Abstract
Acute kidney injury (AKI) is common in patients with cirrhosis and associated with significant mortality. The most common etiologies of AKI in this setting are pre-renal azotemia (PRA), acute tubular necrosis (ATN) and hepatorenal syndrome (HRS). Accurately distinguishing the etiology of AKI is critical as treatments differ markedly. However, establishing an accurate differential diagnosis is extremely challenging. Urinary biomarkers of kidney injury distinguish structural from functional causes of AKI and may facilitate more accurate and rapid diagnoses. We conducted a multi-center, prospective cohort study of patients with cirrhosis and AKI assessing multiple biomarkers for differential diagnosis of clinically adjudicated AKI. Patients (n=36) whose creatinine returned to within 25% of their baseline within 48 hours were diagnosed with PRA. 76 patients with progressive AKI were diagnosed via blinded retrospective adjudication. Of these progressors, thirty-nine (53%) patients were diagnosed with ATN, 19 (26%) with PRA and 16 (22%) with HRS. Median values for neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), liver-type fatty acid binding protein (L-FABP) and albumin differed between etiologies and were significantly higher in patients adjudicated with ATN. The fractional excretion of sodium (FENa) was lowest in patients with HRS, 0.10%, but did not differ between those with PRA, 0.27%, or ATN, 0.31%, p=0.54. The likelihood of being diagnosed with ATN increased step-wise with number of biomarkers above optimal diagnostic cutoffs.
Conclusion
Urinary biomarkers of kidney injury are elevated in patients with cirrhosis and AKI due to ATN. Incorporating biomarkers into clinical decision making has the potential to more accurately guide treatment by establishing which patients have structural injury underlying their AKI. Further research is required to document biomarkers specific to HRS.
Keywords: AKI, etiology, adjudication, urinary markers, ATN
Introduction
Acute kidney injury (AKI) is common in patients with cirrhosis, occurring in 20% of hospitalizations1, and is associated with significant mortality2–4. The most common causes of AKI in this setting are pre-renal azotemia (PRA), acute tubular necrosis (ATN) and hepatorenal syndrome (HRS). Despite the overall poor prognosis for patients with cirrhosis and AKI, viable treatments do exist but differ significantly by AKI etiology. PRA should be treated with aggressive volume expansion5 while such fluid administration is unhelpful and even potentially harmful in patients with ATN6. HRS may be reversed with restoration of renal perfusion, either via vasoconstrictor therapy plus intravenous albumin7 or liver transplant8. Patients with severe ATN may reasonably be treated with dialysis. Unfortunately, current diagnostic strategies are often unable to make the challenging yet crucial distinction between structural and functional disease. HRS is diagnosed via the International Ascites Club (IAC) criteria, now set within a more broad classification system of AKI in cirrhosis proposed jointly by the IAC and the Acute Dialysis Quality Initiative (ADQI)9. However, these criteria are neither sensitive nor specific and may result in misallocation of scarce resources and potentially harmful unnecessary treatments.
It is obvious that new, objective tests to accurately facilitate the distinction of structural from functional AKI in patients with cirrhosis are urgently needed. There is currently tremendous research interest in novel urinary biomarkers of structural kidney injury for early diagnosis, differential diagnosis and prognosis in AKI10. Multiple biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18) and kidney injury molecule-1 (KIM-1) are able to distinguish structural from functional causes of AKI in numerous clinical settings11–13. Such biomarkers, which are specifically reflective of frank structural injury, may be particularly well suited to untangle the frequently vexing diagnostic distinction between ATN and HRS. However, in patients with cirrhosis, where kidney biopsies are uncommonly performed, the very lack of an effective existing diagnostic test or criteria makes the development of new tests challenging as the gold standard against which new tests are to be compared is known to be flawed. Patients whose AKI rapidly resolves can be assumed to have had PRA. However, in patients with progressive AKI, where accurately distinguishing etiology is most therapeutically critical, confidently determining the differential diagnosis it can be extremely challenging. The IAC criteria for HRS are useful for their simplicity in that they can be employed at the bedside to diagnose the etiology of AKI without requiring knowledge of the patient’s entire hospital course but often lack the granularity of data required for distinguishing structural from functional disease. Alternatively, retrospective adjudication by expert clinicians with access to data on the entirety of the course of a patient’s AKI, while obviously not applicable for point of care diagnosis, provides a more robust gold standard for the development of new objective tests which may then themselves be applied at the bedside.
While biomarkers hold tremendous promise to clarify the diagnostic muddle of AKI in cirrhosis, it is unlikely any will result in a clear “positive” or “negative” cut off, tests results will need to be interpreted in light of the overall clinical picture. Similarly, it may be that a combination of multiple markers is more informative than any alone. The few previous studies of AKI biomarkers in cirrhosis have only looked at one marker14–16, used IAC14,15 or unconventional criteria16 as the gold standard and did not explore how results could be incorporated into clinical decision making. We have conducted a prospective, multi-center study of patients with cirrhosis and AKI that measured multiple urinary biomarkers, including NGAL, IL-18, KIM-1, liver-type fatty acid binding protein (L-FABP), albumin and fractional excretion of sodium (FENa). In this analysis, we assess the ability of these biomarkers to improve the differential diagnosis of patients with clinically adjudicated etiologies of AKI. Subsequently, we have employed likelihood ratios to demonstrate how biomarkers results, through the identification of patients with ATN, can clarify uncertain clinical diagnoses.
Materials and Methods
Study design
The details of the cohort and study design have been described previously4. This prospective, multi-center observational cohort study was conducted over 29 months between 2009 and 2011 at four tertiary care academic centers in the US. Eligible patients were admitted with AKI (see “Definition”) or developed it during the course of the hospitalization. Inclusion criteria included a known diagnosis of cirrhosis (see “Definitions”), age ≥ 18 years, and availability of a documented baseline serum creatinine. Exclusion criteria included prior kidney or liver transplant, advanced chronic kidney disease (baseline creatinine > 4.0 mg/dL), acute or chronic renal replacement therapy at the time of enrollment, clinically estimated life expectancy < 3 days, confirmed pregnancy and other known causes of renal insufficiency such as glomerulonephritis or urinary obstruction. Consent was obtained from all patients or their surrogate decision maker. If a patient was unable to provide written consent and a surrogate was unavailable, a urine specimen was nevertheless collected. Over the following seven days, delayed consent was sought from either patient or surrogate. If consent could not be obtained during this period, the urine sample was discarded. All consecutive eligible patients identified during screening were approached for enrollment.. The study was approved by the institutional review board at each of the participating institution.
Sample Collection and Biomarker Measurement
A fresh 10-ml urine sample was collected daily for three days either via clean catch or Foley catheter tubing. Samples were immediately refrigerated and then centrifuged at 5000 × g for 10 minutes at −4°C. Aliquots of 1 ml of supernatant were subsequently stored within 6 hours of collection in cryovials at −80°C for NGAL, IL-18, KIM-1, L-FABP, albumin, sodium and creatinine measurements. No additives or protease inhibitors were utilized. All biomarkers were measured from frozen aliquots that did not undergo any additional freeze-thaw cycles. Laboratory measurements were performed by personnel blinded to patient information.
