Abstract
Upon binding of cortisol, the glucocorticoid receptor (GR) regulates the transcription of specific target genes, including those that encode the stress hormones corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH). Dysregulation of the stress axis is a hallmark of major depression in human patients. However, it is still unclear how glucocorticoid signaling is linked to affective disorders. We identified an adult-viable zebrafish mutant in which the negative feedback on the stress response is disrupted, due to abolition of all transcriptional activity of GR. As a consequence, cortisol is elevated, but unable to signal through GR. When placed into an unfamiliar aquarium (‘novel tank’), mutant fish become immobile (‘freeze’), show reduced exploratory behavior and do not habituate to this stressor upon repeated exposure. Addition of the antidepressant fluoxetine to the holding water and social interactions restore normal behavior, followed by a delayed correction of cortisol levels. Fluoxetine does not affect overall transcription of CRH, the mineralocorticoid receptor (MR), the serotonin transporter Serta or GR itself. Fluoxetine, however, suppresses the stress-induced upregulation of MR and Serta in both wildtype fish and mutants. Our studies show a conserved, protective function of glucocorticoid signaling in the regulation of emotional behavior and reveal novel molecular aspects of how chronic stress impacts vertebrate brain physiology and behavior. Importantly, the zebrafish model opens up the possibility of high-throughput drug screens in search of new classes of antidepressants.
Keywords: Stress, Depression, Anxiety, Glucocorticoid, Serotonin, Fish model
Perception of a threatening environmental stimulus elicits endocrine changes that in turn trigger a cascade of physiological and behavioral processes. This so-called ‘stress response’ is mediated by the hypothalamus-pituitary-adrenal (HPA) axis. Increased synthesis and release of CRH in the hypothalamus promote the release of ACTH (encoded by the pomc gene) from the pituitary gland into the circulation. ACTH stimulates the production of glucocorticoids from the adrenal gland, whose fish homolog is named interrenal organ. In teleost fish and humans, the major glucocorticoid hormone is cortisol (corticosterone in rodents). Cortisol levels not only increase in response to stress, but also exhibit a circadian rhythm, peaking during daytime in both zebrafish and humans1,2.
In humans, hyperactivity of the HPA axis is the most consistent endocrine parameter associated with major depression3, 4. Moreover, even in non-diseased individuals, a high cortisol level in the circulation (hypercortisolemia) is considered a risk factor, predisposing to the development of the disease5. Correction, i. e. lowering, of cortisol is often used clinically to monitor the success of therapeutic intervention4-6. Both extreme short-term stress and mild chronic stress can precipitate affective disorders including depression and pathological anxiety, demonstrating a causal contribution of stress to long-term mood changes. However, it is unclear which component(s) of the HPA axis is/are responsible for the neural circuitry changes that result in depression. There is no obvious link between HPA-related hormones and the pharmacological treatments that have proven to be effective in many forms of depression, such as benzodiazepines (e. g., diazepam = Valium), which modulate GABA-A receptors, and selective serotonin reuptake inhibitors (SSRIs, e. g., fluoxetine = Prozac).
Understanding the molecular crosstalk between the HPA axis and depression is important, as it will inform the search for better therapies. In the brain, cortisol is known to signal through a ligand-dependent transcription factor, the glucocorticoid receptor (GR). Upon binding of cortisol, GR forms homodimers and translocates from the cytoplasm to the nucleus, where it binds specific DNA sequences called glucocorticoid response elements (GREs)7, to regulate the expression of target genes in a tissue-specific manner8, 9. These GREs are often highly conserved and can serve as enhancers or repressors of gene transcription10. GR can also form heterodimers with other transcription factors, largely to repress transcription of target genes. GR is ubiquitously expressed and is only occupied by cortisol during the diurnal peaks or under stress. A related factor, the mineralocorticoid receptor (MR), is more sparsely expressed in the brain and has a tenfold higher ligand affinity than GR. Together, MR and GR act over a wide range of cortisol concentrations. Signaling through GR, within the physiological range, is thought to terminate the stress reaction and facilitate recovery and memory storage11.
Both an excess and a shortage of GR signaling might be detrimental to brain function. Some evidence exists in rodents that glucocorticoids have negative effects on neurogenesis and synaptic plasticity in the hippocampus and that these effects are reversed by SSRI administration over several weeks12. On the other hand, GR activity appears to be protective of the brain by dialing down the stress response. In the latter view, depression is characterized by ‘glucocorticoid resistance’, and the disease-causing culprit is excess of some other hormone, perhaps CRH8,9,13. In humans with functioning HPA axis, administration of Dexamethasone (Dex, a synthetic ligand of GR) suppresses cortisol. In depressed patients with hyperactivated HPA axis, however, this effect is blunted11. This is evidence in favor of glucocorticoid resistance in at least some forms of major depression.
We have identified an adult viable zebrafish mutant, grs357 in which a single base-pair change completely disrupts GR transcriptional regulation of its target genes. We show that fish homozygous for the grs357 mutation display a hyperactivated HPA axis, blunted suppression of cortisol by Dex and increased depression-like behavior in response to mild stress. Diazepam and Fluoxetine treatments, as well as social interactions, reverse the abnormal behavior. These results reveal a phylogenetically conserved link between the HPA axis and affective disorders in vertebrates and strongly support the view that glucocorticoid resistance, and not excessive GR signaling, contribute to the development of depression.
Materials and Methods
Positional cloning and genotyping of grs357
By linkage mapping using 1,230 meioses, the s357 mutation was mapped to chromosome 14, between microsatellite markers z9017 (0.08 cM) and z22094 (0.16 cM). Four partly overlapping BAC clones, zK10H23, zC221F10, zC143O2 and zC119P14 covered this region (Zebrafish Genome Fingerprinting Project; http://www.sanger.ac.uk/). By blasting GenScan-predicted peptide sequences in this region to the NCBI protein database (http://www.ncbi.nlm.nih.gov/), nine genes were identified. The zebrafish kohtalo/TRAP230 mutant complemented grs357, excluding this gene as a candidate locus. Coding regions from the other eight genes were RT-PCR-amplified and sequenced. One mutation was found in the GR cDNA, which was also confirmed in the genomic sequence. The WT cDNA sequence of GR matched the one submitted by Ozawa et al. (NCBI accession number AB218424).
All animals used for behavioral analysis were genotyped by using the markers z9017 and z22094, which flank the gr locus, on genomic DNA template extracted from fin clips or by mismatch PCR restriction-fragment length polymorphism (RFLP) strategy on cDNA template. PCR primers [Forward 5’GGAAGAACTGACCTGCCTGT3’; reverse 5’TATCCGGCATAGAGGGTGTC3’] were designed to add a DrdI site to the WT allele adjacent to the mutation site. PCR products were then digested with DrdI and separated by 2% agarose gel electrophoresis to identify carriers.
Cell culture, transient transfection and luciferase assay
Transcriptional activity was measured in two cell lines, U2OS and COS-7. To transfect cells, WT (pCDNA3-GRWT) and mutant (pCDNA3-GRR443C) forms of Flag-tagged GR constructs were made. Anti-FLAG antibody (Sigma) was used to detect GR by fluorescent immunocytochemistry. GRE-luciferase reporter gene was co-transfected, and luciferase activity was measured following standard procedures.
To transfect U2OS cells, the following reporter plasmids were used: AP1-Luc containing a single consensus AP-1 site upstream of the TK promoter, pOS-344-Luc containing the -344/+33 fragment of the osteocalcin gene, and NFκB-Luc containing a single NFκB-site. U2OS human osteosarcoma cells were maintained in DMEM (GIBCO) supplemented with 5% FBS. For reporter activity assays, cells were seeded into 24-well plates in DMEM/5% FBS at approximately 20,000 cells per well. The following day, the cells were transfected in FBS-free DMEM using 0.8 μl of Lipofectamine and 1.6 μl of PLUS reagent (Invitrogen) per well, with 20 ng reporter and 20 ng of lacZ plus 100 ng empty plasmid p6R. After transfection (3h), cells were re-fed with DMEM/5% FBS, allowed to recover for 3h, and either re-fed with DMEM/5% FBS containing 100 nM Dex or ethanol vehicle (AP1 and osteocalcin reporters) for twelve hours, or treated the next day with ethanol vehicle, TNFa (5ng/ml) or TNFa (5ng/ml) + 100 nM Dex for 6 hrs (NFkB reporter). After treatment, cells were lysed in 100 μl per well of 1x lysis buffer (PharMingen) and assayed for luciferase and β-galactosidase activity.
