Abstract
Objectives
Hypoglycorrhachia, or a low glucose level in the cerebrospinal fluid (CSF), can suggest bacterial, fungal, or tuberculous meningitis. When tests for these common infectious etiologies are negative, many clinicians are unsure of which diagnoses to consider, resulting in delayed treatment. We analyzed the diagnoses associated with hypoglycorrhachia to determine their relative frequencies at our institution, as well as summarizing all the diagnoses associated with hypoglycorrhachia in the literature.
Methods
Retrospective analysis of adults with hypoglycorrhachia at a tertiary care teaching hospital over a 5 year period. Inclusion criteria included CSF glucose <40 mg/dL and age ≥ 18 years old. Exclusion criteria included CSF/serum glucose ≥ 0.6.
Results
89 unique hypoglycorrhachia episodes were identified. The most common etiologies amongst all episodes of hypoglycorrhachia were bacterial meningitis (24%), fungal meningitis (15%), stroke/bleeds (13%), malignancy (11%), viral meningitis (6%), neurosarcoidosis (4%), neurosyphilis (4%), and cerebral toxoplasmosis (3%). The most common etiology was fungal meningitis (38%) among HIV–infected patients, and bacterial meningitis (62%) among neurosurgery patients. However, in patients without HIV or neurosurgical history, noninfectious etiologies (stroke/bleed (24%) and malignancy (22%)) were most common.
Conclusions
Many diagnoses, both infectious and noninfectious, lead to hypoglycorrhachia and must be considered in the differential diagnosis.
Keywords: hypoglycorrhachia, CSF, cerebrospinal fluid, glucose
INTRODUCTION
Hypoglycorrhachia, a low glucose level in the cerebrospinal fluid (CSF), is commonly associated with infections such as bacterial, fungal, and tuberculous meningitis 1. When tests for these infectious causes are negative, the diagnosis can be elusive. Available diagnostic tests for tuberculous meningitis, the most common cause of chronic meningitis, are not very sensitive (even the newer interferon gamma release assays, which are generally more sensitive than older methods, have reported sensitivities ranging from 44% to 71%)2, 3. Consequently, many clinicians advocate empiric treatment for tuberculous meningitis in patients with hypoglycorrhachia, meningeal symptoms, and negative diagnostic tests4. However, this approach can lead to a delay in correct diagnosis and treatment 1, 5, 6. As it has been shown that hypoglycorrhachia is an independent risk factor for poor clinical outcome among patients with meningeal symptoms and negative CSF gram stain, it is very important that these patients are diagnosed and treated promptly7.
Individual case reports have described a number of infectious and non-infectious diagnoses that can be associated with hypoglycorrhachia 1, 5, 6, 8-14. However, to our knowledge, there has never been a study evaluating the differential diagnosis of hypoglycorrhachia in adults at a single institution. Further, the one study investigating the differential diagnosis of hypoglycorrhachia in children at a single institution was done in 1976, before many current diagnostic tests were available 15.
We conducted a retrospective study of adult patients with hypoglycorrhachia at a tertiary-care hospital in Virginia to determine the most common diagnoses associated with hypoglycorrhachia in general, and among patients with specific risk factors. We also conducted a systematic literature review and summarized the diagnoses associated with hypoglycorrhachia reported in the literature.
PATIENTS AND METHODS
This was a retrospective chart review of adult patients with hypoglycorrhachia (defined as CSF glucose <40 mg/dL) who were treated at Sentara Norfolk General Hospital (SNGH) in Norfolk, Virginia after the implementation of the electronic medical record system in July 2007 through February 22, 2012. Sentara Norfolk General Hospital is a 525-bed tertiary care facility and the only level 1 trauma center in the area. Inclusion criteria included a CSF glucose < 40 mg/dL, age ≥ 18 years old, and treatment at Norfolk General Hospital during the aforementioned period. Exclusion criteria included a CSF/serum glucose ratio ≥ 0.6 (to exclude hypoglycorrhachia solely due to hypoglycemia) and lack of basic information through chart review. This study was approved by the institutional review boards at Eastern Virginia Medical School and SNGH.