Sekisui Diagnostics LLC developed assays for KIM-1 and L-FABP. Capture antibodies were bound to Multi-Assay 96 well plates (MesoScale Discovery [MSD], Gaithersburg, MD) and detection antibodies were biotinlyated. Signal generation relied on strepavidin coupled Sulfo-Tag (MSD). The Sulfo-Tag includes ruthenium(II)-tris-bipyridine, which in combination with a triproplyamine read buffer generates an electrochemical signal detected by a Sector Imager 2400™ (MSD). Sekisui Diagnostics LLC also developed the rabbit anti-KIM-1 antibodies (for capture and detection) and recombinant hKIM-1 (for standards and controls). CMIC (Tokyo, Japan) supplied monoclonal antibodies and rec hL-FABP standards. The detection range for KIM-1 is .056–60 ng/mL while L-FABP is .057–400 ng/mL. The intra-assay coefficient of variation is ≤10% for both assays. ELISA methods, coefficient of variation and the detection ranges were as described previously for the measurement of NGAL17 and IL-1818. Urine creatinine was measured by the modified Jaffe reaction.
Adjudication
Adjudication of the cause of AKI was performed by a committee of two nephrologists and one hepatologist after the patient was discharged or expired. Adjudicators were selected to provide a breadth of experience and primary site of clinical practice (University vs Veterans Administration). Only those patients whose AKI progressed to a higher AKIN stage were adjudicated. This decision was made for reasons of practicality and because the greatest diagnostic confusion is typically seen in patients whose AKI continues to progress despite initial standard management. If patients who presented with Stage 3 AKI by creatinine criteria but not requiring renal replacement therapy subsequently required dialysis, this was considered as progression. Adjudicators were provided with a standardized data form containing key variables related to the patients’ medical history, hospital presentation, general medical and cirrhosis specific hospital events, medical therapies and renal function. Additionally, data were provided detailing vital signs and fluid balance for a period of 10 days surrounding biomarker collection. Options for diagnosis included PRA, HRS and intrinsic kidney disease, to be specified as ATN or other pathologies. Final diagnosis was contingent on the agreement of at least two adjudicators. Adjudicators were blinded to measurements of NGAL, IL-18, KIM-1, L-FABP and albumin but had access to urine sodium values if these were measured in the course of clinical care.
Variables
Independent Variables
Cirrhosis
Patients were eligible who carried an existing documented diagnosis of cirrhosis based on liver biopsy, when available, or on a combination of clinical, biochemical, imaging and endoscopic findings.
AKI
AKI was defined as arise in creatinine of 0.3 mg/dL or 50% from baseline as recommended by a working group composed of members of the IAC and the ADQI who based this cut-off on Stage 1 of the acute kidney injury network (AKIN) criteria19.
Baseline serum creatinine
Baseline serum creatinine was defined as the most recent stable measurement within a year prior to admission for the index hospitalization. The use of outpatient values for establishing baseline creatinine has been shown to result in less misclassification of AKI incidence, severity and prognosis compared to utilizing hospital admission, hospital nadir or imputed values20. When possible, outpatient measurements were utilized though values were also used from previous admissions not complicated by AKI. The median and inter-quartile range (IQR) for the interval between the creatinine utilized for baseline and hospital admission was 26 (9–73) days. In rare cases, patients without an outpatient measurement were included in the analytic cohort if, prior to onset of AKI, they manifested at least 5 initial days from admission of stable values within the normal creatinine range. In these instances, the creatinine at admission was considered the baseline.
Other variables
Glomerular filtration rate (GFR) was estimated via the CKD-EPI equation using the baseline creatinine value21. Chronic kidney disease was defined as estimated GFR < 60 ml/min/1.73m2 present for at least 3 months. Model of end-stage liver disease (MELD) and Child-Pugh scores were calculated on the day of first sample collection.
Outcomes
Our primary outcome was AKI diagnosis. Patients were diagnosed with PRA either via adjudication in those patients whose AKI progressed or by the designation of PRA in patients whose AKI did not progress and whose serum creatinine returned to within 25% of baseline within 48 hours of developing AKI. HRS and ATN were diagnosed via adjudication in patients with progressive AKI.
Statistics
Categorical variables were expressed as proportions and compared using Chi-square and Fisher’s exact test, as appropriate. Normally or near-normally distributed variables were reported as means with standard deviations (SD) and compared by Student’s t-test. Non-normally distributed continuous variables were reported as medians with IQR and compared by the Wilcoxon rank sum test. Normality was assessed using the Kolmogorov-Smirnov test. NGAL values were bounded at an upper limit of 1000 ng/mL with no lower bound. All patients with values above 1000 ng/mL were assigned a value of 1000. KIM-1 was bounded at an upper limit of 60 ng/ml and a lower limit of 0.056 ng/ml. L-FABP was bounded at an upper limit of 400 ng/ml and a lower limit of 0.57 ng/ml. IL-18 did not have a bounded upper limit but the lower limit of detection for the assay was 25 pg/mL. All patients below this threshold were assigned a value of 15 pg/mL.
The primary analysis evaluated biomarkers ability to identify patients with ATN. Areas under the curve (AUC) with 95% confidence intervals (CI) were calculated to evaluate biomarkers for risk discrimination. Optimal cutoffs were determined for diagnosing ATN versus non-ATN. Utilizing these cutoffs, biomarker performance was assessed through the calculation of sensitivity, specificity and positive and negative likelihood ratios. Likelihood ratios were then applied to examples wherein pretest probability for ATN is converted to posttest. Those biomarkers whose levels differed significantly between diagnoses were selected for a panel and relative risks for ATN were calculated based on number of markers above their optimal cutoffs. To determine internal validity of the results, a leave-10-out cross validation was performed using SAS Proc Surveyselect. In a secondary analysis, biomarkers were also evaluated for their ability to distinguish the three distinct diagnoses of PRA, HRS and ATN. A 2-sided p<0.05 was considered significant for all analysis. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Cary, NC). The conditional probability curves were constructed using the spreadsheet devised by MacEneaney and Malone22.