Behavioral testing and image analysis
Experimental fish were recorded in 4-7 day intervals, once or twice a week. Since the HPA axis is under circadian regulation all behavioral tasting were restricted to the early afternoon (5-8 hours after light onset; 13:00-16:00). Movies were recorded with a digital camera (HandyCam, Sony), transferred by Firewire to a computer, and saved on the hard disk. Offline analysis was carried with custom-written software in LabVIEW 8 (National Instruments) and Matlab 6.5 (The MathWorks Inc.).
Drug treatments
Long-term drug treatments with fluoxetine hydrochloride (Sigma, 0.8 μM), bupropion hydrochloride (Sigma, 3 μM) and RU-486 (mifepristone, Sigma, 2.5-10μM) were conducted on groups of fish (n=4 to 5) housed in 2 l tanks (1.5 l water volume) with solvent for the drug serving as the control. Drug-treated fish were fed once a day with live Artemia salina, followed by replacement of the water and drug one hour later. Short-term treatment with diazepam (Sigma, 5μM) was carried out by adding the drug to the fish tank 30 min before the assay. All drugs were also added to the water of the tank in which the behavioral test was carried out. Treatment with dexamethasone (Dex, Sigma, 25 μM) was carried out by adding the drug to the fish tank at 18:00 hours the day before the cortisol extraction. The next morning (9:00), fish were fed and Dex-containing water was replaced with system water.
cDNA isolation and quantitative RT-PCR
Adult fish were killed by immersion in Tricaine (Western Chemicals Inc, WA, USA). RNA, extracted from fresh brains, was dissolved immediately in Trizole reagent (Invitrogen) and kept on ice until processed (10-40 min). If brains were taken immediately following behavioral testing, heads were first frozen on dry ice and stored in -80°C before dissection and RNA extraction. cDNAs (Invitrogen) were prepared, and qRT-PCR (Applied Biosystems Inc.) was performed according to the manufacturers’ instructions. All RT-PCR expression data were normalized to zebrafish ppia-1 (NM199957).
Whole-mount RNA in situ hybridization and vibratome sections
Adult fish were killed by immersion in Tricaine and fixed in 4% paraformaldehyde (Ted Pella, Inc.) in PBS overnight in 4°C or for 4h at room temperature. Whole brains were removed under a dissecting scope and stored in 100% methanol at -20°C. SP6 and T7 clones with fragments from the coding sequence of pomca (NM_181438), crh (NM_0001007379) and serta (NM_001039972) were used as templates to prepare digoxigenin (DIG)-labeled antisense riboprobes (Roche). Large probes were further digested to 200-300bp fragments. Whole mount in situ hybridizations were performed following a standard protocol. Following hybridization, brains were washed and mounted in 4% agarose (low melting temperature, Invitrogen) in PBS and sectioned at 100μm using a vibratome (Leica). BM purple (Roche) substrate was used for AP staining. Images were captured with a CCD camera, mounted on Leica stereoscope.
Cortisol measurement
Fish were anesthetized on ice, superficially dried with a paper towel, and the tail was removed. Blood was collected from the caudal vein with a heparinized capillary (Fisher Scientific), prefilled with sample buffer (1% BSA (Sigma), 2 TIU Aprotinin (Sigma) in PBS). Either blood was blown out into a tube with sample buffer to a final amount of 20μl, or capillaries were directly centrifuged in a 1.5 ml tube, and plasma was taken out of the capillary using a gel loading tip. Plasma was isolated by centrifugation at 4°C, 3000g for 10 min, and stored at -80°C. Cortisol plasma concentrations were determined using an enzyme immunoassay (EIA) kit (Cayman Chemicals Co, MI, USA, or Demeditec Diagnostics GmbH, Kiel-Wellsee, Germany) or a Cortisol RIA kit (COAT-A-COUNT, Siemens Healthcare Diagnostics Ltd., Frimley, Camberley, UK). In order to reach the sensitive range of the standard curve, plasma was diluted 1:25-60 (gr+/+ and grs357/+) or 1:500-1000 (grs357/s357) with EIA buffer or zero calibrator.
Statistical analysis
Statistical analysis was carried out with JMP8 software (SAS Institute Inc.). In all graphs, error bars represent SEM. For statistical comparisons, one-way or two-way ANOVA, followed by paired Student’s t-tests, were used. The Shapiro–Wilk test was used to determine normal distributions.
Results
The grs357 mutation generates an R to C substitution in the DNA binding domain of GR
The grs357 mutant (originally named utoutos357) was identified in a large-scale forward genetic screen for chemically-induced point mutations that disrupt larval behavior14. The mutant larvae move less actively than wildtype (WT) at 6 days after fertilization (6 dpf), but are morphologically indistinguishable from WT at all stages into adulthood. We employed standard genetic mapping techniques to identify the mutation in the zebrafish genome. Homozygous grs357 mutant larvae are somewhat darker than their WT siblings, owing to dispersed melanin pigment in their melanophores. This phenotype, which may be related to misregulation of alpha-melanocyte-stimulating hormone (α–MSH, a peptide hormone encoded by the pomc gene) was used to sort mutants from WT. A panel of polymorphic simple-sequence repeats were scanned to identify those that co-segregated with the mutant phenotype. About 600 individual homozygous mutants from a WIK/TL hybrid cross (see Materials and Methods) were then used to refine the chromosomal location.
Narrowing the locus to a small region on chromosome 14 and sequencing of positional candidates identified gr, the gene encoding GR, as the mutated locus (Fig. 1a). The zebrafish genome contains one copy of GR with high protein identity (47%) to the human GR protein, especially in the DNA-binding domain (97%) and the ligand-binding domain (73%)15. Sequencing of the mutant cDNA showed a missense mutation that replaces an arginine (R) at position 443 with a cysteine (C) in the DNA-binding domain. This domain is essential for transcriptional regulation by GR16-20 (Fig. 1b-d). The protein structure model of GR (http://www.rcsb.org/pdb/) predicts that the positive charge of this arginine, which corresponds to R496 in rat, R484 in mouse and R477 in human GR, is critically important for binding of the second zinc finger knuckle to the negatively charged phosphate groups of the DNA backbone21 (Fig. 1d). This suggested (and was verified below) that the grs357 mutation generated a strong hypomorph or null allele of GR.
Figure 1. Forward genetic identification and biochemical characterization of a mutation that disrupts transcriptional activity of the zebrafish glucocorticoid receptor.