In instances where the same patient had multiple episodes of hypoglycorrhachia, these were counted as one episode if the hypoglycorrhachia could be attributed to the same diagnosis in all episodes, and the lowest CSF glucose level was selected for analysis. In some cases the same patient had separate episodes of hypoglycorrhachia that were clearly due to different diagnoses (different species growing on CSF culture), and these were counted as separate episodes.
Most diagnoses were retrieved from progress and discharge notes from the patient’s encounter with their primary medical team or consultant at SNGH. In situations in which there were competing diagnostic causes for hypoglycorrhachia, the final etiology was chosen based on the consistency of lab values and other objective tests with a particular diagnosis. Diagnoses were divided into 9 subgroups, including 1) bacterial meningitis, 2) fungal meningitis, 3) malignancy (consisting of carcinomatosis, or lymphoma or leukemia with CNS involvement), 4) neurosarcoidosis, 5) neurosyphilis, 6)stroke/bleed, 7) toxoplasmosis, 8) viral meningitis, 9) other, and 10) indeterminate. Diagnosis of bacterial meningitis included 18 patients with positive CSF bacterial cultures as well as 3 patients with a presumptive diagnosis who had negative CSF bacterial cultures but who had received antibiotics prior to the CSF culture, responded to antibiotics, and had a neutrophilic dominance of their CSF pleocytosis. An indeterminate etiology was ascribed in situations where cause of hypoglycorrhachia could not be determined by discharge and/or at any subsequent hospital visits.
Subjects were considered to be HIV-infected if they either had a positive HIV test or report of HIV infection on chart review. Subjects with either a negative HIV test or without history of HIV testing or diagnosis were classified as HIV-negative. Subjects with any history of surgery in the brain or spine were classified as having a neurosurgical history.
Laboratory values were extracted from all the laboratory results at SNGH for that patient, with the values closest to the time point of hypoglycorrhachia being used. Brain imaging (including magnetic resonance imaging and computed tomography scans) were taken from the official radiology reports. Signs and symptoms were extracted from the admission and progress notes of the primary medical team and consultants, and were considered present if mentioned in any of the notes. Descriptive statistics were used to analyze the data.
The literature review was conducted by doing a PubMed search using the term “hypoglycorrhachia”, by searching Mandell’s Principles and Practice of Infectious Diseases 4 for any mention of the term “hypoglycorrhachia”, and by searching the references included in hypoglycorrhachia case reports. Case reports not indexed in PubMed that were known to the authors were also included. When multiple reports described the same diagnosis, only one was referenced.
RESULTS
Out of 2723 CSF samples for which glucose levels were measured during the study period, 153 (6%) had glucose levels < 40 mg/dL. Of these 153 samples, 64 were excluded: 33 occurred in individuals < 18 years old, 26 were repeat episodes in the same individual due to the same diagnosis, 3 had a CSF/serum glucose ratio ≥ 0.6, and 2 had inadequate available information due to either the patient leaving against medical advice or the lumbar puncture alone being done in the hospital with no other notes available. Our study included the remaining 89 episodes from 87 patients (two patients had separate episodes with different diagnoses).