Results
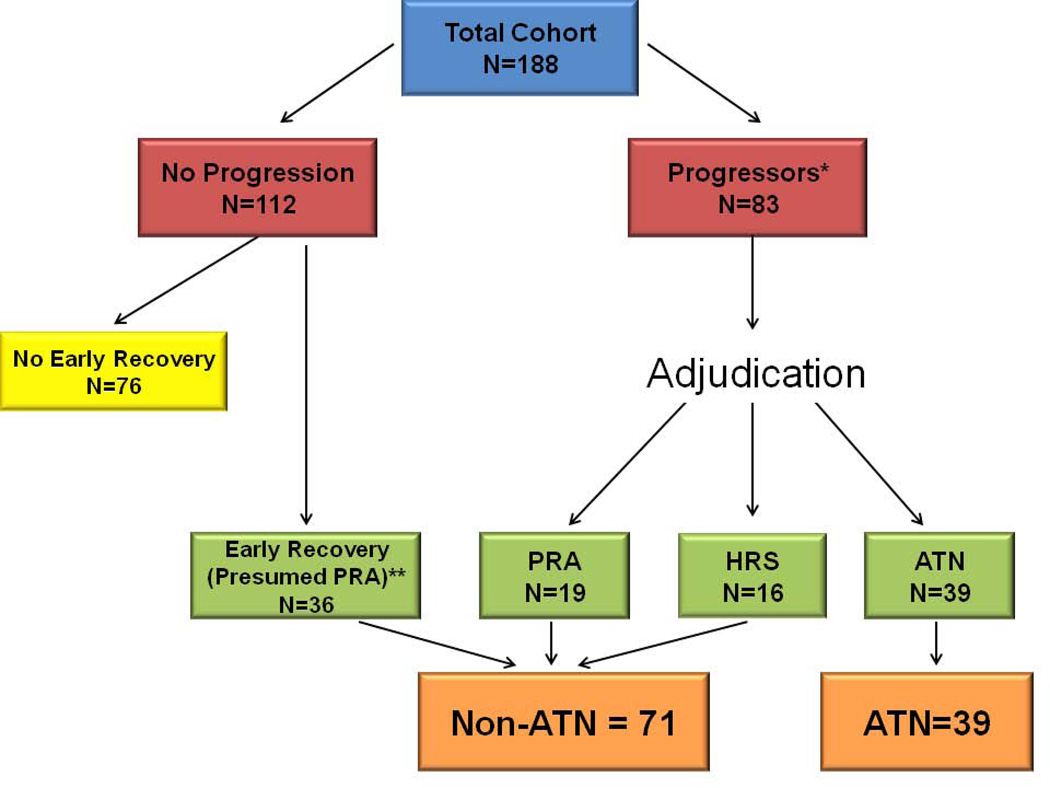
A total of 188 patients with cirrhosis and AKI with available urinary biomarkers were enrolled in the study. Of these, 83 experienced progression of their AKI. The distribution of adjudicators diagnoses is shown in Supplemental Table 1. Thirty-nine (53%) patients were diagnosed with ATN, 19 (26%) with PRA and 16 (22%) with HRS. 36 additional patients were assigned a diagnosis of PRA due to their creatinine returning to within 25% of baseline within 48 hours. The breakdown of patient diagnosis is shown in Figure 1. Baseline demographic, clinical and laboratory data for all adjudicated patients and for those with and without ATN are shown in Table 1. There was no difference in cirrhosis etiology or previous complications of cirrhosis between groups. The reason for admission was similar between the two groups excepting jaundice and infections other than spontaneous bacterial peritonitis which were more common in patients diagnosed with ATN. Median baseline estimated GFR was lower in patients without ATN than in those with ATN (67 vs 84ml/min/1.73m2) though this did not reach statistical significance (P = 0.09). Serum creatinine at the time of sample collection differed significantly between groups and was higher in patients diagnosed with ATN. Patients with ATN had more advanced cirrhosis as assessed both by the model for end-stage liver disease (MELD) score (31 vs 24) and Child-Pugh (11 vs 10). Though intravenous albumin administration was near ubiquitous in all groups, patients adjudicated with ATN were treated more frequently with midodrine and octreotide. The number of IAC criteria fulfilled for the diagnosis of HRS (5/6) was identical between the two groups.
Figure 1. Process for Determination of Differential Diagnosis.
The process by which patients with cirrhosis and AKI had the etiology of their AKI determined.
*7 patients who progressed were enrolled during the pilot phase of the study and had incomplete data collection. These patients were excluded from adjudication to avoid information bias. In addition, 2 patients who did not have 2/3 adjudicator diagnostic agreement were excluded. Of the remaining 74, 3/3 adjudicators agreed for 37 patients and 2/3 for 37 patients.
**Of the non-progressors with rapid recovery who were assigned a diagnosis of PRA, 6 (17%) were additionally adjudicated and all 6/6 were adjudicated as having PRA.
Table 1.
Baseline and Clinical Characteristics of All Patients and Those With and Without ATN
| Total N = 110 |
Not ATN N=71 |
ATN N=39 |
P* | |
|---|---|---|---|---|
| Age in years - mean ± SD | 55.3 ± 9.8 | 56.4 ± 9.4 | 53.3 ± 10.3 | 0.24 |
| Male sex – n (%) | 76 (69) | 48 (68) | 28 (72) | 0.65 |
| BMI – median (IQR) | 30.4 (25.6–35) | 28.1 (25–33.3) | 32.2 (28.6–36.1) | |
| Race – n (%) | ||||
| White | 83 (75) | 56 (79) | 27 (69) | 0.26 |
| Black | 13 (12) | 7 (10) | 6 (15) | 0.39 |
| Hispanic | 12 (11) | 7 (10) | 5 (13) | 0.75 |
| Diabetes – n (%) | 30 (27) | 18 (25) | 12 (31) | 0.54 |
| Active Cancer – n (%) | 13 (12) | 8 (11) | 5 (13) | 0.81 |
| Renal function | ||||
| Baseline creatinine mg/dL – median (IQR) | 1 (0.8–1.3) | 1.1 (0.8–1.3) | 1.0 (0.7–1.2) | 0.27 |
| CKDa | 30 (27) | 21 (30) | 9 (23) | 0.46 |
| Baseline GFR ml/min – median (IQR) | 72 (58–98) | 67 (53–95) | 84 (60–102) | 0.09 |
| Proteinuria – n (%)b | 11 (10) | 5 (7) | 6 (15) | 0.16 |
| Creatinine mg/dL – median (IQR)c | 2.1 (1.5–3.5) | 1.8 (1.4–2.5) | 3.3 (2.3–4.1) | <0.001 |
| BUN mg/dL – median (IQR)c | 44 (29–62) | 41 (27–54) | 49 (33–72) | 0.06 |
| Peak creatinine mg/dL - median (IQR) | 2.6 (1.8–4.2) | 2.2 (1.6–3.4) | 4.1 (2.9–5.2) | <0.001 |