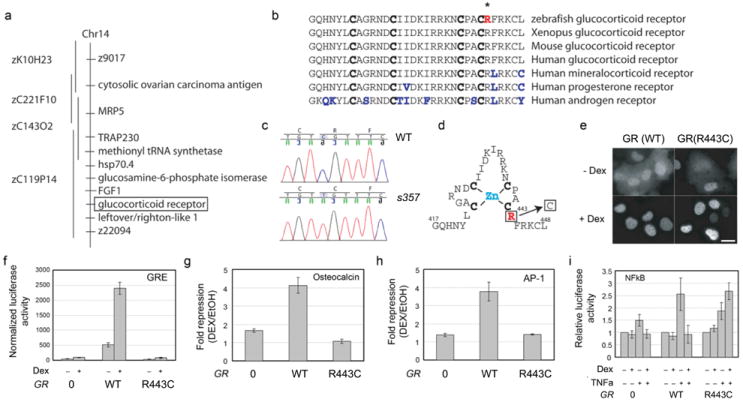
a, BAC contig, spanning the interval between two microsatellite markers, z9017 and z22094. The region contains nine predicted genes. b, Genomic sequence alteration identified in the gr gene. c, Sequence comparison of the zinc finger motif near the mutated amino acid residue (in red, marked with *). Four conserved cysteines in the zinc finger are in bold. Non-conserved amino acids are in blue. d, Schematic of the zinc-finger and the location of the R to C substitution. e, Subcellular localization of Flag-tagged WT and R443C GR protein, expressed in COS-7 cells and detected with anti-Flag antibody. Both showed nuclear translocation in the presence of 1 μM Dex. Scale bar is 10 μm. f-i, GR (R443C) is defective in transcriptional repression. U2OS cells lacking endogenous GR, were co-transfected with either empty vector (‘0’), or expression constructs for either wildtype GR (WT) or mutant GR (R443C) and reporter constructs for PRE-tk (GRE) (n=2, p<0.0001) (f); Osteocalcin (nGRE) (n=5, p<0.0001) (g); AP1 (n=3, p=0.018) (h) or NFκB (n=4, p=0.0037) (i). Cells were treated overnight with 100 nM Dex (PRE-tk, AP1 and Osteocalcin) or for 6 h with either ethanol vehicle, TNFα or TNFα plus 100 nM Dex (NFκB). Reporter activity was expressed as fold repression of Dex treated/EtOH vehicle (g and h) or relative luciferase units (f and i).
The mutation spares hormone binding and nuclear translocation, but eliminates transcriptional activity of GR
To determine which protein functions were disrupted by the R443C mutation, we expressed Flag-tagged GR, with and without the amino acid substitution, in COS-7 cells. In the absence of ligand, anti-Flag antibody detected GR protein in both nucleus and cytoplasm. In the presence of the synthetic GR ligand Dex, both WT and mutant GRs were now exclusively found in the nucleus (Fig. 1e), indicating that neither hormone binding nor subsequent nuclear translocation were disrupted by the mutation. This is consistent with previous structure-function studies showing that nuclear translocation does not depend on the protein domain carrying the substitution16-20.
To assay the effect of the R443C mutation on transcriptional regulation by GR, WT or mutant GR were co-transfected with reporter plasmids that recapitulated GR-dependent transcriptional activation (GRE and GRE-tk) or repression (Osteocalcin, AP-1, NF-kB) in cell lines not expressing GR. Transcriptional activation through direct binding to a strong GRE was abrogated in two different cell lines (COS7 and U2OS), even at a Dex concentration that exceeded the maximal effect in WT tenfold (Fig. 1f; Suppl. Fig. S1). Similarly, transcriptional repression, which can either be mediated by direct DNA binding (Osteocalcin; negative GRE) or by interaction with other transcription factors, which tether GR to DNA (AP-1 and NF-kB), was also disrupted (Fig. 1g-i). Together our findings indicate that transcriptional activity of GR is largely eliminated by the s357 mutation.
The stress axis is chronically elevated and dysregulated in the mutant
Disruption of GR is expected to abrogate cortisol-mediated negative feedback of physiological stress signals. Indeed we found substantially elevated cortisol levels in homozygous grs357 mutants (Fig. 2a). Cortisol varied by a factor of three, between individual animals, but was always several fold higher in mutants (1-3 μg/ml) than in WT sibling fish from the same tank (0.02-0.2 μg/ml). In WT, cortisol was slightly increased in response to acute confinement stress (keeping an adult fish in a narrow glass tube for 10 minutes) (Fig 2a). Homozygous mutants did not exhibit a further increase in cortisol following stress treatment, suggesting a ceiling effect. Heterozygous carriers had higher cortisol levels than WT in the non-stressed condition and responded more strongly to confinement stress, but never reached the levels seen in homozygous mutants (Fig. 2a, insert). Stress did not further raise cortisol levels in mutants, perhaps owing to a ceiling effect. To investigate if negative feedback by GR on the HPA axis is blunted by the mutation, we carried out a ‘Dex suppression test’. Blood cortisol levels were measured in the early afternoon (13:00-16:00, matching the time of the day in which behavioral measurements were made, see below), following administration of Dex or vehicle in the evening (18:00) of the previous day (lights were on from 7:00 to 21:00). Whereas in WT and heterozygous carriers Dex treatment abolished the diurnal cortisol increase, cortisol levels remained high in mutants (Fig. 2b).
Figure 2. Hyperactivity of the HPA axis in grs357 mutant zebrafish.
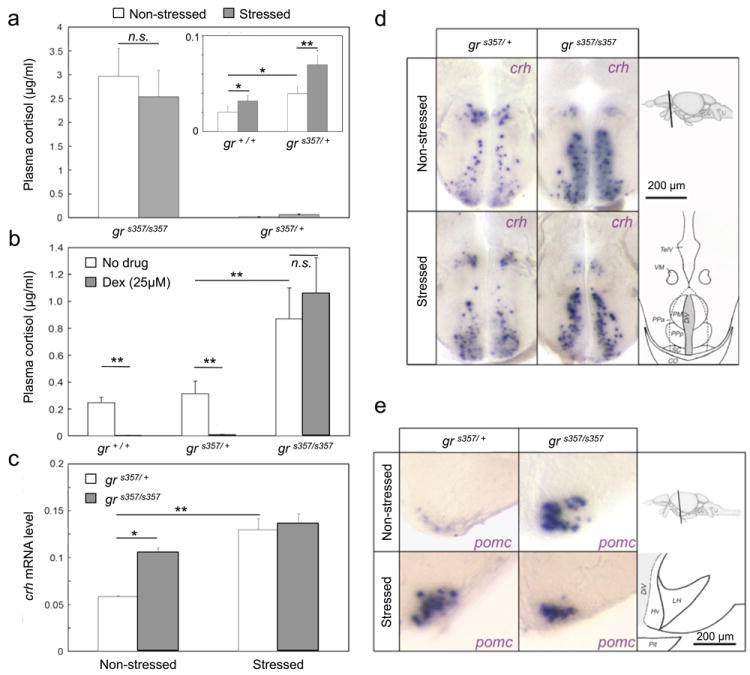
a, Plasma cortisol levels determined by EIA before (non-stressed) and after confinement stress (stressed) in mutants (grs357/s357) compared to heterozygotes (grs357/+). Insert shows cortisol of heterozygous (grs357/+) and WT fish (gr+/+) at different scale (n=4-10 each). Cortisol is highest in mutants. Cortisol is higher in heterozygotes than in WT. Stress increases cortisol in heterozygotes and WT (difference between grs357/s357 and grs357/+: p<10-4, ANOVA F ratio = 160.24; difference between grs357/+ and gr+/+: *p=0.05; difference between non-stress and stress: **p=0.0051, ANOVA F ratio = 8.81; combined effect genotype × stress p=0.002, ANOVA F ratio=10.99; pooled data ANOVA: degrees of freedom = 3, F ratio = 60.82, p<10-4). b, Dex suppression test. Dex was added at 18:00 the day before. Cortisol was measured at 13:00-16:00. Light on was at 7:00. In WT and heterozygotes, the diurnal cortisol spike is greatly suppressed (**p<10-3). Mutants show no reduction (p>0.05;). c, RT-PCR expression of crh. RNA was extracted from the front part of the brain (including telencephalon and anterior hypothalamus) in non-stressed and stressed fish (n=3-9). crh mRNA is increased in mutants (*p<0.05) and following confinement stress (**p<10-3). d, Sagital sections showing crh-expressing cells in hypothalamic preoptic area. More signal is seen in mutants. Drawings on the right indicate position of section in a side view of the zebafish brain (top) and boundaries of brain nuclei and ventricle at the level of the stained section (bottom). e, Sagital sections showing pomca-expressing cells in the lateral tuberal nucleus (NLT) of the hypothalamus. Mutants and stressed fish show stronger signal. Drawings on the right, as in (d). Abbreviations: PPa and PPp, parvocellular preoptic nucleus, anterior and posterior parts; PM, magnocellular preoptic nucleus; VM, ventromedial thalamic nucleus; TelV, telencephalic ventricle; DiV, Diencephalic ventricle; SC, suprachiasmatic nucleus; CO, optic chiasm; LH, lateral hypothalamic nucleus; Hv, ventral zone of periventricular hypothalamus; pit, pituitary.