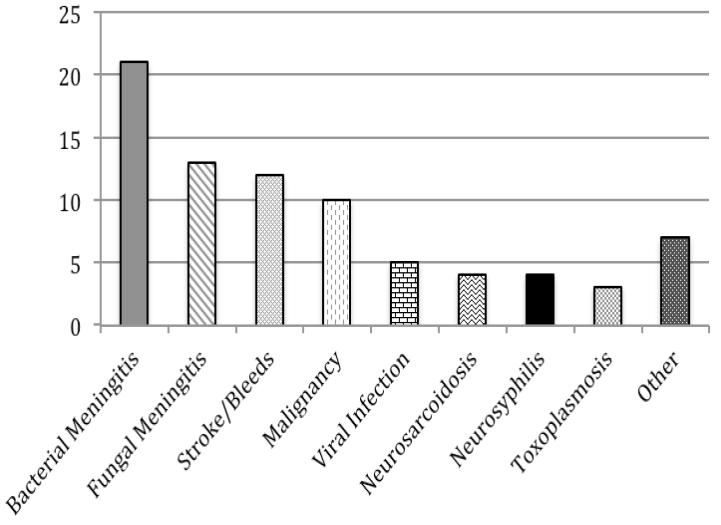
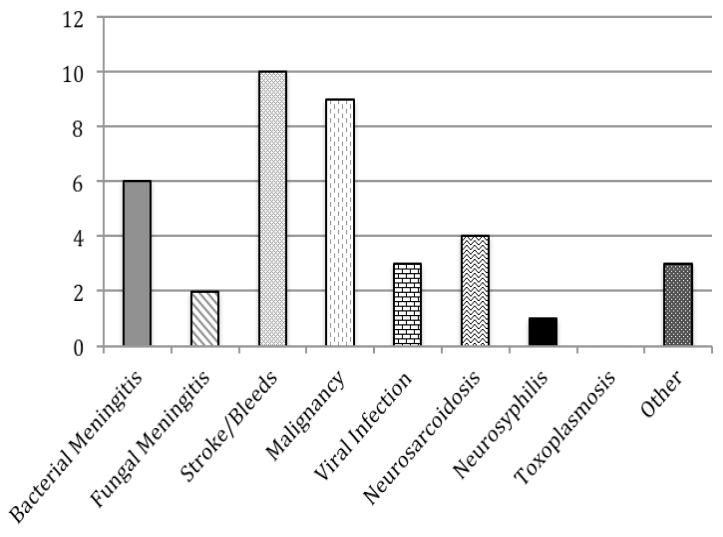
Diagnoses were identified for 79 of the 89 episodes of hypoglycorrhachia (89%). Although the most common overall diagnosis was bacterial meningitis, it accounted for only 26% of the 79 episodes with known diagnoses, while noninfectious etiologies (stroke/bleeds, malignancy, and neurosarcoidosis) accounted for 33%. The most common diagnoses for the 41 patients without documented HIV infection or neurosurgical history were stroke/bleed (24%) and malignancy (22%). Figure 1 shows the relative frequency of different diagnoses among all patients who had an identified diagnosis and among patients with different risk factors.
Figure 1. Frequency of Different Known Diagnoses Seen in Patients with Hypoglycorrhachia.
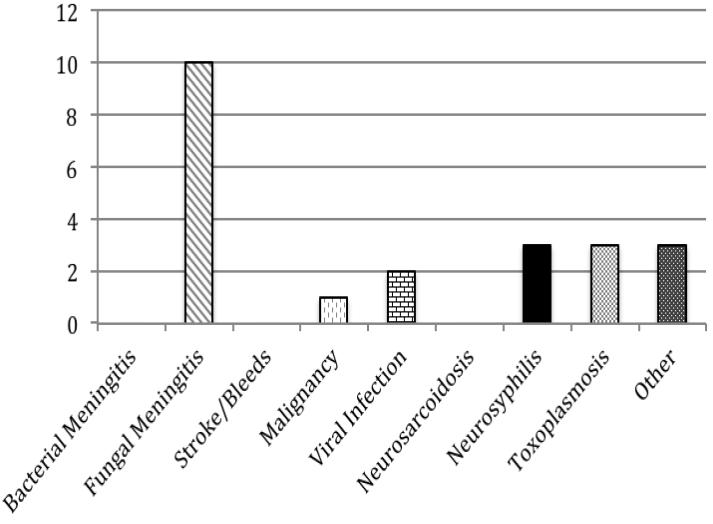
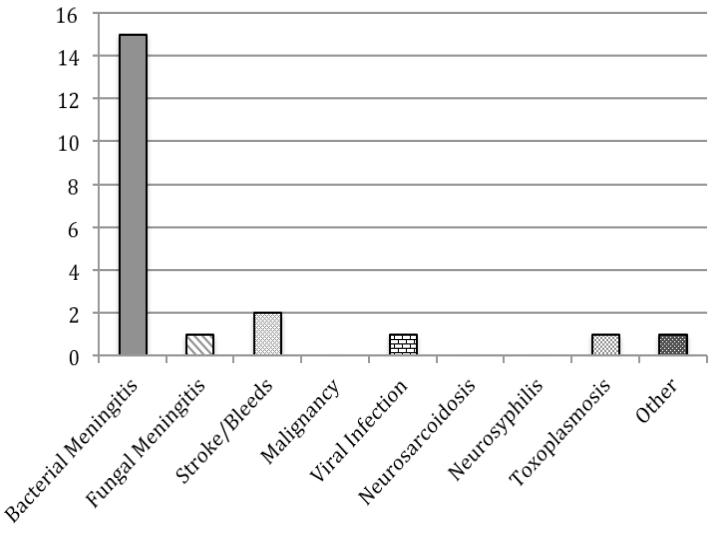
a. All Patients (n=79) b. HIV-Infected Patients (n=22) c. Patients with History of Neurosurgery (n=21) d. Patients without HIV or Neurosurgical History (n=38)
The Y axis represents number of cases with each diagnosis. 2 subjects with a history of neurosurgery also had HIV. Subjects with an indeterminate diagnosis (10 total, including 4 HIV-infected and 3 with history of neurosurgery) were not included.
Among the 26 HIV-infected subjects with hypoglycorrhachia, fungal meningitis followed by neurosyphilis and cerebral toxoplasmosis were the most common diagnoses, and bacterial meningitis was not seen. Of note, most of the HIV-infected subjects in our study were severely immunosuppressed. Of the 25 HIV-infected subjects with a documented CD4 count, 92% had a CD4 count <200, and 52% had a CD4 count <50.
Most of the 24 subjects with hypoglycorrhachia and a neurosurgical history had bacterial meningitis. Of note, neurosurgery was recent (within the past two months) in 21 of the 23 subjects (91%) for which timing of neurosurgery was known. 12 of the 24 subjects (50%) had CSF-draining hardware in place, such as a ventriculoperitoneal shunt.