| Dialysis – n (%) | 27 (25) | 9 (13) | 18 (46) | <0.001 |
| Cirrhosis etiology – n (%) | ||||
| Alcohol | 30 (27) | 19 (27) | 11 (28) | 0.87 |
| Alcohol and HCV | 30 (27) | 22 (31) | 8 (21) | 0.24 |
| HCV | 20 (18) | 14 (20) | 6 (15) | 0.57 |
| NASH | 11 (10) | 9 (13) | 2 (5) | 0.32 |
| Cryptogenic | 6 (5) | 3 (4) | 3 (8) | 0.66 |
| Autoimmune | 8 (7) | 4 (6) | 4 (10) | 0.37 |
| Other | 5 (5) | 0 (0) | 5 (13) | 0.004 |
| Previous complications of cirrhosis – n (%) | ||||
| Ascites | 83 (76) | 57 (80) | 26 (68) | 0.17 |
| Hepatic encephalopathy | 72 (65) | 49 (69) | 23 (59) | 0.29 |
| Variceal bleed | 24 (22) | 18 (25) | 6 (15) | 0.23 |
| SBP | 13 (12) | 10 (14) | 3 (8) | 0.37 |
| Reason for admission – n (%) | ||||
| Hepatic encephalopathy | 23 (21) | 17 (24) | 6 (15) | 0.29 |
| Refractory ascites/edema | 16 (15) | 13 (18) | 3 (8) | 0.16 |
| Abdominal pain | 11 (10) | 7 (10) | 4 (10) | 0.95 |
| AKI | 14 (13) | 10 (14) | 4 (10) | 0.77 |
| GI bleed | 10 (9) | 6 (8) | 4 (10) | 0.75 |
| Jaundice | 8 (7) | 2 (3) | 6 (15) | 0.02 |
| Infection other than SBP | 3 (3) | 0 (0) | 3 (8) | 0.04 |
| SBP | 3 (3) | 1 (1) | 2 (5) | 0.27 |
| Other | 22 (20) | 13 (18) | 9 (23) | 0.55 |
| Cirrhosis severityc | ||||
| Child-Pugh Class - n (%) | 0.006 | |||
| A | 3 (3) | 3 (4) | 0 (0) | |
| B | 35 (32) | 29 (41) | 6 (15) | |
| C | 72 (65) | 39 (55) | 33 (85) | |
| Child-Pugh score – median (IQR) | 10 (9–12) | 10 (8–12) | 11 (10–12) | 0.005 |
| MELD score – mean ± SD | 26.4 ± 9.5 | 24 ± 7.9 | 31 ± 10.5 | <0.001 |
| Sodium – mean ± SD | 133 ± 6 | 133 ± 5 | 134 ± 6 | 0.78 |
| Hyponatremiad – n (%) | 33 (30) | 22 (31) | 11 (28) | 0.76 |
| MAP (max) - mmHg mean ± SD | 85 ± 14 | 83 ± 12 | 90 ± 17 | 0.05 |
| MAP (min) - mmHg mean ± SD | 67 ± 12 | 68 ± 11 | 67 ± 14 | 0.43 |
| IAC criteria – median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.43 |
| Hospital complications – n (%) | ||||
| HEENC | 58 (53) | 34 (48) | 24 (62) | 0.17 |
| Ascites | 91 (83) | 58 (82) | 33 (85) | 0.70 |
| SBP | 22 (20) | 9 (13) | 13 (33) | 0.01 |
| EVB | 10 (9) | 7 (10) | 3 (8) | 1.00 |
| Pneumonia | 19 (17) | 6 (8) | 13 (33) | 0.001 |
| Bacteremia | 18 (16) | 8 (11) | 10 (26) | 0.05 |
| GI Bleed | 26 (24) | 13 (24) | 13 (33) | 0.08 |
| UTI | 27 (25) | 16 (23) | 11 (28) | 0.50 |
| Therapies – n (%) | ||||
| Albumin | 97 (88) | 61 (86) | 36 (92) | 0.32 |
| Midodrine | 53 (48) | 29 (41) | 24 (62) | 0.04 |
| Octreotide | 56 (51) | 28 (39) | 28 (72) | 0.001 |
Abbreviations: N, number; PRA, pre-renal azotemia; HRS, hepatorenal syndrome; ATN, acute tubular necrosis; SD, standard deviation; BMI, body mass index; IQR, inter-quartile range; CKD, chronic kidney disease; GFR, glomerular filtration rate; BUN, blood urea nitrogen; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; AKI, acute kidney injury; GI, gastrointestinal; SBP, spontaneous bacterial peritonitis; MELD, model for end-stage liver disease; MAP, mean arterial pressure; HEENC, hepatic encephalopathy; EVB, esophageal variceal bleed; UTI, urinary tract infection
CKD is defined as estimated GFR 60 < ml/min by CKD-EPI equation
Microalbuminuria (30mg/dL) or greater on dipstick or quantitative measurement prior to admission
BUN, creatinine and indices of cirrhosis severity are on day of sample collection
Serum sodium <130 mEq/L
Universal f-test
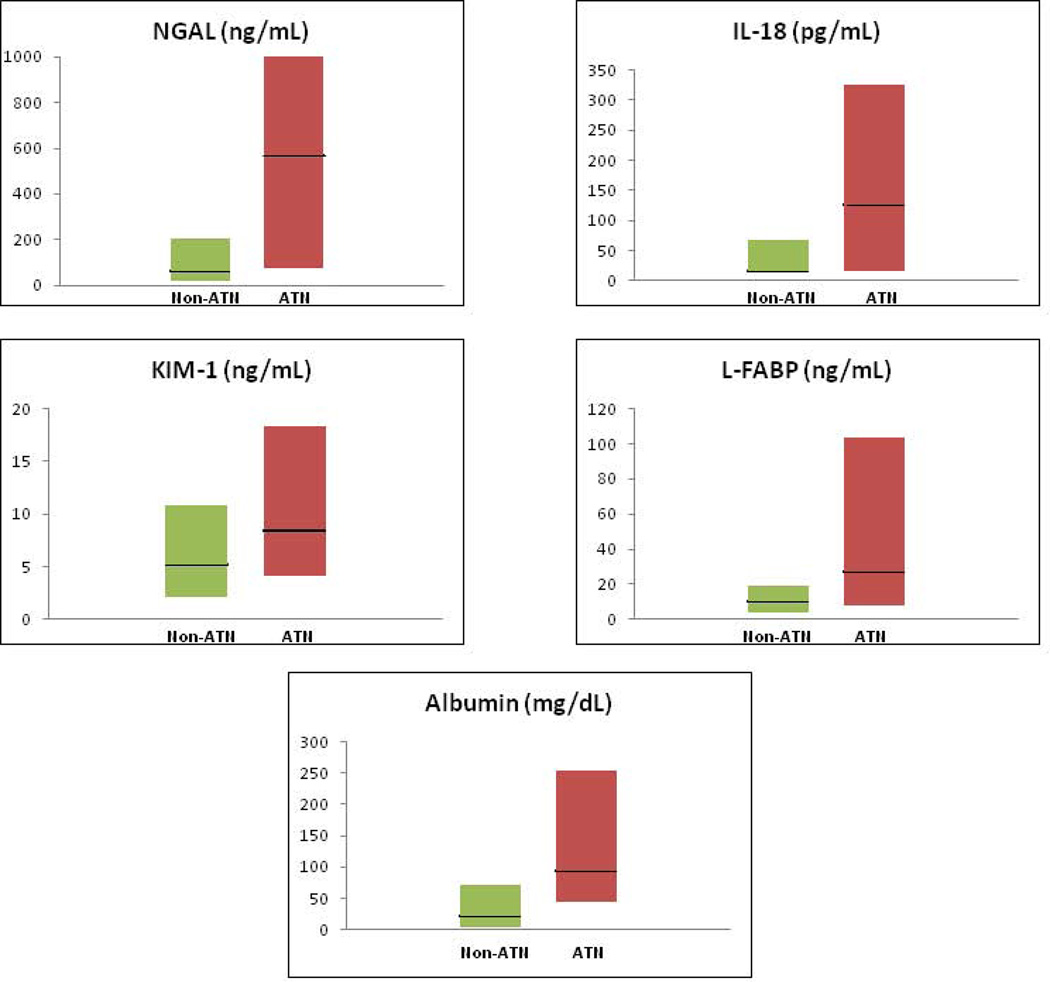
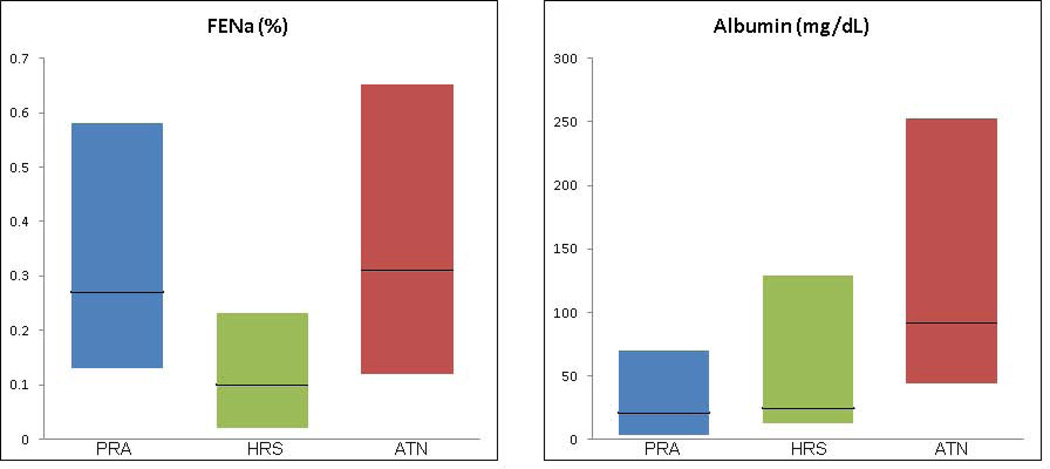