Quantitative real-time PCR on total RNA taken from the front part of the brain (including telencephalon and anterior hypothalamus) demonstrated chronic, two-fold increases of crh transcript levels in mutants compared to heterozygotes (Fig. 2c). Elevated levels of crh mRNA were seen in the preoptic area of the hypothalamus (the homologue of the mammalian paraventricular nucleus)22 (Fig. 2d) and the lateral tuberal nucleus (Suppl. Fig. S2). pomca mRNA was likewise globally increased in mutants (data not shown), particularly in the lateral tuberal nucleus (Fig. 2e). Confinement stress resulted in an increase of crh and pomca transcripts by RT-PCR and in situ hybridization in heterozygotes, but had little, if any, effect in mutants (Fig. 2c-e). Together, these changes in peptide hormone expression indicate a lack of negative feedback on gene transcription in the HPA axis.
Mutants freeze when placed in a novel tank and fail to habituate to repeated stress treatments
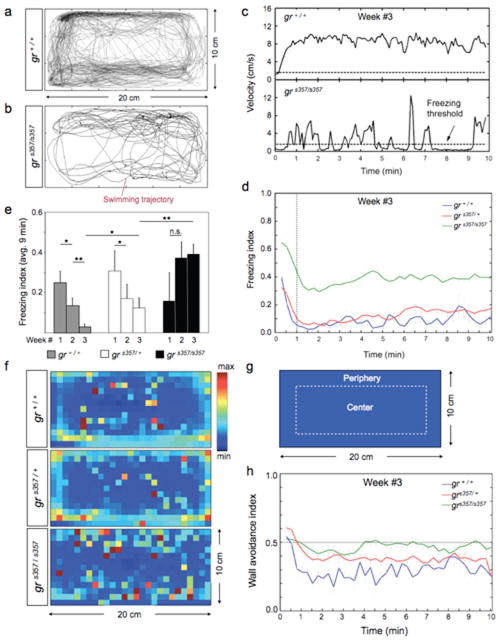
To test if grs357 mutants showed abnormal stress-related behavior, we observed the responses of WT, heterozygotes and mutants to a mildly anxiogenic environment23, 24. Single male fish were placed into a novel tank, which was well lit and had opaque, dark, non-reflective walls. The novel-tank test was repeated twice a week over three weeks. For each test, the fish’s swimming trajectory was recorded with an overhead video camera, graphed over time and evaluated with respect to the animal’s location and speed (Fig. 3a, b). After ten minutes, the fish were returned to their familiar community tanks. After being transferred into the novel tank, the fish often stopped swimming and sank to the bottom of the tank for 10 to 60 sec, before resuming normal swimming for the remainder of the observation period (Fig. 3c; top panel shows the velocity profile of a representative WT fish after five previous tests, in the third week after initial exposure to the novel tank). Most fish reacted most strongly in the first 60 s and then habituated. In some fish, however, ‘freezing’ bouts could occur through the entire observation period. Then fish could be immobile for up to 6 min out of the 10 min observation period and show only short swimming bouts across the tank (Fig. 3c; bottom panel shows velocity profile of a homozygous mutant with prior exposure identical to the WT fish above).
Figure 3. Exaggerated freezing responses and altered location preference in zebrafish grs357 mutants after isolation in a novel tank.
Naïve fish were tested in the novel tank on day 0 (week #1) and then tested again every 4-7 days during the next two weeks (n=4-8). Movements were recorded every 15 s over 10 min. a, Trajectory of a representative WT fish after 5 exposures to the novel tank. b, Trajectory of a representative mutant after 5 exposures. The mutant swims significantly less than WT. c, Plot of velocities over 10 min observation time. WT (top) shows short-term habituation within the first min and then swims at approx. constant speed for 9 min. Mutant (bottom) is immobile half of the time and swims in short spurts. Peak velocities are similar to WT. d, Freezing index (time spent immobile/observation time) of experienced fish in novel tank. Data were pooled and averaged (gr+/+, n=47; grs357/+, n=115; grs357/s357 n=134). The index drops in the first min but remains high in mutants. WT freeze slightly less than heterozygotes. Freezing threshold (dotted line) is 1.6 cm/s. e, Freezing index, averaged over 9 min. The first minute of the observation period (short-term habituation) was not included. WT and heterozygotes show long-term habituation over three weeks; they freeze gradually less. Mutants become more sensitized or are unchanged (*p<0.05; **p<10-3). f, Heat maps, showing the spatial preferences of animals in the novel tank after 5 exposures. The spatial bin size is 10×10 pixels. Warmer colors represent preferred locations (see scale on the right). Mutants (bottom) spend less time near the perimeter of the tank than WT (top) or heterozygotes (middle) (note the yellow and cyan pixels near the walls in the WT and heterozygotes). Orange and red pixels indicate places where the fish froze. A greater number of red pixels are seen in the mutants, and they tend to be at a distance from the walls. g, Quantification of place preference. Video images of the tank were divided into two equally large compartments, as indicated. The wall-avoidance index was calculated as the time spent in the central compartment divided by total observation time. h, Wall avoidance in experienced fish. Mutants remain in the central compartment (index ca. 0.5). WT prefers the periphery, after short-term habituation index drops from 0.5 to 0.3. Heterozygotes show intermediate preferences. Error bars ± SEM.
While the behavior was variable between individuals, particularly the initial freezing response (minute 1 of the test), we observed strong population differences between the behavioral patterns of mutant and WT fish. After, >3 test, each separated by 4 to 7 days of recovery, mutants froze significantly more than WT and heterozygous carriers. They spent greater percentage of time in an immobile state during the entire 10 min (see Fig. 3c). We calculated the freezing index as the time spent without moving, divided by the total observation time. Plotting the freezing index showed significant difference between mutants and WT (Fig. 3d). Genotype differences were not detectable at the very first exposure to the novel tank, but gradually developed as a result of experience. WT fish froze less with each exposure, apparently habituating to repeated isolation (Fig. 3e; Suppl. Fig. S3a). Heterozygotes followed the WT trend, although their freezing index revealed a slower time course of habituation than WT (Fig. 3e). Similarly experienced mutants showed the opposite trend over the course of three weeks; they spent increasingly longer time freezing with each exposure, suggesting that they became sensitized to the novel tank (Fig. 3e; Suppl. Fig. S3b). Thus, zebrafish with one or two WT copies of GR appear to exhibit long-term (inter-trial) habituation to a novel tank, while homozygous mutants become sensitized to the aversive effects of this stressor. The conditioning regime chosen here thus amplified behavioral differences between the genotypes.
Mutants show reduced wall exploration in the novel tank
We evaluated the locations of fish in the novel tank over the 10 min test period, as a function of genotype and prior experience. ‘Heat maps’ were generated that allowed us to visualize the cumulative positions of the fish, with warmer colors representing preferred areas of the tank and cooler colors representing areas that the fish only rarely visit (Fig. 3f). Place preference was calculated by dividing the time spent in the central 50% of the tank volume (away from the walls; as indicated in Fig. 3g) by the total observation time. This metric was termed “wall avoidance index”. Wildtype and heterozygotes showed no preference for the walls during the first exposures. (Suppl. Fig. S3c). With repeated exposures, WT fish became increasingly likely to swim near the walls (index drops from >0.5 to around 0.2; Fig. 3h; Suppl. Fig. S3c, data collected from two different cohorts of fish). Mutants exhibited a greater degree of wall avoidance and did not habituate to weekly exposures, being equally likely to be found near the perimeter as in the inner area of the tank (index 0.5; Fig. 3h; Suppl. Fig. S3d). Thus, fish with presumably higher levels of anxiety tended to avoid the walls of the novel tank.