The specific etiologies of the diagnoses associated with hypoglycorrhachia include the following. The 21 cases of bacterial meningitis included 5 infections with coagulase-negative staphylococci, 4 with enterobacter species, 3 with streptococci (included one mixed infection with Staphylococcus hominis), 2 with Staphylococcus aureus, and one each with Burkholderia cepacia, Haemophilus influenza, Klebsiella pneumonia, Serratia marcescens, and Enterococcus. 12 of the 13 cases of fungal meningitis were with Cryptococcus neoformans, and the other was with Histoplasma capsulatum. The 10 cases of malignancy-associated hypoglycorrhachia included 6 cases of carcinomatosis (3 breast cancer, 2 lung cancer, 1 colon cancer), and 1 case each of acute lymphoblastic leukemia, acute myelogenous leukemia, diffuse large B cell lymphoma, and follicular lymphoma. Of note, all cases of malignancy-associated hypoglycorrhachia had either CSF cytology or CSF flow cytometry positive for malignant cells. The 12 cases of stroke/bleed included 8 cases of subarachnoid hemorrhage, 2 of cerebellar hemorrhage, and 2 of ischemic stroke. The 5 cases of viral meningitis included 2 infections with herpes simplex virus and one each of coxsackie B virus, cytomegalovirus, and varicella zoster virus. The 7 “other” diagnoses included 3 patients clinically diagnosed with aseptic meningitis who did not have tests sent for a specific viral etiology, 2 patients for whom the hypoglycorrhachia was attributed to HIV infection when other diagnostic tests were negative, 1 patient with HIV for whom the hypoglycorrhachia was attributed to immune reconstitution inflammatory syndrome without a primary pathogen identified, and 1 patient with a known parameningeal infection.