Biomarker values for patients diagnosed with ATN and non-ATN are shown in Table 2a and Figure 2a. Values for patients with PRA, HRS and ATN are presented in Table 2b and, for albumin and FENa, the only widely commercially available of the biomarkers under study, in Figure 2b. Urine samples for biomarker analysis were collected a median of two days following onset of AKI and a median of 26 days from the establishment of patients’ baseline creatinine. Biomarkers were collected over three consecutive days. The values for all biomarkers did not differ over the days of sample collection and results from the first day of collection are presented. Sensitivity analysis of results using raw biomarker values and those corrected for urine creatinine showed minimal variation (data not shown). To facilitate cross-study comparison of results with published literature, NGAL, IL-18, KIM-1, L-FABP and albumin are therefore presented as raw values. Median values for NGAL, IL-18, KIM-1, L-FABP and albumin were significantly higher in patients adjudicated with ATN vs non-ATN. FENa did not differ between the two groups. When assessing the three distinct diagnoses, all biomarkers except FENa were able to distinguish ATN from PRA but only NGAL, IL-18, albumin and FENa differed significantly between patients with ATN and HRS. Critically, FENa was the only biomarker to distinguish HRS from PRA, 0.1% vs 0.27%, p=0.01.
Table 2.
| a. Summary Statistics for Urine Biomarkers in Patients With and Without ATN | |||
|---|---|---|---|
| Non-ATN N=71 |
ATN N=39 |
p | |
| Tubular injury markers | |||
| NGAL (ng/ml) | 59 (22–203) | 565 (76–1000) | <0.001 |
| IL-18 (pg/ml) | 15 (15–65) | 124 (15–325) | <0.001 |
| KIM-1 (ng/ml) | 5.1 (2.1–10.7) | 8.4 (4.1–18.3) | 0.02 |
| L-FABP (ng/ml) | 10 (4–19) | 27 (8–103) | 0.001 |
| Tubular function marker | |||
| FENa (%) | 0.24 (0.06–0.48) | 0.31 (0.12–0.65) | 0.29 |
| Glomerular injury marker | |||
| Albumin (mg/dL) | 21 (4–70) | 92 (44–253) | <0.001 |
| b. Summary Statistics for Urine Biomarkers by Diagnosis | ||||
|---|---|---|---|---|
| PRA N=55 |
HRS N=16 |
ATN N=39 |
p | |
| Tubular injury markers | ||||
| NGAL (ng/ml) | 54 (17–180) | 115 (51–373) | 565 (76–1000)***, ## | <0.001 |
| IL-18 (pg/ml) | 15 (15–49) | 37 (15–90) | 124 (15–325)***, # | <0.001 |
| KIM-1 (ng/ml) | 4.4 (1.8–11.7) | 7.6 (4.5–10.1) | 8.4 (4.1–18.3)** | 0.03 |
| L-FABP (ng/ml) | 9 (4–18) | 14 (6–20) | 27 (8–103)*** | 0.002 |
| Tubular function marker | ||||
| FENa (%) | 0.27 (0.13–0.58) | 0.10 (0.02–0.23)** | 0.31 (0.12–0.65)## | 0.01 |
| Glomerular injury marker | ||||
| Albumin (mg/dL) | 21 (4–70) | 24 (13–129) | 92 (44–253)***, # | <0.001 |
Abbreviations: ATN, acute tubular necrosis; NGAL, neutrophil gelatinase associated lipocalin; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; FENa, fractional excretion of sodium
Abbreviations: PRA, pre-renal azotemia; HRS, hepatorenal syndrome; ATN, acute tubular necrosis; NGAL, neutrophil gelatinase associated lipocalin; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; FENa, fractional excretion of sodium
Values significantly different from pre-renal azotemia indicated with
p < 0.05;
p ≤ 0.01;
p ≤ 0.001
Values significantly different from HRS indicated with
p < 0.05;
p ≤ 0.01;
p ≤ 0.001
Figure 2.
a. Biomarker Values for Patients With and Without ATN
Biomarker values are shown for patients with and without ATN. Dark horizontal lines represent medians while the shaded boxes represent interquartile ranges. Biomarkers values are statistically significantly higher in patients with ATN for all biomarkers.
b. FENa and Albumin for Patients with PRA, HRS and ATN
FENa and albumin values are shown for patients with PRA, HRS and ATN. Dark horizontal lines represent medians while the shaded boxes represent interquartile ranges. FENa is statistically significantly lower in patients with HRS as compared to both PRA and ATN while albumin is significantly higher in patients with ATN than in those with either PRA or HRS.