The behavioral differences between the genotypes, both in their freezing responses and in their location preferences, could not be attributed to a locomotor defect, as mutant fish swam with peak velocities very similar to WT, although their average velocities and their total distances traveled were suppressed (Suppl. Fig. S4a, b).
Social interactions reduce freezing responses of the mutant
Zebrafish are highly social animals25. According to the social buffering paradigm26, human subjects are less prone to the anxiogenic effects of stressful environments when allowed interaction with other non-stressed group members and, inversely, show increased stress responses when isolated. We asked if freezing and place preference in the novel tank could be modified by exposure to a conspecific. For this experiment, we varied the configuration, leaving one wall of the tank unoccluded, enabling visual interaction with a WT fish in an adjacent tank (Fig. 4a). An empty tank served as a control. Analysis of swimming trajectories (Fig. 4b), cumulative ‘heat maps’ (Fig. 4c) and measurement of average positions (Fig. 4d) all revealed a clear tendency of zebrafish, regardless of genotype, to spend more time near the transparent wall. This tendency was stronger when the neighboring tank contained a conspecific. Intriguingly, when allowed visual interaction with other fish, mutants spent less time freezing, approaching the levels of the heterozygotes (Fig. 4e). Reduced freezing was not associated with measurably lower cortisol levels (Fig. 4f). We conclude that social interactions reversed freezing behavior in the mutant and did do so without correcting the HPA axis.
Figure 4. Social interactions reduce freezing and wall avoidance in zebrafish grs357 mutants.
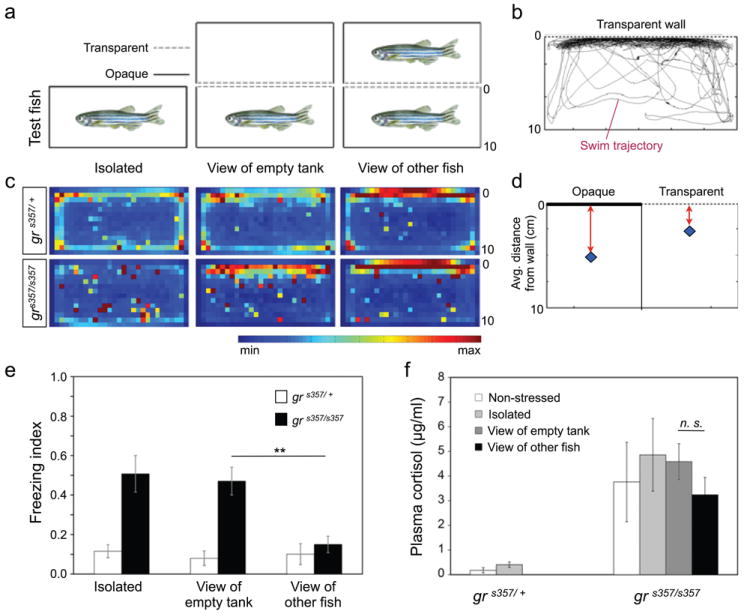
a, Top view of the experimental setup. The tested fish are depicted in the bottom row of tanks. Behavior was tested in the tank with one transparent wall, allowing visual interaction with a non-mutant conspecific in a neighboring tank (‘view of other fish’, right panel). A standard tank with four opaque walls (‘isolated’, left panel) and a tank with one transparent wall with view of an empty tank (‘view of empty tank’, middle panel) were used as controls. b, Representative trajectory of a mutant fish with view of a conspecific. c, Heat maps, showing place preferences as in Fig. 4a. Heterozygotes (top panels) prefer the walls and corners of the tanks and particularly the wall that apposes the empty tank or the tank that contains the other fish. Isolated mutants show no preference for the walls. However, they prefer to swim close to the transparent wall, particularly when the neighboring tank contains another fish. d, Average fish distance from the opaque vs. transparent wall. SEMs are smaller than the size of the marker diamond. e, Freezing indices for two genotypes (n=9-19) as a function of their visual environment. Experienced mutants freeze much less when able to view a conspecific (**p=0.0013, ANOVA F ratio = 11.26; pooled data ANOVA: degrees of freedom = 5, F ratio = 4.64, p=0.001). Other differences were not statistically significant. f, Plasma cortisol, determined by EIA. Hormone levels do not change in the behaviorally rescued mutants (n=2-4).
Acute diazepam and long-term fluoxetine treatments reverse depression-like behavior
To further investigate if freezing and reduced wall exploration behavior involves neural circuits associated with depression and/or anxiety, we treated fish with drugs known to have antidepressant or anxiolytic effects in mammals. Diazepam binds to the benzodiazepine binding site of the GABA-A receptor, where it acts like a positive allosteric modulator. Addition of diazepam (5 μM for 30 min) to the tank water lowered the freezing response of experienced mutants to levels seen in untreated heterozygotes (Fig. 5a; Suppl. Fig. S5a). Wall exploration was likewise increased to levels seen in untreated heterozygotes (Fig. 5c; Suppl. Fig. S5b). Heterozygotes treated with diazepam showed the lowest-possible freezing index (0; Fig. 5a) and the lowest-measured wall avoidance index (ca. 0.1; see Fig. 5c, top-right panel). Treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine (0.8μM) had a similar effect, when administered over four days, but not after shorter treatments (Fig. 5b; Suppl. Fig. S5c, d). Neither diazepam nor fluoxetine altered average swimming speeds in either mutants or heterozygotes (Suppl. Fig. S4c), indicating that these drugs did not exert sedative or other undesirable effects at the concentrations used. The selective norepinephrine and dopamine reuptake inhibitor bupropion (3 μM) had no effect on freezing behavior (Suppl. Fig. S6a). As expected, the potent GR antagonist RU486 (5 μM) had no behavioral effects on the mutants (Suppl. Fig. S6b-d). However, RU486 increased freezing responses in WT, without affecting swimming speed or place preference (Suppl. Fig. S6e-g), thus partially phenocopying the mutation.
Figure 5. Effect of diazepam and fluoxetine on zebrafish behavior and gene expression.