There was significant overlap in the demographics and objective findings in subjects with different etiologies for their hypoglycorrhachia. The mean age of the subjects was 47 years, and 57% of the population was male. All cases of cerebral toxoplasmosis, and 75% of the cases of neurosyphilis, took place in HIV-infected individuals, and 11/ 13 (85%) of individuals with fungal meningitis were immunocompromised (10 were HIV-infected, and 1 was on immunosuppressive medications for Wegener’s granulomatosis). Fifteen of the 21 cases of bacterial meningitis had a neurosurgical history. Abnormalities on brain imaging were found in all hypoglycorrhachia subjects with stroke/bleed, neurosarcoidosis, and toxoplasmosis. Bacterial meningitis, stroke/bleed, and cerebral toxoplasmosis were most often associated with a CSF pleocytosis with neutrophilic predominance. Fungal meningitis, malignancy, neurosarcoidosis, neurosyphilis, and viral meningitis were most often associated with a CSF pleocytosis with a lymphocytic predominance. Similar to what is reported in the literature4, the 4 cases of carcinomatosis associated with hypoglycorrhachia with known CSF cell count had a nearly normal CSF WBC count, but so did the subjects with neurosyphilis and cerebral toxoplasmosis. Four subjects had isolated hypoglycorrhachia with normal CSF protein and cell count: 2 subjects categorized with an “other” diagnosis (hypoglycorrhachia attributed to HIV infection), and 2 subjects with indeterminate diagnoses.
There was also significant overlap in the signs and symptoms seen by subjects with varying diagnoses. Both neck stiffness and photophobia were uncommon symptoms in our subjects (10% and 5.6%, respectively) even among those with bacterial (14% with neck stiffness and none with photophobia), fungal (15.4% with neck stiffness and 15.4% with photophobia) and viral meningitis (none). Fever was documented in subjects with both infectious and some noninfectious diagnoses. Headache was the predominant symptom for viral meningitis, bacterial meningitis, fungal meningitis, and stroke/bleeds. Altered mental status was the predominant symptom for hypoglycorrhachia associated with malignancy.
Table 1 is a summary of the etiologies of hypoglycorrhachia reported in the literature. Infectious etiologies commonly associated with hypoglycorrhachia that were reported in the literature but not seen in this study include mycobacterial (tuberculous) meningitis and amebic encephalitis4. The only noninfectious etiology reported in the literature as commonly associated with hypoglycorrhachia that was not seen in this study was GLUT1 deficiency syndrome 16.
Table 1.
Common and Uncommon Etiologies of Hypoglycorrhachia in the Literature
| ETIOLOGIES COMMONLY ASSOCIATED WITH HYPOGLYCORRHACHIA |
ETIOLOGIES UNCOMMONLY ASSOCIATED WITH HYPOGLYCORRHACHIA (reported but not typical) |
|---|---|
| Infectious Causes | |
| Non-Infectious Causes | |
Etiologies reported to cause severe hypoglycorrhachia, generally defined as CSF glucose ≤10 mg/dL.
DISCUSSION
Our study investigated the differential diagnosis of hypoglycorrhachia in adult patients at a tertiary care hospital in Virginia. While bacterial meningitis was the most common diagnosis associated with hypoglycorrhachia in our study, it accounted for only one fourth of overall cases, most among subjects with a history of neurosurgery. One third of hypoglycorrhachia episodes were associated with noninfectious causes such as strokes/bleeds, carcinomatosis, lymphomatosis/leukemia, and neurosarcoidosis. Subjects with a recent history of neurosurgery and hypoglycorrhachia most often had bacterial meningitis, while HIV-infected subjects with hypoglycorrhachia most often had fungal meningitis followed by cerebral toxoplasmosis and neurosyphilis. Surprisingly, the most common diagnoses associated with hypoglycorrhachia in subjects without HIV-infection or neurosurgical history were noninfectious. While there were some distinguishing patterns seen between different diagnoses among laboratory findings, imaging, and presenting signs and symptoms, there was also significant overlap. Our study demonstrates that a wide range of diagnoses, both infectious and noninfectious, are associated with hypoglycorrhachia and should be considered in the differential.