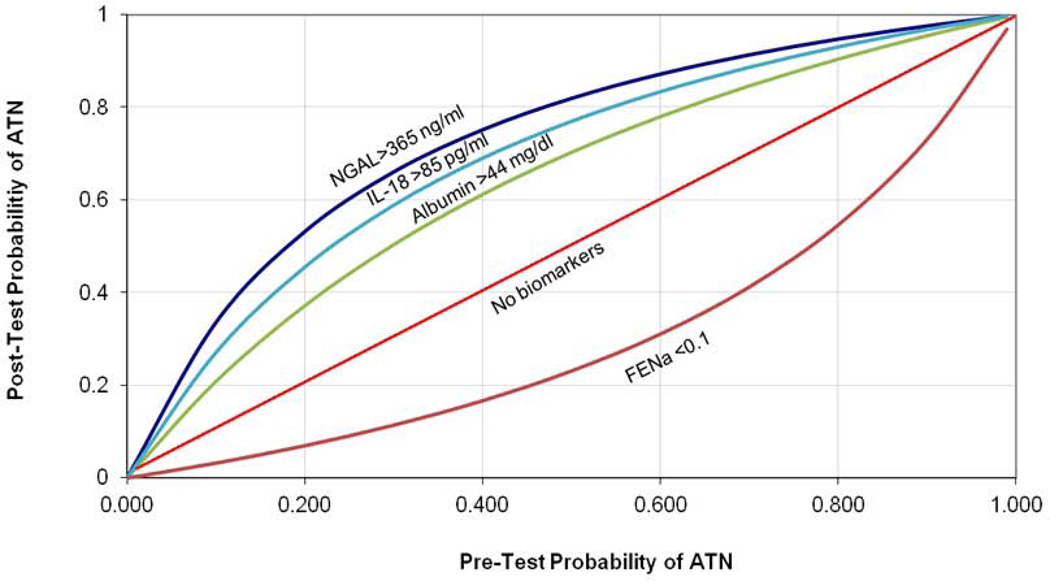
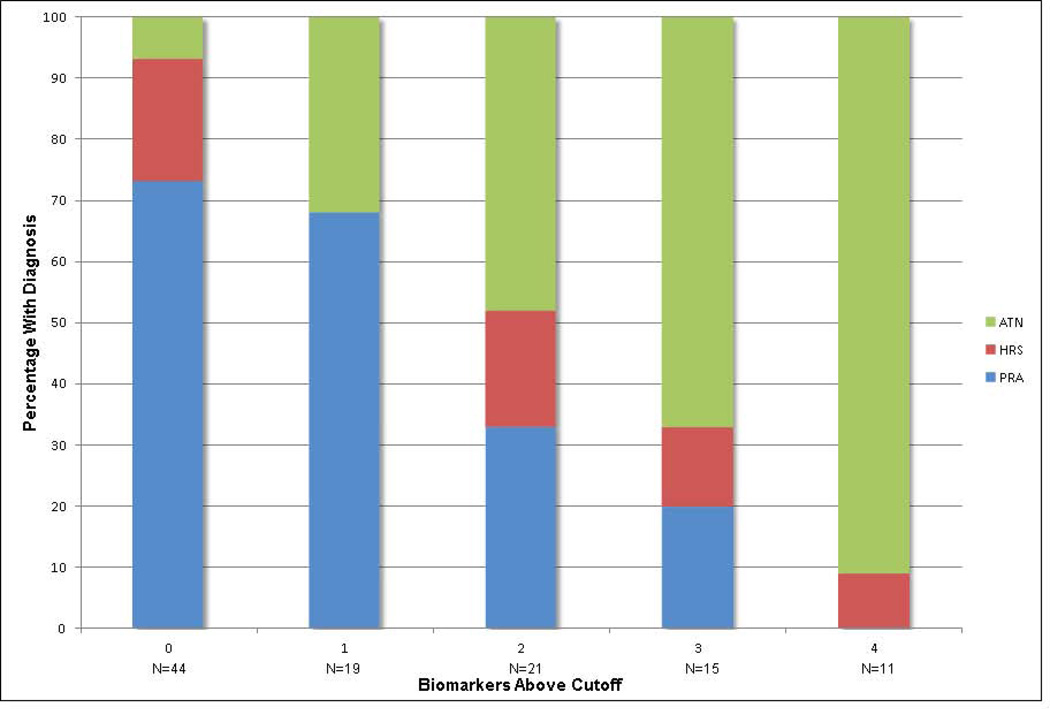
AUC’s and optimal cutoffs of each biomarker for the diagnosis of ATN vs non-ATN are depicted in Table 3. AUCs derived from leave-10-out cross validation are presented alongside those for the entire cohort. The potential practical utility of the three biomarkers with the best discrimination, NGAL, IL-18 and albumin, as well as FENa, when incorporated into clinical decision making is demonstrated through the application of likelihood ratios to determine post-test probabilities for ATN (Figure 3). The post-test probability is calculated using positive and negative likelihood ratios assuming the biomarker level is above or below the optimal cutoff, respectfully. For example, in a patient with a pre-test probability of 40% for the diagnosis of ATN, the finding of a urinary NGAL level above 365 ng/mL would raise the post-test probability to 76%. Similarly, the finding of FENA below 0.1% would lower the post-test probability for ATN to 16%. To examine the utility of biomarkers in combination, the four biomarkers which distinguished ATN from non-ATN with a p-value of < 0.01 (NGAL, IL-18, L-FABP and albumin) were selected and the relative risk for ATN was calculated for successive numbers of these biomarkers above their optimal cutoff, relative to none of the four being elevated (Table 4). The proportion of patients diagnosed with PRA, HRS and ATN with increasing numbers of these biomarkers above their optimal cutoff for ATN cutoff is shown in Figure 4.
Table 3.
Measures of Test Performance Characteristics
| Optimal Cut Point |
Proportion Over Cut Point with ATN |
AUC (95% CI) | Validation AUC* |
|
|---|---|---|---|---|
| Tubular injury markers | ||||
| NGAL (ng/ml) | 365 | 25/35 (71%) | 0.78 (0.69–0.88) | 0.787 |
| IL-18 (pg/ml) | 85 | 21/33 (64%) | 0.71 (0.61–0.81) | 0.711 |
| KIM-1 (ng/ml) | 15.4 | 15/24 (63%) | 0.64 (0.53–0.75) | 0.639 |
| L-FABP (ng/ml) | 25 | 21/30 (70%) | 0.69 (0.57–0.80) | 0.688 |
| Tubular function marker | ||||
| FENa (%) | 0.1 | 22/62 (35%) | 0.56 (0.45–0.68) | 0.563 |
| Glomerular injury marker | ||||
| Albumin (mg/dL) | 44 | 29/52 (56%) | 0.73 (0.64–0.83) | 0.734 |
Abbreviations: ATN, acute tubular necrosis; AUC, area under the curve; CI, confidence interval; NGAL, neutrophil gelatinase associated lipocalin; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; FENa, fractional excretion of sodium
Validation AUCs derived from leave-10-out cross validation performed with SAS Proc Surveyselect
Figure 3. Graph of Conditional Probabilities For Urine Biomarkers.
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; ILK-18, interleukin-18; FENa, fractional excretion of sodium; ATN, acute tubular necrosis
Figure depicts the conditional probabilities for the diagnosis of ATN utilizing biomarkers at their optimal cutoff. For each pre-test probability, a post-test probability is calculated utilizing a positive (NGAL, IL-18, albumin) or negative (FENa) likelihood ratio23.
Formula: Likelihood ratio− = (1-sensitivity)/specificity; Likelihood ratio+ = sensitivity/(1-specificity); pretest odds = pretest probability/(1-pretest probability); posttest odds = pretest odds×LR; posttest probability = posttest odds/(posttest odds + 1)
Table 4.