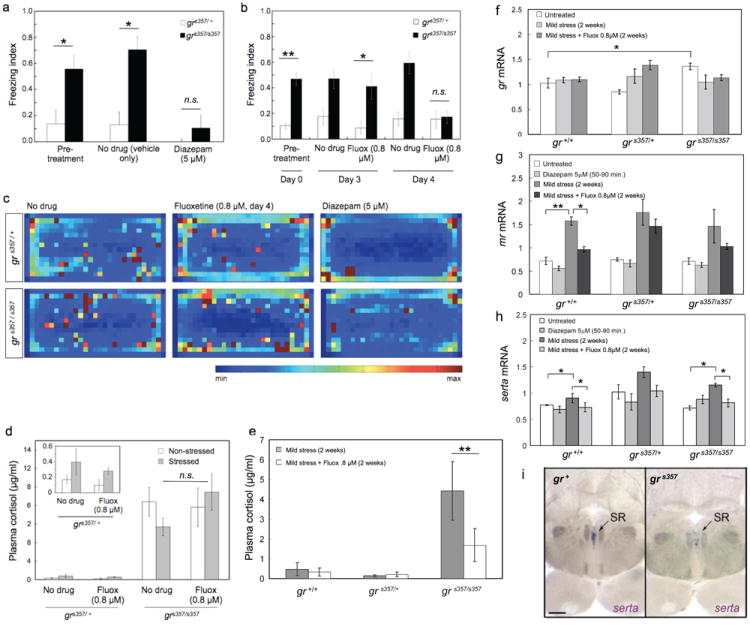
a, Freezing indices of experienced fish before (‘pretreatment’) and after diazepam (5μM, 30 min) or vehicle (‘no drug’, 30 min) treatments (n=5, significant difference between mutant and wildtype pre-treatment, *p=0.019, ANOVA F ratio = 6.17; no significant difference between treated mutants and wildtype, p=0.066, ANOVA F ratio = 2.97; no significant combined effect genotype × treatment, p=0.3464, F ratio = 1.09; pooled data ANOVA: degrees of freedom = 5, F ratio = 2.87, p<0.03). b, Freezing indices of experienced fish after fluoxetine treatment (0.8μM, 4 days). Same controls as in the diazepam experiment (n=10-36; significant difference between mutant and wildtype pre-treatment genotype difference, **p<0.0012, ANOVA F ratio = 11.0; no significant difference between treated mutants and wildtype, p=0.097, ANOVA F ratio = 2.38; no significant combined effect genotype × treatment, p=0.62, F ratio = 0.62; pooled data ANOVA: degrees of freedom = 5, F ratio = 6.91, p=10-4). c, Heat maps of location preferences. The heterozygous fish shows a mild preference for the corners and walls, which is greatly increased following fluoxetine and diazepam treatments. Diazepam-treated fish (upper right panel) spent most of the time in the corners of the tank. The mutant is similarly affected, although its wall avoidance does not reach the levels of the diazepam-treated heterozygote (lower right panel). d, Plasma cortisol levels of experienced mutants and heterozygotes, determined by EIA before (‘non-stressed’) and after behavioral testing (‘stressed’) following 4 days of fluoxetine (0.8μM) or vehicle (‘no drug’) treatments (n=2-8). Insert shows heterozygote levels on a different Y-axis scale. Fluoxetine normalizes behavior without correcting cortisol levels (p>0.05). e, Plasma cortisol levels of naïve WT, mutants and heterozygotes, determined by RIA following 2 weeks of fluoxetine (0.8μM) or vehicle (‘untreated 2 weeks’) treatments (n=3-7). Cortisol is partially corrected after two weeks of fluoxetine administration (**p<0.001). f-h, RT-PCR expression data of gr, mr and serta mRNA in total RNA extracted from whole brain of non-stressed fish (‘Untreated’); following two weeks of chronic mild stress (‘2 weeks CMS’); 50-90 min of 5μM diazepam treatment (‘Diazepam 5μM’); or two weeks of CMS with 0.8μM fluoxetine treatment (‘2 weeks CMS + Fluoxetine 0.8μM’) (n=3-9 fish per group; *p<0.01, **p<0.0001). gr mRNA is largely unaltered by stress and drug treatments (f). mr mRNA is similar between genotypes, but upregulated in stressed fish of all genotypes and normalized by fluoxetine (g). At the whole-brain transcript level, there is no significant difference in serta expression between experimental groups (h), but see (i). i, In situ hybridizations on hindbrain sagittal sections reveal the absence or strong reduction of serta-expressing cells in the superior raphe nucleus (SR) of the grs357 mutant.
We next asked if fluoxetine treatment lowered cortisol levels in stressed fish and/or corrected cortisol in behaviorally rescued mutants. After four days of treatment, i. e., immediately after the behavioral test, cortisol was unchanged (Fig. 5d). However, following two weeks of continuous fluoxetine treatment, cortisol was substantially reduced, albeit not to the comparatively low levels seen in heterozygotes and wildtype (Fig. 5e). Therefore, cortisol correction follows on the heels of, but is apparently not necessary for, behavioral improvement.
Fluoxetine and the grs357 mutation reveal crosstalk between glucocorticoid signaling and the serotonergic system
To better understand how SSRIs impinge on the stress axis, we measured the expression levels of GR and MR by quantitative RT-PCR, following chronic mild stress (CMS) treatment. CMS was produced by isolating a fish for up to two weeks, with regular ad libitum feeding and daily handling. We found that gr mRNA itself was slightly but consistently increased (by ca. 25%) in the grs357 mutant and was not detectably changed by CMS und the experimental conditions used here. Fluoxetine had subtle, if any, effects on gr expression (Fig. 5f). Transcript levels of mr, the gene encoding the mineralocorticoid receptor, were similar for all genotypes, suggesting that MR is not regulated by GR (Fig. 5g). Following chronic mild stress, mr mRNA was significantly increased (by ca. 100%); fluoxetine administration blunted this increase (Fig. 5g). It is possible that an attenuation of the MR-mediated stress response accounts for some of the beneficial effect of fluoxetine on behavior.
Chronic stress may affect expression of genes important for serotonergic signaling. We therefore analyzed the level and expression pattern of brain serotonin transporter (serta). Whole brain qRT-PCR revealed a subtle increase in serta expression between stressed and non-stressed states in wildtype, mutants and heterozygotes (Fig. 5h). This trend is detectable by 4 days of CMS and is significant by 2 weeks (Suppl. Fig. S7). Fluoxetine (4 days or 2 weeks) lowers serta levels to baseline levels in CMS-exposed fish, whereas diazepam does not, even when applied continuously for 4 days (Suppl. Fig. S7). Thus, regulation of serta transcription could be one mechanism, by which the therapeutic effects of fluoxetine and diazepam differ. Our measurements also suggested that serotonin regulation during stress and the effect of SSRIs are not disrupted by the grs357 mutation. In situ hybridization, however, offered a more complex picture. Here, serta mRNA appeared to be absent from the superior raphe nucleus of the mutants (Fig. 5i). Pretectal nuclei expressed apparently normal levels of serta (Suppl. Fig. S8). Together, the observed fluoxetine effects on behavior, GR and MR expression, as well as apparent serta misregulation in the grs357 mutant, are consistent with the notion that glucocorticoid signaling is cross-linked with the serotonergic transmitter system and that a serotonin imbalance predisposes depression-like behavior in zebrafish.
Discussion
We have presented evidence that disruption of GR causes a syndrome in adult zebrafish that resembles an affective disorder. The syndrome carries the molecular signature of chronic stress and the behavioral profile of depression, including decreased exploratory behavior and impaired habituation to repeated exposure to an anxiogenic environment. Molecularly, the mutation results in substitution of a highly conserved R to C in the second zinc finger motif of the GR protein, which is essential for DNA binding. This change abolishes both transcriptional repression and activation of target genes in a broad range of in vitro assays, including those requiring binding of GR homodimers to positive or negative GREs and heterodimerization with NF-kB or AP-1. We conclude from these data that the mutation has generated a null for most, if not all, genomic functions of GR. In agreement with our findings on the molecular consequences of this mutation in zebrafish, a naturally occurring mutation of the equivalent residue (R477) in human GR20, 27, 28 also disrupted transcriptional regulation by GR. Moreover, mutation of the corresponding amino acid residue in the androgen receptor (R615) causes androgen insensitivity syndrome in humans29 suggesting that this conserved arginine is essential for the function of steroid hormone receptors in general.
We established that disruption of GR genomic activity results in a hyperactivated HPA axis. In particular CRH, ACTH and cortisol, are chronically elevated. Consistent with an exaggerated stress response, mutants overreact to an anxiogenic environment, a novel tank in which they are isolated from conspecifics. Wildtype and heterozygous fish habituate both in the short term (within the 10 minutes of the individual trial) and long term (between trials) to this stressful environment. Their behavior shifts from freezing and location indifference to strong exploratory swimming behavior near the walls. Homozygous mutants, on the other hand, largely remain in a freezing posture and do not show place preference between and during freezing bouts; they barely habituate within a trial and even become sensitized between trials. Treatments with diazepam and fluoxetine reduce freezing duration and wall avoidance behavior in the mutants and also augment and accelerate habituation in wildtype and heterozygotes.
In zebrafish, wall-hugging behavior, or thigmotaxis, in the novel tank appears to be an indicator of motivated, exploratory behavior. In agreement with previous studies30-32, we observed that habituation to the novel tank results in a reduction of wall avoidance. It has been reported that intermediate doses of ethanol increased several indicators of anxiety, while thigmotaxis decreased33. On the other hand, d-Lysergic Acid Diethylamide (LSD) reduced freezing and other anxiety-related behaviors, and simultaneously increased thigmotaxis34. We show her that anxiolytic and antidepressant treatments similarly result in pronounced swimming near the walls. So, a consensus is emerging that, at least in the small, well-lit containers used by us and others, ‘thigmotaxis’ increases as stress levels drop, and vice versa. This may appear counterintuitive, since it is opposite to what has been reported in rat and mouse, but could be owed to ecological differences between diurnal telosts and nocturnal rodents. Zebrafish are visual specialists. For them, moving to the open field when threatened, where they cannot be ambushed by a hidden predator and can seek conspecifics to shoal with, may be an adaptive strategy. Further work is needed to resolve this important issue.