To our knowledge, the only other study evaluating the differential diagnosis of hypoglycorrhachia at a single institution was done in children in 1976. Silver et al investigated the diagnoses of 181 children with hypoglycorrhachia, aged 1 week to 14 years, and also found multiple etiologies, including bacterial meningitis (19%), hypoglycemia (9%), aseptic meningitis (8%), meningeal carcinomatosis (6%), subarachnoid hemorrhage (4%), and “other” (43%)15. Of note, 5 of the aseptic meningitis cases had positive CSF culture for enterovirus, and one had positive CSF culture for mumps virus. While our study excluded hypoglycorrhachic events due to hypoglycemia, the 2 age groups shared similar common infectious and noninfectious diagnoses. The lower number of indeterminate diagnoses in our study is likely due to the improved diagnostic tests that have been developed since 1976.
Surprisingly, since tuberculous meningitis is considered to be the most common cause of chronic meningitis 4, we did not find any cases of tuberculous meningitis among our subjects with hypoglycorrhachia. This is likely because the incidence of tuberculosis is low in Virginia, particularly southeast Virginia where this study was conducted. The state of Virginia in 2011 reported a total of 221 cases of tuberculosis, or a rate of 2.7 per 100,000, which is less than the U.S. national average of 3.4 per 100,000 17. The eastern region of the state, which includes the city of Norfolk, saw a total of 26 cases in 2011, while the city of Norfolk itself had only 4 documented cases 17. As tuberculous meningitis only accounts for 1 % of tuberculosis cases 18, the likelihood of seeing any cases of tuberculous meningitis at our hospital is very low.
The maintenance of CSF glucose levels is controlled by several mechanisms, including glucose transport into and out of the CSF as well as glucose utilization by cells. Glucose enters the CSF through the blood-brain barrier at the choroid plexus with the help of glucose transport proteins (such as GLUT1) and can either be used by cells or exit through the arachnoid villi into the venous system 1, 19. The pathophysiology behind hypoglycorrhachia is not fully understood but is likely multifactorial. Possible contributors include decreased glucose delivery to the choroid plexus because of reduced blood flow, decreased transport across the blood-brain barrier, increased metabolism in the brain, and increased glucose transport out of the CSF into the venous system 1. While it was once thought that an increased rate of glycolysis by bacterial or immune cells was the primary cause of hypoglycorrhachia, this is no longer conventional thought1, 20, 21. A patient with prolonged hypoglycorrhachia due to leptomeningeal carcinomatosis showed no improvement in the hypoglycorrhachia after an IV glucose loading test despite a marked increase in blood glucose levels, suggesting abnormal transport of glucose into the CSF as the likely cause 22.
Our study had limitations. Due to the geography and the patient population at our hospital, certain diseases that have been reported to cause hypoglycorrhachia in the literature were not found in this study, such as tuberculous and coccidioides meningitis. This study also had the inherent limitations of retrospective chart reviews, which are dependent on the established medical record. The diagnosis associated with the hypoglycorrhachia was not always established. Signs and symptoms were not always recorded by the providers. Certain tests, most notably an HIV test, were not conducted on all of the patients, which may have led to miscategorization of HIV-infected patients as HIV-negative.
Conclusion
Despite the limitations of our study, we report, to our knowledge, the first study investigating the differential diagnosis of hypoglycorrhachia in hospitalized adults at a single institution. We report many infectious and noninfectious etiologies of hypoglycorrhachia that should be considered in the diagnostic workup, with significant overlap in laboratory tests and signs and symptoms. It would be interesting to see if future studies conducted in other areas of the world or in a pediatric population with modern diagnostic tests had similar results. We hope the findings from our study, along with our literature review, will serve as a diagnostic tool to aid clinicians in correctly diagnosing and treating patients with this finding.