Association Between Biomarker Panel and the Diagnosis of ATN
| Relative Risk* | |
|---|---|
| 0 Markers Positive | 1.00 |
| 1 Marker Positive | 4.63 (1.29–16.61) |
| 2 Markers Positive | 6.98 (2.14–22.75) |
| 3 Markers Positive | 9.78 (3.10–30.86) |
| 4 Markers Positive | 13.33 (4.40–40.39) |
Abbreviations: ATN, acute tubular necrosis
Biomarker cutoffs: NGAL, 365 ng/ml; IL-18, 85 pg/mL; L-FABP, 25 ng/mL; Albumin 44 mg/dL
Unadjusted
Figure 4. Association Between Biomarker Elevation and Diagnosis.
The percentage of patients with pre-renal azotemia (PRA), hepatorenal syndrome (HRS) and acute tubular necrosis (ATN) by the number of biomarkers of structural injury above their optimal cutoff for the diagnosis of ATN.
Discussion
In this study, we have demonstrated that multiple urinary biomarkers of kidney injury have the ability to distinguish ATN from non-ATN in patients with cirrhosis and progressive AKI. Patient diagnoses were rigorously established via expert adjudicators based upon clinical data and blinded to biomarker values. While injury biomarkers were highest in patients with ATN, levels were similar between patients with PRA and HRS. FENa in patients with ATN was significantly higher than in those with HRS but did not differ from PRA. Using likelihood ratios, we have shown that injury biomarkers including urine albumin have the potential to significantly modify clinicians’ post-test probability for the diagnosis of ATN in a patient with cirrhosis and AKI.
Distinguishing patients with ATN from those with HRS or PRA is often clinically challenging but carries profound significance for both patient care and research. While the diagnostic criteria proposed by the IAC23 are consistent with our understanding of the pathophysiology of HRS, patients with ATN can, and often do, fulfill all six criteria. Indeed, the median number of IAC criteria fulfilled was 5/6 across all three diagnoses. The inability to make this distinction is critical as for HRS, unlike much of AKI, there exists specific therapies tailored to the physiology of the renal dysfunction. Reversal of cirrhotic physiology and restitution of renal blood flow, either via vasoconstrictors7 or liver transplantation8, has been shown to reverse AKI. However, without objective tests, there is evidence that, despite clinicians’ best efforts, significant misclassification occurs. 50% of patients treated with terlipressin do not experience renal recovery while 12% of patients receiving placebo do recover7. It is likely many of these may, in fact, have ATN. Patients who have suffered AKI for greater than 6–8 weeks are thought to be unlikely to spontaneously recover renal function following liver transplant and are therefore listed for a combined transplant24,25. However, 24% of patients with cirrhosis requiring dialysis for 8–12 weeks prior to solitary liver transplant recover renal function post-operatively26 while 27% of patients who receive a combined liver-kidney transplant have a measured native kidney GFR of > 30ml/min 1 year post-operatively27.
The key distinction is not so much whether a patient with cirrhosis and AKI is dichotomously labeled as “having” ATN or HRS, but rather determining if their acute drop in GFR is primarily due to frank structural injury or a functional failure of filtration. Kidney injury biomarkers, which are efficacious for differential diagnosis of renal dysfunction in multiple clinical settings11–13, would seem to hold particular promise in patients with cirrhosis and AKI where both functional and structural diseases can manifest with severe, progressive AKI. While the performance of novel biomarkers in our cohort is indeed encouraging, the ability of albumin to identify patients with ATN and FENa to distinguish HRS from ATN and PRA is particularly significant as these point-of-care tests are currently readily available. The utility of FENa in patients with cirrhosis and AKI has often been dismissed as the majority of patients fall below 1%, regardless of whether their AKI is structural or functional. It appears however that the intense sodium avidity characteristic of HRS may in fact be identifiable with FENa. Though further research is required to validate the specific cutoffs, reappraisal of albumin and FENa in patients with AKI and cirrhosis has the potential to immediately impact challenging diagnostic cases.
Critically, the discriminatory performance of new diagnostic tests is contingent not only on the sensitivity and specificity of the test under investigation but also those of the gold standard against which it is compared. A new biomarker that is in fact 100% sensitive and specific for a disease state can appear to function poorly when evaluated against an even modestly fallible gold standard28. Given the limitations of the IAC criteria, we therefore chose to use expert, retrospective clinical adjudication for our gold standard diagnoses.
Despite utilizing different diagnostic methods, other investigators examining biomarkers in patients with cirrhosis and AKI have found results similar to ours. NGAL levels in our study were similar to those seen by these investigators for patients with HRS and ATN, though we found higher levels in patients with PRA, 78 ng/ml (16–206), than those seen by Verna et al., 20 ng/ml (15–45)14, or Fagundes et al. 30 µg/g (20–59)15. IL-18 has also shown promise, demonstrating an AUC of 0.88 to distinguish ATN from function AKI in 94 ICU patients with cirrhosis and AKI16.
While HRS is classically considered as a purely functional disease, it is interesting to note that both Verna et al. and Fagundes et al. found NGAL levels in patients with HRS to be significantly higher than in those with PRA. The finding of injury biomarkers in HRS as intermediary between PRA and ATN is potentially consistent with recent speculation that HRS may in fact contain some degree of structural injury29. Given this spectrum of pathology, there is likely to be overlap in injury marker levels between HRS and mild ATN. While the presence of elevated injury markers in our study is consistent with ATN, their absence does not necessarily imply HRS. It appears then that the most immediate clinical application of our findings regarding biomarkers of injury will be to identify significant ATN, not to identify HRS or PRA. Injury biomarkers can therefore serve not to identify those patients who should receive HRS specific therapy but, instead, to exclude those with significant structural injury who are unlikely to respond or benefit from treatment, sparing potential unnecessary side effects and optimizing resource utilization and organ allocation. The tantalizing potential of FENa to identify HRS will require validation in future studies.
For this reason, we sought to demonstrate the utility of using likelihood ratios for ruling in or out ATN. Likelihood ratios estimate how much clinicians should shift clinical suspicion for a disease based on a given test result and are derived from a test’s sensitivity and specificity. Incorporating biomarker results through the use of cutoffs and likelihood ratios can greatly assist clinicians confronted with diagnostic and therapeutic conundrums. For example, when deciding whether to utilize vasoconstrictors in a patient with cirrhosis and AKI where the diagnosis is very unclear and there is a 50% probability of ATN, the finding of NGAL above or albumin below their cutoffs strongly re-stratifies them in favor of, 82% probability, or away from, 25%, the diagnosis of ATN, respectively. If the pre-test probability was higher or lower, the post-test probabilities would be even more definitive. Irrespective of pre-test probability, 91% patients with cirrhosis and AKI with NGAL, IL-18, L-FABP and albumin above their respective cutoffs had ATN while only 7% of those without any marker positive did so. Our findings also hold tremendous promise for research where the use of biomarkers to identify ATN would allow investigators enrolling patients for a trial of new HRS therapy to exclude patients with ATN, avoiding misclassification bias and improving study power.