Is the freezing behavior observed here more closely related to anxiety or to depression? The pharmacology leaves this question unanswered, since freezing is reversed by both anxiolytic (diazepam) and antidepressant drugs (fluoxetine). So we have to consider behavioral parameters. Anxiety in zebrafish is characterized by darting across the tank and active exploration of escape routes23,24,30. We did not observe this behavior in our mutants. Also, behavioral differences between mutants and wildtypes became only detectable after repeated exposures, suggesting long-lasting, experience-dependent effects akin to learned helplessness. While we cannot know the emotions of a fish, we therefore interpret the freezing behavior and immobility of grs357 mutants as manifestation of a syndrome more similar to depression than to anxiety. It cannot be excluded, however, that the mutant’s behavior may reflect an extreme form of anxiety or that the boundaries of anxiety, panic, catatonia, despair and depression may be fluid for teleost fish.
Successful treatment with fluoxetine, as well as social interactions with other zebrafish leave the high cortisol levels of the mutant initially unchanged, apparently bypassing the HPA axis. Only after two weeks of fluoxetine treatment did we observe a reduction of cortisol. Thus, while disruption of GR-mediated transcriptional control is sufficient to cause an affective disorder in this zebrafish model, subsequent correction of cortisol levels is not necessary for normalization of the behavior. Instead, other stress-associated mechanisms, presumably in the emotional centers of the brain, are independently targeted by SSRIs and other successful treatments. Cortisol reduction occurs with a delay and appears to be consequence, rather than cause, of the successful therapy.
The neural mechanism by which HPA dysregulation conditions behavioral responses in zebrafish is currently unknown, but there is strong evidence that interactions between CRH and the serotonergic system may be involved. A subsensitivity of the serotonin system is strongly associated with depression in mammals35,36. In teleosts and amphibians, intracerebral injection of CRH modulates locomotor activity and place preference in a context-dependent manner. These CRH-induced behaviors are strongly potentiated or inhibited by co-administration of fluoxetine or a 5-HT1A receptor antagonist, respectively37,38. In our zebrafish grs357 mutant, brain serotonin transporter (Serta) expression appears to be decreased in the superior raphe nucleus. Serotonergic neurons from the raphe nucleus project broadly to many areas of the CNS and are important modulators of brain plasticity and neurogenesis, as well as locomotion and affective behaviors40. The therapeutic potency of SSRIs observed above could be related to restoration of normal serotonin function in the superior raphe nucleus with distributed effects on forebrain or brainstem motor circuits. In addition, as suggested by their slow time course of action, SSRIs might affect gene transcription, e. g., dampen the stress-induced expression of MR, as shown here in zebrafish.
Several mouse lines with conditionally altered GR expression or function have been generated40,41. (Constitutive knockout of GR is embryonic lethal in the mouse.) Depressive behaviors were observed in mice with heterozygotic GR knockout42 or forebrain-restricted GR knockout43. However, total neuronal or glial GR knockouts44, or antisense GR knockdown45 either resulted in reduced anxiety or had no measurable effect on behavior. Paradoxically, a transgenic mouse with overexpression of GR in the forebrain showed increased anxiety and/or depression-like behavior after exposure to chronic mild stress46. Thus, behavioral results in the mouse cannot be easily reconciled with data in human patients, maybe because tissue-restricted manipulations have incomplete or complex effects on the HPA axis and/or the limbic system.
Transcriptional regulation by GR is not the only mediator of cortisol signaling in the brain. Transgenic overexpression of MR in the forebrain was recently shown to dampen anxiety-related behavior in mouse47. In addition to the classical genomic activity of GR and MR, an alternative rapid, non-genomic signaling route for cortisol was shown, which may or may not involve GR or MR48. Apparently, this signaling is crucial for mediating glucocorticoid effects on neuronal activity and plasticity, facilitating or inhibiting signaling of ion channels and neurotransmitter receptors49-51. Both cortisol’s non-genomic effects and MR-dependent signaling are expected to be intact in the grs357 mutant or may even be exaggerated due to increased cortisol levels. Since grs357 mutants lack all GR genomic activity it may be a useful tool for disentangling the contributions of these other signaling pathways in the stress response.
Together, our findings demonstrate the striking degree of evolutionary conservation in the neuroendocrine circuits regulating emotion in vertebrates. Our data strongly support the hypothesis8 that excessive activation of the HPA axis, by acquired or (in the case of this novel fish mutant) inherited glucocorticoid resistance, is an important contributor to the development of depression. As a diurnal species with cortisol rhythms similar to humans1, zebrafish may provide a useful model system for neuropsychiatric research, complementary to the widely used rodent models. In a future application, our zebrafish gr mutant could potentially be used as a small-molecule screening tool52 for the discovery of novel antidepressant pharmacologies.
Supplementary Material
Transcriptional activation by GR (R443C) is largely abolished in COS-7 cells. Cells were transfected with GR (WT) or GR (R443C) and GRE-luciferase DNAs.
Hypothalamic crh expression is increased in response to stress and chronically elevated in grs357 mutants.
crh mRNA-expressing cells in two consecutive sagital sections (100μm) showing the nucleus lateralis tuberis (NLT) (blue arrowhead) and posterior tuberal nucleus (PTN) (white arrowhead). crh is undetectable in the NLT of non-stressed heterozygotes (top left panel). crh is elevated in the NLT of WT following stress and in mutants. Drawing on the right indicates position of sagittal section in the zebrafish brain.
Development of fish behavior in the novel tank over three weeks.
Freezing and wall avoidance indices were measured in 15 s bins over the 10 min observation period. This measurement was repeated for the same cohort of fish in weekly intervals. a, Freezing index of WT. b, Freezing index of mutants. c, Wall avoidance index of WT. d, Wall avoidance index of mutants.
Swim velocity and distance traveled in the novel tank.
Velocity was measured for each episode of swimming faster than 1.6 cm/s (the freezing threshold) and averaged. Distances were measured by calculating the length of the trajectories over the 10 min observation period. a, Mutants appear to swim on average more sluggishly than WT or heterozygotes, although the difference was not significant in this experiment. b, Total distance traveled by WT, heterozygotes and homozygous mutants in the novel tank. c, Fluoxetine and diazepam treatments do not alter swim velocities at the concentrations used. *p<0.01, **p<0.0001.
Freezing indices and wall avoidance behavior of drug-treated fish.
Behavior of experienced fish was measured and plotted as in Suppl. Fig. S3. a, Freezing index of diazepam-treated fish. b, Freezing index of fluoxetine-treated fish. c, Wall avoidance index of diazepam-treated fish. d, Wall avoidance index of fluoxetine-treated fish.
Effect of bupropion antidepressant and cortisol antagonist, RU486, on fish behavior in the novel tank test.
a, Freezing indices of experienced fish before (‘pre treatment’, day 0) and after 3 or 4 days of treatment with bupropion (3μM) or vehicle (‘no drug’) (n=10-36 fish per group). Behavior is apparently unchanged by bupropion. b-g, Effect of RU486 on behavior of the mutants (b-d) and WT (e-g). Experienced fish were treated for two consecutive days with several doses (2.5-10mM) of RU486, or with ethanol vehicle, and tested the next day (see Methods) (n=5-6). RU486 does not alter freezing (b), swimming velocity (c) or wall avoidance (d) of the mutants (n=5-6). However, RU486 increased freezing responses of WT (n=3-8 animals per group; *p<0.05). Error bars represent ± SEM.
Whole-brain serta RNA expression in response to 4 day diazepam treatment.