Footnotes
Conflicts of Interest and Sources of Funding: Dr. Troy received salary support from the United States National Institute of Allergy and Infectious Diseases [K23 AI093678] and the Doris Duke Charitable Foundation [Grant #2012061] while conducting this study. Neither author reports any conflicts of interest.
Bibliography
- 1.Viola GM. Extreme hypoglycorrhachia: not always bacterial meningitis. Nat Rev Neurol. 2010;6(11):637–641. doi: 10.1038/nrneurol.2010.126. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Cho OH, Park SJ, et al. Rapid diagnosis of tuberculous meningitis by T cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Infect Dis. 2010;50(10):1349–1358. doi: 10.1086/652142. [DOI] [PubMed] [Google Scholar]
- 3.Vidhate MR, Singh MK, Garg RK, et al. Diagnostic and prognostic value of Mycobacterium tuberculosis complex specific interferon gamma release assay in patients with tuberculous meningitis. J Infect. 2011;62(5):400–403. doi: 10.1016/j.jinf.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J, Dolin R, Douglas R, Mandell G. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th edition Churchill Livingstone/Elsevier; Philadelphia: 2010. [Google Scholar]
- 5.Troy S, Chary A, Polesky A, Bhatia G. A Woman With Hydrocephalus and Severe Hypoglycorrhachia. Infectious Diseases in Clinical Practice. 2009;17(5):323–325. [Google Scholar]
- 6.Troy SB, Blackburn BG, Yeom K, Caulfield AK, Bhangoo MS, Montoya JG. Severe encephalomyelitis in an immunocompetent adult with chromosomally integrated human herpesvirus 6 and clinical response to treatment with foscarnet plus ganciclovir. Clin Infect Dis. 2008;47(12):e93–96. doi: 10.1086/593315. [DOI] [PubMed] [Google Scholar]
- 7.Khoury NT, Hossain MM, Wootton SH, Salazar L, Hasbun R. Meningitis with a negative cerebrospinal fluid gram stain in adults: risk classification for an adverse clinical outcome. Mayo Clin Proc. 2012;87(12):1181–1188. doi: 10.1016/j.mayocp.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin RH, Ross BC, Taylor KI, Yung AP, Johnson PD. Chronic meningitis due to herpes simplex virus in an immunocompetent host. Eur J Clin Microbiol Infect Dis. 1996;15(8):650–653. doi: 10.1007/BF01691151. [DOI] [PubMed] [Google Scholar]
- 9.Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash in an immunocompetent woman. J Neurovirol. 2009;15(2):206–208. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severien C, Jacobs KH, Schoenemann W. Marked pleocytosis and hypoglycorrhachia in coxsackie meningitis. Pediatr Infect Dis J. 1994;13(4):322–323. doi: 10.1097/00006454-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Borhani Haghighi A, Ashjazadeh N. Acute disseminated encephalomyelitis-like manifestations in a patient with neuro-Behçet disease. Neurologist. 2009;15(5):282–284. doi: 10.1097/NRL.0b013e31818e096e. [DOI] [PubMed] [Google Scholar]
- 12.Yaşar Anlar F, Yalçin S, Seçmeer G. Persistent hypoglycorrhachia in neurobrucellosis. Pediatr Infect Dis J. 1994;13(8):747–748. doi: 10.1097/00006454-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Sarva H, Chapman R, Omoregie E, Abrams C. The challenge of profound hypoglycorrhachia: two cases of sarcoidosis and review of the literature. Clin Rheumatol. 2011;30(12):1631–1639. doi: 10.1007/s10067-011-1834-y. [DOI] [PubMed] [Google Scholar]
- 14.Erdem G, Topçu M, Topaloğlu H, Bertan V, Arikan U. Dermoid tumor with persistently low CSF glucose and unusual CT and MRI findings. Pediatr Neurol. 1994;10(1):75–77. doi: 10.1016/0887-8994(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 15.Silver TS, Todd JK. Hypoglycorrhachia in pediatric patients. Pediatrics. 1976;58(1):67–71. [PubMed] [Google Scholar]
- 16.Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009;31:545–552. doi: 10.1016/j.braindev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 17.VDH Division of Disease Prevention [Accessed December 18, 2012];Tuberculosis Control Number of Reported TB Cases and Rates per 100,000: 2007-2011. Available at: http://www.vdh.virginia.gov/Epidemiology/DiseasePrevention/Programs/Tuberculosis/documents/TBCR2011_FINAL_file_revised_7_24_2012.pdf.