Our study has several strengths. The use of rigorous clinical adjudication provides the best possible diagnostic gold standard outside of biopsy, which is rarely performed in this setting. By evaluating multiple biomarkers, we have demonstrated that a panel of markers may be most efficient for identifying ATN. Our findings will require validation in an external cohort. However, the strikingly consistent AUC point estimates seen with leave-10-out cross validation indicates robust internal validity. Finally, we chose to adjudicate only those patients with progressive AKI. Such patients are typically the most challenging for clinicians with treatment decisions being both critical and fraught with confusion. Indeed, while at least 2 out of 3 adjudicators agreed on 74/76 (97%) patients, there was 3 out of 3 agreement in only 37/76 (49%), emphasizing again the critical need for objective diagnostic tests. Despite these strengths, our study is not without limitations. Though clinical adjudication offers the best possibility of accurately phenotyping patients, the true gold standard is a kidney biopsy. However, while studies suggest that biopsies can safely be performed in many patients with cirrhosis30, they are rarely executed for concerns of bleeding risk. Finally, as an observational study, treatment of patients was not standardized and thus we could not assess the relationship between biomarker levels and treatment response.
In conclusion, multiple urinary biomarkers show the ability to distinguish clinically adjudicated ATN in patients with progressive AKI and cirrhosis. Further research is required to determine if such biomarkers can improve outcomes by more accurately phenotyping the pathophysiology of AKI and thereby triaging only those patients with primarily functional disease to HRS specific treatments. Ultimately, a panel combining markers for both vasoconstriction and structural injury may provide the greatest granularity for determining where on the spectrum of functional to structural disease a patient with cirrhosis and AKI lies.
Supplementary Material
Acknowledgments
Financial Support:
The project was supported by the grant from the National Institutes of Health (NIH) R21-DK078714 to Dr. Parikh. Dr. Belcher was supported on the institutional fellowship training grant from NIH and an American Society of Nephrology Research Fellowship grant.
Abbreviations
- AKI
acute kidney injury
- PRA
pre-renal azotemia
- ATN
acute tubular necrosis
- HRS
hepatorenal syndrome
- IAC
International Ascites Club
- ADQI
Acute Dialysis Quality Initiative
- NGAL
neutrophil gelatinase-associated lipocalin
- IL-18
interleukin-18
- KIM-1
kidney injury molecule-1
- L-FABP
liver-type fatty acid binding protein
- FENa
fractional excretion of sodium
- AKIN
acute kidney injury network
- IQR
inter-quartile range
- GFR
glomerular filtration rate
- MELD
model of end-stage liver disease
- SD
standard deviations
- AUC
area under the curve
- CI
confidence interval
Contributor Information
Justin M. Belcher, Email: justin.belcher@yale.edu.
Arun J. Sanyal, Email: asanyal@mcvh-vcu.edu.
Aldo J. Peixoto, Email: aldo.peixoto@yale.edu.
Mark A. Perazella, Email: mark.perazella@yale.edu.
Joseph Lim, Email: joseph.lim@yale.edu.
Heather Thiessen-Philbrook, Email: heather.thiessenphilbrook@lhsc.on.ca.
Naheed Ansari, Email: Naheed.ansari@nbhn.net.
Steven G. Coca, Email: steven.coca@yale.edu.
Guadalupe Garcia-Tsao, Email: guadalupe.garcia-tsao@yale.edu.
Chirag R. Parikh, Email: chirag.parikh@yale.edu.
References
- 1.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 2.Cárdenas A, Ginés P, Uriz J, Bessa X, Salmerón JM, Mas A, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34(4):671–676. doi: 10.1053/jhep.2001.27830. [DOI] [PubMed] [Google Scholar]
- 3.Tandon P, Garcia-Tsao G. Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2011;9(3):260–265. doi: 10.1016/j.cgh.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57(2):753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroyo V, Fernandez J. Pathophysiological basis of albumin use in cirrhosis. Ann Hepatol. 2011;10(Suppl 1):S6–S14. [PubMed] [Google Scholar]
- 6.Schier RW. Fluid administration in critically in patients with acute kidney injury. Clin J Am Soc Nephrol. 2010;5(4):733–739. doi: 10.2215/CJN.00060110. [DOI] [PubMed] [Google Scholar]
- 7.Dobre M, Demirjian S, Sehgal AR, Navaneethan SD. Terlipressin in hepatorenal syndrome: a systematic review and meta-analysis. Int Urol Nephrol. 2011;43(1):175–184. doi: 10.1007/s11255-010-9725-8. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W et al. Impact of etiology of acute kidney injury on outcomes following liver transplantation: Acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18(5):539–548. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- 9.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 10.Belcher JM, Edelstein CL, Parikh CR. Clinical applications of biomarkers for acute kidney injury. Am J Kidney Dis. 2011;57:930–940. doi: 10.1053/j.ajkd.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 13.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57(9):2362–2370. doi: 10.1007/s10620-012-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagundes C, Pepin MN, Guevara M, Barreto R, Casals G, Sola E, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57(2):267–273. doi: 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Tsai MH, Chen YC, Yang CW, Jenq CC, Fang JT, Lien JM, et al. Acute Renal Failure in Cirrhotic Patients with Severe Sepsis: Value of Urinary Interleukin-18. J Gastroenterol Hepatol. 2012 doi: 10.1111/j.1440-1746.2012.07288.x. [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 18.Shibata M, Hirota M, Nozawa F, Okaba A, Kurimoto M, Ogawa M, et al. Increased concentrations of plasma IL-18 in patients with hepatic dysfunction after hepatectomy. Cytokine. 2000;12(10):1526–1530. doi: 10.1006/cyto.2000.0740. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacEneaney PM, Malone DE. The meaning of diagnostic test results: A spreadsheet for swift data analysis. Clinical Radiology. 2000;55:227–235. doi: 10.1053/crad.1999.0444. [DOI] [PubMed] [Google Scholar]
- 23.Salerno F, Gerbes A, Ginés P, Wong F, Arroyo V. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis: a consensus workshop of the international ascites club. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 25.Simpson N, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS database. Transplantation. 2006;82(10):1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]
- 26.Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010;16(4):440–446. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
- 27.Levitsky J, Baker T, Ahya SN, Levin ML, Friedewald J, Gallon L, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2949–2957. doi: 10.1111/j.1600-6143.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- 28.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande P, Rausa K, Turner J, Johnson M, Golestaneh L. Acute kidney injury as a causal factor in mortality associated with hepatorenal syndrome. Hepatol Int. 2012;5:751–758. doi: 10.1007/s12072-011-9269-8. [DOI] [PubMed] [Google Scholar]
- 30.Wadei HM, Geiger XJ, Cortese C, Mai ML, Kramer DJ, Rosser BG, et al. Kidney allocation to liver transplant candidates with renal failure of undetermined etiology: role of percutaneous renal biopsy. Am J Transplant. 2008;8:2618–2626. doi: 10.1111/j.1600-6143.2008.02426.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.