;12(4 Suppl):S32–8. doi: 10.1007/s10461-008-9386-0. [DOI] [PubMed] [Google Scholar]
- 49.Wei C, McFarland W, Colfax GN, Fuqua V, Raymond HF. Reaching black men who have sex with men: a comparison between respondent-driven sampling and time-location sampling. Sex Transm Infect. 2012;88(8):622–6. doi: 10.1136/sextrans-2012-050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerr LR, Mota RS, Kendall C, de Pinho AA, Mello MB, Guimaraes MD, et al. HIV among MSM in a large middle-income country. AIDS. 2013;27(3):427–35. doi: 10.1097/QAD.0b013e32835ad504. [DOI] [PubMed] [Google Scholar]
- 51.Johnston LG, Vaillant TC, Dolores Y, Vales HM. HIV, hepatitis B/C and syphilis prevalence and risk behaviors among gay, transsexuals and men who have sex with men, Dominican Republic. Int J STD AIDS. 2013;24(4):313–21. doi: 10.1177/0956462412472460. [DOI] [PubMed] [Google Scholar]
- 52.Johnston LG, Khanam R, Reza M, Khan SI, Banu S, Alam MS, et al. The effectiveness of respondent driven sampling for recruiting males who have sex with males in Dhaka, Bangladesh. AIDS Behav. 2008;12(2):294–304. doi: 10.1007/s10461-007-9300-1. [DOI] [PubMed] [Google Scholar]
- 53.Reisner SL, Mimiaga MJ, Johnson CV, Bland S, Case P, Safren SA, et al. What makes a respondent-driven sampling “seed” productive? example of finding at-risk Massachusetts men who have sex with men. J Urban Health. 2010;87(3):467–79. doi: 10.1007/s11524-010-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Truong HH, Grasso M, Chen YH, Kellogg TA, Robertson T, Curotto A, et al. Balancing theory and practice in respondent-driven sampling: a case study of innovations developed to overcome recruitment challenges. PLoS ONE. 2013;8(8):e70344. doi: 10.1371/journal.pone.0070344. [DOI] [PMC free article] [PubMed] [Google Scholar]