- 18.Leonard J. [Accessed December 18, 2012];Central nervous system tuberculosis. UpToDate. 2012 Available at: http://www.uptodate.com/contents/central-nervous-system-tuberculosis?source=search_result&search=central+nervous+system+tuberculosis&selectedTitle=1%7E43.
- 19.Klepper J, Voit T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain-- a review. Eur J Pediatr. 2002;161(6):295–304. doi: 10.1007/s00431-002-0939-3. [DOI] [PubMed] [Google Scholar]
- 20.Menkes JH. The causes for low spinal fluid sugar in bacterial meningitis: another look. Pediatrics. 1969;44(1):1–3. [PubMed] [Google Scholar]
- 21.PETERSDORF RG, HARTER DH. The fall in cerebrospinal fluid sugar in meningitis. Arch Neurol. 1961;4:21–30. doi: 10.1001/archneur.1961.00450070023003. [DOI] [PubMed] [Google Scholar]
- 22.Jann S, Comini A, Pellegrini G. Hypoglycorrhachia in leptomeningeal carcinomatosis. A pathophysiological study. Ital J Neurol Sci. 1988;9(1):83–88. doi: 10.1007/BF02334413. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Anderegg KA, Yu VL. Significance of hypoglycorrhachia in patients with AIDS and cytomegalovirus meningoencephalitis. Clin Infect Dis. 1993;17(2):283–284. doi: 10.1093/clinids/17.2.283. [DOI] [PubMed] [Google Scholar]
- 24.Dayan NE, Rubin LG, Di John D, Sood SK. Hypoglycorrhachia in Lyme meningitis. Pediatr Infect Dis J. 2004;23(4):370–371. doi: 10.1097/00006454-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz SL, Bentson JR, Benson F, Davos I, Pressman B, Gottlieb MS. CNS toxoplasmosis in acquired immunodeficiency syndrome. Arch Neurol. 1983;40(10):649–652. doi: 10.1001/archneur.1983.04050090085015. [DOI] [PubMed] [Google Scholar]
- 26.Teshima T, Akashi K, Shibuya T, et al. Central nervous system involvement in adult T-cell leukemia/lymphoma. Cancer. 1990;65(2):327–332. doi: 10.1002/1097-0142(19900115)65:2<327::aid-cncr2820650224>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Makino M, Ueda Y, Nakajima K. Spinal subarachnoid hemorrhage presenting as disturbance of consciousness with severe hypoglycorrhachia. Nihon Ronen Igakkai Zasshi. 1998;35(12):924–928. doi: 10.3143/geriatrics.35.924. [DOI] [PubMed] [Google Scholar]
- 28.Gibson T, Myers AR. Nervous system involvement in systemic lupus erythematosus. Ann Rheum Dis. 1975;35(5):398–406. doi: 10.1136/ard.35.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reik L, Grunnet ML, Spencer RP, Donaldson JO. Granulomatous angiitis presenting as chronic meningitis and ventriculitis. Neurology. 1983;33(12):1609–1612. doi: 10.1212/wnl.33.12.1609. [DOI] [PubMed] [Google Scholar]
- 30.Label LS, Tandan R, Albers JW. Myelomalacia and hypoglycorrhachia in malignant atrophic papulosis. Neurology. 1983;33(7):936–939. doi: 10.1212/wnl.33.7.936. [DOI] [PubMed] [Google Scholar]