Real-time PCR expression data for serta transcripts in total RNA extracted from the front part of the brain (including telencephalon, and anterior hypothalamus) following CMS for 4 days with and without continuous diazepam (5 μM) treatments (n= 4 animals per group).
serta expression in the pretectum.
serta mRNA expression in two consecutive sagital sections (100 μm) showing serta expression in the pretectal diencephalic cluster, the parvocellular preoptic nucleus (PP), of experienced fish. Expression strengths appear similar between the two genotypes in these areas of the brain.
Acknowledgments
We thank J. Kitamoto and M. Suzawa for technical assistance on some of our preliminary experiments, E. Gahtan, N. M. Shah, R. Carpenter, R. Fletterick and M. Dallman for advice and comments, S. Hong and I. Dawid for sending us the kohtalo mutant for complementation testing, G. Rechavi for support of L. Z., and members of the Baier lab for discussions.
Footnotes
Conflict of Interest
The authors declare that they do not have competing financial interests.
References
- 1.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 2.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, et al. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 3.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 5.Belmaker RH, Agam G. Mechanisms of disease: major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 6.Carroll BJ. The Dexamethasone suppression test for melancholia. British Journal of Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- 7.Chandler VL, Maler BA, Yamamoto KR. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983;33:489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- 8.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. Plos Genetics. 2007;3:927–938. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 11.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 12.Sahay A, Hen R. Hippocampal neurogenesis and depression. Novartis Found Symp. 2008;289:152–60. doi: 10.1002/9780470751251.ch12. [DOI] [PubMed] [Google Scholar]
- 13.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety – insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, et al. Forward genetic analysis of visual behavior in zebrafish. Plos Genetics. 2005;1:575–588. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaaf MJM, Chatzopoulou A, Spaink HP. The zebrafish as a model system for glucocorticoid receptor research. Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology. 2009;153:75–82. doi: 10.1016/j.cbpa.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Danielsen M, Northrop JP, Ringold GM. The mouse glucocorticoid receptor - mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986;5:2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM. Functional domains of the human glucocorticoid receptor. Cell. 1986;46:645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- 18.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 19.Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11:S65–S72. doi: 10.1038/sj.cdd.4401413. [DOI] [PubMed] [Google Scholar]
- 21.Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yammamoto KR, Siegler PB. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352:497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 22.Bernier NJ, Lin XW, Peter RE. Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. Gen Comp Endocrinol. 1999;116:461–477. doi: 10.1006/gcen.1999.7386. [DOI] [PubMed] [Google Scholar]
- 23.Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouvela A., Jr Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Current Biology. 2004;14:881–884. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society B-Biological Sciences. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz M, Lind U, Gafvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L, et al. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001;55:363–371. doi: 10.1046/j.1365-2265.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- 28.Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP. Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGR alpha R477H and hGR alpha G679S associated with generalized glucocorticoid resistance. Journal of Clinical Endocrinology & Metabolism. 2006;91:1535–1543. doi: 10.1210/jc.2005-1893. [DOI] [PubMed] [Google Scholar]
- 29.Marcelli M, Zoppi S, Grino PB, Griffin JE, Wilson JD, McPhaul MJ. A mutation in the DNA-binding domain of the androgen receptor gene causes complete testicular feminization in a patient with receptor-positive androgen resistance. J Clin Invest. 1991;87:1123–1126. doi: 10.1172/JCI115076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res. 2010;214:332–342. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Blaser RE, Chadwick L, McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Maximino C, de Brito TM, Colmanetti R, Pontes AA, de Castro HM, de Lacerda RI, Morato S, Gouveia A., Jr Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res. 2010;210:1–7. doi: 10.1016/j.bbr.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C, Wong K, Elegante M, Elkhayat S, Tan J, Gilder T, Wu N, Dileo J, Cachat J, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214:277–284. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 35.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Clements S, Moore FL, Schreck CB. Evidence that acute serotonergic activation potentiates the locomotor-stimulating effects of corticotropin-releasing hormone in juvenile chinook salmon (Oncorhynchus tshawytscha) Horm Behav. 2003;43:214–221. doi: 10.1016/s0018-506x(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter RE, Watt MJ, Forster GL, Øverli Ø, Bockholt C, Renner KJ, et al. Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity. Horm Behav. 2007;52:600–611. doi: 10.1016/j.yhbeh.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 40.Chourbaji S, Gass P. Glucocorticoid receptor transgenic mice as models for depression. Brain Res Rev. 2008;57:554–560. doi: 10.1016/j.brainresrev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Kolber BJ, Wieczorek L, Muglia LJ. Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress. 2008;11:321–338. doi: 10.1080/10253890701821081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. Journal of Neuroscience. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 45.Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, et al. Long-term antidepressant treatment reduces behavioral deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995;7:841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 46.Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- 49.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. Journal of Neuroscience. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Wang CN, Ni X, Sun JH. A rapid inhibition of NMDA receptor current by corticosterone in cultured hippocampal neurons. Neurosci Lett. 2007;420:245–250. doi: 10.1016/j.neulet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcriptional activation by GR (R443C) is largely abolished in COS-7 cells. Cells were transfected with GR (WT) or GR (R443C) and GRE-luciferase DNAs.
Hypothalamic crh expression is increased in response to stress and chronically elevated in grs357 mutants.
crh mRNA-expressing cells in two consecutive sagital sections (100μm) showing the nucleus lateralis tuberis (NLT) (blue arrowhead) and posterior tuberal nucleus (PTN) (white arrowhead). crh is undetectable in the NLT of non-stressed heterozygotes (top left panel). crh is elevated in the NLT of WT following stress and in mutants. Drawing on the right indicates position of sagittal section in the zebrafish brain.
Development of fish behavior in the novel tank over three weeks.
Freezing and wall avoidance indices were measured in 15 s bins over the 10 min observation period. This measurement was repeated for the same cohort of fish in weekly intervals. a, Freezing index of WT. b, Freezing index of mutants. c, Wall avoidance index of WT. d, Wall avoidance index of mutants.
Swim velocity and distance traveled in the novel tank.
Velocity was measured for each episode of swimming faster than 1.6 cm/s (the freezing threshold) and averaged. Distances were measured by calculating the length of the trajectories over the 10 min observation period. a, Mutants appear to swim on average more sluggishly than WT or heterozygotes, although the difference was not significant in this experiment. b, Total distance traveled by WT, heterozygotes and homozygous mutants in the novel tank. c, Fluoxetine and diazepam treatments do not alter swim velocities at the concentrations used. *p<0.01, **p<0.0001.
Freezing indices and wall avoidance behavior of drug-treated fish.
Behavior of experienced fish was measured and plotted as in Suppl. Fig. S3. a, Freezing index of diazepam-treated fish. b, Freezing index of fluoxetine-treated fish. c, Wall avoidance index of diazepam-treated fish. d, Wall avoidance index of fluoxetine-treated fish.
Effect of bupropion antidepressant and cortisol antagonist, RU486, on fish behavior in the novel tank test.
a, Freezing indices of experienced fish before (‘pre treatment’, day 0) and after 3 or 4 days of treatment with bupropion (3μM) or vehicle (‘no drug’) (n=10-36 fish per group). Behavior is apparently unchanged by bupropion. b-g, Effect of RU486 on behavior of the mutants (b-d) and WT (e-g). Experienced fish were treated for two consecutive days with several doses (2.5-10mM) of RU486, or with ethanol vehicle, and tested the next day (see Methods) (n=5-6). RU486 does not alter freezing (b), swimming velocity (c) or wall avoidance (d) of the mutants (n=5-6). However, RU486 increased freezing responses of WT (n=3-8 animals per group; *p<0.05). Error bars represent ± SEM.
Whole-brain serta RNA expression in response to 4 day diazepam treatment.
Real-time PCR expression data for serta transcripts in total RNA extracted from the front part of the brain (including telencephalon, and anterior hypothalamus) following CMS for 4 days with and without continuous diazepam (5 μM) treatments (n= 4 animals per group).
serta expression in the pretectum.
serta mRNA expression in two consecutive sagital sections (100 μm) showing serta expression in the pretectal diencephalic cluster, the parvocellular preoptic nucleus (PP), of experienced fish. Expression strengths appear similar between the two genotypes in these areas of the brain.